Breast cancer (BC) is the most common malignant tumour among women, with its incidence and mortality rank first worldwide in 2018(Reference Bray, Ferlay and Soerjomataram1). In 2020, BC cases and deaths will account for 30 % and 15 % of diagnosed female cancers and cancer-related deaths, respectively, in America, while in China, the percentages in 2015 were approximately 15 % and 7 %, respectively(Reference Siegel, Miller and Jemal2,Reference Zheng, Sun and Zhang3) . Although the cases and death proportions in China were much lower than in America, there has been an upward trend in recent years(Reference Chen, Zheng and Baade4).
The malignant transformation of normal cells is analogous to somatic cell reprogramming, and the malignant transformation yields cells with unlimited self-renewal potential(Reference Suvà, Riggi and Bernstein5). DNA methylation inhibits epigenetic reprogramming either by silencing transcription factors or by promoting oncogenesis by impeding anti-proliferation genes(Reference Kim and Costello6). Studies have verified that DNA methylation as a relatively stable epigenetic modification type can maintain cell phenotype through continuous cell cycles(Reference Halley-Stott and Gurdon7). Traditional research on the relationship between DNA methylation and cancerogenesis is often based on tumour tissues. However, considering the availability of samples and the acceptability of subjects, peripheral blood leukocytes (PBL) DNA methylation may be hall markers in recent cancer molecular epidemiology studies(Reference Woo and Kim8). Kuchiba et al. (Reference Kuchiba, Iwasaki and Ono9) determined that the global methylation level of PBL DNA is low in BC cases, which may be a potential epigenetic marker for predicting the risk of developing BC.
The basic characteristic of cancer is the over proliferation and apoptosis suppression of cells(Reference Elmore10). Experimental studies have indicated that many natural dietary products, and their bioactive components could affect the development of cancer by mediating cell proliferation and apoptosis(Reference Li, Li and Meng11). Theresa et al. (Reference Hastert, Beresford and Patterson12) analysed the BC risk and dietary recommendations, proposed by the World Cancer Research Fund and the American Institute for Cancer Research, and found that meeting the World Cancer Research Fund/American Institute for Cancer Research cancer prevention recommendations of alcohol, body fatness and plant foods is associated with reduced postmenopausal BC risk. Natural dietary products could also interfere with DNA methylation by altering the methyl donor, or the activity of DNA methyltransferases. This phenomenon contributes to phenotype alteration and susceptibility to cancer(Reference Bai, Bai and Zhu13). Jana et al. confirmed that soya isoflavone daidzein specifically stimulates the expression of BRF2 (TF III B-related factor 2, a transcription factor required for synthesis of a small group of non-coding RNAs by RNA polymerase III) through promoter demethylation and then induces the proliferation of human BC cells(Reference Koo, Cabarcas-Petroski and Petrie14). Thus, the potential relationship of epigenetic events associated with dietary factors may afford new BC prevention strategies(Reference Khan, Aumsuwan and Khan15).
Cadherin-4 (CDH4), a member of the cadherin family, encodes Ca2+-dependent cell–cell adhesion glycoproteins(Reference Kools, Vanhalst and van Roy16). CDH4 has been confirmed to inhibit the proliferation, invasion and migration of cells in many cancers(Reference Xie, Feng and Lin17,Reference Ceresa, Alessandrini and Bosio18) . Georgia et al. reported that the overexpression of retinal cadherin (R-cad), encoded by CDH4, could induce glandular morphogenesis, inhibit invasiveness, tumour formation and lung colonisation in BC(Reference Agiostratidou, Li and Suyama19). Elena et al. verified that CDH4 methylation is observed in PBL DNA in gastrointestinal tumour patients and is an early event in carcinogenesis progression(Reference Miotto, Sabbioni and Veronese20). However, the relation between CDH4 methylation and BC risk has never been studied.
Herein, we explored the correlations of CDH4 methylation in PBL DNA, dietary factors and their combined and interactive effects with the risk of developing BC. We also explored the associations between CDH4 methylation in breast tumour tissue DNA and PBL DNA and clinicopathological characteristics. The relations of dietary factors, lifestyle and CDH4 methylation in BC tissues were also investigated.
Materials and methods
Study subjects
A case–control study including 380 cases and 439 controls was designed. Cases were newly diagnosed female BC patients from The Third Affiliated Hospital of Harbin Medical University from 2010 to 2014. Anyone who has undergone radiotherapy or chemotherapy was excluded. Controls were 208 female patients from the Department of Orthopaedics and Ophthalmology in The Second Affiliated Hospitals of Harbin Medical University and 231 female volunteers from Xiangfang District Centers for Disease Control and Prevention. The individuals who had a history of BC or malignancies, and benign neoplasms were excluded from the control group. All subjects who were pregnant, postpartum or breast-feeding were also excluded from cases and controls. Approximately 5 ml fasting peripheral venous blood was collected from case patients prior to surgery or from controls in enrolling.
A case-only study of 335 BC patients was also designed. A tumour tissue specimen from each patient was obtained during surgery, then immediately stored in liquid nitrogen after marking and finally frozen in a refrigerator at –80°C.
Data collection
A structured questionnaire was designed in accordance with quantitative reporting results(Reference Freudenheim, Marshall and Vena21,Reference Cho, Spiegelman and Hunter22) . For foods that are frequently ingested, such as cereal, vegetables, pork and eggs, were quantitatively collected based on the dietary habits of people in Northeast China(Reference Bai, Bai and Zhu13,Reference Koo, Cabarcas-Petroski and Petrie14) . Other food information with lower intake frequency, such as fish, milk and overnight foods, were collected according to the frequency. The question included the following information from each subject: demographic characteristics (age, marital status, occupation, etc.), lifestyle (drinking, smoking, physical activity, etc.) and frequency of food item consumption (vegetable, milk, fish, etc.). Lifestyle and dietary information for 1 year prior to a diagnosis or enrollment were collected. All subjects were interviewed face to face by well-trained interviewers. Clinicopathological information of each patient was obtained from medical records, including TNM stage, ER status, PR status, etc.
Genomic DNA extraction and sodium bisulphite modification
Genomic DNA was extracted from blood samples and tumour tissues by QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) and PureLinkTM Genomic DNA Kit (Thermo Fisher Scientific, Carlsbad, USA) and immediately stored at −80°C. Totally, 25 mg of tissue was prepared by cutting the tissue sample into small pieces in a tissue grinder before DNA extraction. Bisulfite modification was carried out using 2 μg of genomic DNA and EpiTect Bisulfite Kit (Qiagen, Hilden, Germany) and stored at −80°C.
All experimental procedures were strictly in line with the manufacturer’s guidelines. DNA quantity was determined using the Nanodrop 2000 Spectrophotometer (Thermo Scientific) before and after the genomic DNA extraction and bisulfite conversion.
Plasmid and standard curve construction
The cloning process was performed by TOPO TA Cloning for Sequencing kit (Invitrogen, America) using freshly purified specific PCR products and TOP10 competent cells based on the manufacturer’s instructions. The plasmid DNA was detected by sequencing after extraction, and then diluted to serial concentrations (10 to 107 copy number) to establish a standard curve for quantitative methylation-specific PCR(Reference Ramalho-Carvalho, Henrique and Jerónimo23).
Quantification of DNA methylation with quantitative methylation-specific PCR
The quantitative methylation-specific PCR procedure can confirm the specificity of the amplified product after the PCR reaction using melting curve analysis. The methylation of samples and plasmid DNA were detected in duplicate by LightCycler 480 (Roche Applied Science, Mannheim, Germany) equipped with Gene Scanning software (version 2.0). The primers of CDH4 and a housekeeping gene, MyOD, designed by Primer Premier 5.0 software were as follows: CDH4; forward primer, TGAGGGTGAGTGTAGATTTTTTTCG and reverse primer, CTCATTACAAAAAAAATAAAATCGCG; MyOD; forward primer, CCAACTCCAAATCCCCTCTCTAT and reverse primer, TGATTAATTTAGATTGGGTTTAGAGAAGGA.
The amplification reactions were performed in a total volume of 20 μl which consisted of: 10 μl SYBR Green Supermix, 0·6 μl of primers (final concentration: 600 nmol/l), 7·8 μl RNase-free water and 1 μl bisulphite converted DNA (concentration: PBL DNA, 15 ng/μl; tumour tissue DNA, 5 ng/μl). The amplification protocol was as follows: 6 min at 95°C for 1 cycle, 42 cycles at 94°C for 30 s, gene annealing temperature for 30 s, 72°C for 30 s and finally 30 s at 72°C for 1 cycle. The annealing temperature of CDH4 and MyOD was 63°C and 56°C, respectively. Duplicate reactions, negative controls and blank controls were designed for each experiment, and the method of methylation detection was repeated for 10 % of the samples.
The R 2 value of a standard curve between 0·97 and 1·00 indicated a good fit(Reference Ramalho-Carvalho, Henrique and Jerónimo23). The methylation level of each sample was calculated by the following formula: methylation level = (copy number of CDH4/copy number of MyOD) × 100 %.
A standard curve of CDH4 was obtained using quantitative methylation-specific PCR detection. A serial of 10 to 107 copy number of plasmid DNA and corresponding bisulphite modified samples were used as templates in this process. The R 2 value of the standard curve is 0·99. All plots were logarithmically amplified until a plateau was observed, and all samples have only one lysed peak, which is consistent with the plasmid DNA (online Supplementary Fig. 1).
Statistical analysis
We analysed the statistical difference of demographic characteristics between BC patients and controls with PBL DNA, CDH4 hypermethylated and hypomethylated BC patients with tumour tissue DNA. A χ 2 test was used in this calculation. OR and 95 % CI were calculated by univariate and multivariate unconditional logistic regression to analyse the association of CDH4 methylation in PBL DNA, dietary factors and lifestyle with BC risk. The same statistical methods were also used to determine the effects of dietary factors and lifestyle on CDH4 methylation in tumour tissue DNA. A cross-over study and multivariate logistic regression were used to assess the combined and interactive effects between CDH4 methylation, dietary factors and lifestyle in BC cases(Reference Wu, Yang and Zhang24). The correlation between CDH4 methylation in tumour tissue DNA, PBL DNA and clinicopathological characteristics were evaluated by a χ 2 test. All the calculations were performed using SPSS version 23.0, and P-value <0·05 was considered to have statistical significance.
Results
Association of Cadherin-4 methylation in peripheral blood leukocyte DNA and breast cancer risk
A total of 380 BC patients and 439 cancer-free controls with PBL DNA were included in this study. The demographic features are summarised in Table 1. The proportion of married women in the case group was significantly lower than the controls (89·74 % v. 94·05 %, P = 0·023). There were significant differences in educational level (P < 0·001), occupation (P < 0·001), family history of cancer (P < 0·001) and history of hormone therapy (P = 0·024) between the case group and controls. No significant differences were observed with regard to age (P = 0·27), BMI (P = 0·19) and reproductive history (P = 0·81).
Table 1. Demographic characteristics of breast cancer patients and controls with peripheral blood leukocyte DNA
(Number and percentages)

* We analysed the statistical difference of demographic characteristics between breast cancer patients and controls with PBL DNA by χ 2 test.
† Missing data: Marriage status: 2 controls; educational level: 2 controls; occupation: 34 cases, 74 controls; family history of cancer: 5 cases, 54 controls; BMI: 1 case, 2 controls; number of pregnancies: 3 cases, 5 controls; history of hormone therapy: 5 cases, 1 control.
‡ Marriage status: Single is a person who is unmarried, widowed (not remarried), separated (due to discord or long distance) and divorced (not remarried). Married is a person who is married or cohabitated.
§ BMI (weight/height2).
Table 2 summarises the relation between CDH4 methylation and BC risk. CDH4 methylation was divided into hypermethylation and hypomethylation based on the methylation cut-off value (0·778) when the Youden index is largest. Hypermethylated BC patients and controls in CDH4 were 175 (46·05 %) and 115 (26·20 %), respectively. After adjusting by marital status, education level, occupation and family history of cancer, the hypermethylation of CDH4 was significantly associated with increased BC risk in PBL DNA (ORadjusted (ORadj = 2·70, (95 % CI 1·90, 3·83), P < 0·001).
Table 2. Association of CDH4 methylation in peripheral blood leukocyte DNA and breast cancer risk
(Numbers and percentages)

ORcrude, odds ratio generated by univariate logistic regression; ORadj, odds ratio generated by univariate logistic regression, adjustment factors included marital status, education level, occupation, family history of cancer and history of hormone therapy.
* Methylation status, the methylation values were tested from peripheral blood leukocyte DNA of fasting peripheral venous blood. The methylation cut-off value (0·778) was selected according to the maximum Youden index.
Subgroup analyses
Supplementary Table 1 showed the demographic characteristics of luminal A patients and controls with PBL DNA. We observed significant differences between luminal A patients and controls regarding age (P = 0·020) and occupation (P = 0·040). Thus, the two factors were used as adjusted factors in the unconditional logistic regression. However, no significant differences were found between CDH4 methylation and luminal A BC risk (online Supplementary Table 2).
Then we analysed the demographic characteristics of luminal B patients and controls with PBL DNA (online Supplementary Table 3). There were significant differences in marital status (P = 0·046), occupation (P = 0·001) and family history of cancer (P < 0·001). Supplementary Table 4 showed the association of CDH4 methylation in PBL DNA and luminal B BC risk. Marital status, occupation and family history of cancer were adjusted in this calculation. CDH4 hypermethylation was found to be associated with increased luminal B BC risk (OR = 2·93, (95 % CI 1·89, 4·56), P < 0·001).
Association between dietary factors, lifestyle and breast cancer risk
The results of the univariate and multivariate analyses for the association between dietary factors, lifestyle and BC risk are shown in Supplementary Table 5. Educational level, occupation, marital status, family history of cancer and history of hormone therapy patients were used as adjusted factors in these calculations. In the multivariate analyses, vegetables (≥500 g/d v. <500 g/d, ORadj = 0·59, (95 % CI 0·41, 0·84)), allium vegetables (>3 times/week v. ≤3 times/week, ORadj = 0·37, (95 % CI 0·26, 0·53)), fish (≥3 times/month v. <3 times/month, ORadj = 0·45, (95 % CI 0·25, 0·81)), milk (≥1 times/week v. <1 time/week, ORadj = 0·57, (95 % CI 0·35, 0·93)) and physical activity (≥1times/month v. <1 time/month, ORadj = 0·57, (95 % CI 0·39, 0·83)) were significantly associated with decreased BC risk. However, overnight food (>3 times/week v. 0 time/week, ORadj = 2·38, (95 % CI 1·44, 3·92)) and pork (≥250 g/week v. 0 g/week, ORadj = 1·92, (95 % CI 1·14, 3·24)) were significantly associated with increased BC risk.
Combined and interactive effects of Cadherin-4 methylation and dietary factors, lifestyle in breast cancer
The combined and interactive effects between CDH4 methylation, dietary factors and lifestyle in BC are shown in Table 3. Among those factors, vegetables (<500 g/week v. ≥500 g/week, ORadj = 4·33, (95 % CI 2·63, 7·10)), allium vegetables (≤3 times/week v. >3 times/week, ORadj = 7·00, (95 % CI 4·17, 11·77)), fish (<3 times/week v. ≥3 times/week, ORadj = 7·92, (95 % CI 3·79, 16·53)) and milk (<1 time/week v. ≥1 times/week, ORadj = 6·30, (95 % CI 3·41, 11·66)) were significantly related to increased BC risk when combined with CDH4 hypermethylation. The same combined effects were also found between CDH4 hypermethylation and overnight food (>3 times/week v. ≤3 times/week, ORadj = 4·63, (95 % CI 2·69, 7·99)), pork (≥250 g/week v. <250 g/week, ORadj = 5·59, (95 % CI 2·94, 10·62)) and physical activity (<1 time/month v. ≥1 times/month, ORadj = 4·72, (95 % CI 2·87, 7·76)). No valuable interactive effect was found between any dietary factor, lifestyle with CDH4 methylation.
Table 3. Combined and interactive effects between CDH4 methylation and dietary factors and lifestyle in breast cancer
(odd ratios and 95 % confidence intervals)
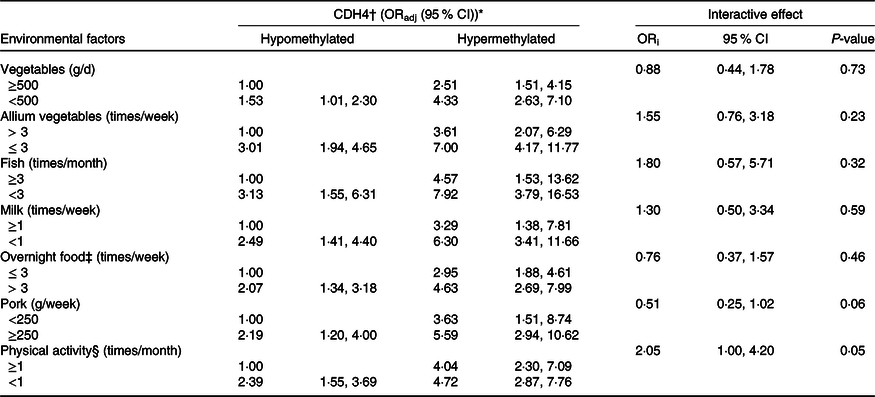
ORadj: adjusted for marital status, education level, occupation, family history of cancer and history of hormone therapy. ORi: ORinteraction, interactive effects.
* Bold values indicate significance after Bonferroni correction. P value is 0·05/14 = 0·003571.
† CDH4, the methylation values were tested from peripheral blood leukocyte DNA of fasting peripheral venous blood. And the methylation cut-off value (0·778) was selected according to the maximum Youden index.
‡ Overnight food, the cooked vegetables, eggs or meats that were left overnight either in the fridge or outside of the fridge.
§ Physical activity, Yes/No means exercise ≥ 70 min/<70 min of moderate-intensity aerobic physical activity; or ≥30 min/<30 min of vigorous-intensity aerobic physical activity, such as running, swimming and participating in ball games.
Effects of dietary factors and lifestyle on Cadherin-4 methylation in tumour tissue DNA
The distribution of demographic characteristics among BC patients with tumour tissue DNA is displayed in Table 4. The CDH4 methylation was defined as hypermethylated or hypomethylated by 55·5 % based on the 50 % percentile. Age, marital status, educational level, occupation, family history of cancer, BMI, reproductive history and history of hormone therapy were analysed in this calculation. No statistical association was found for any demographic characteristics (P > 0·05).
Table 4. Demographic characteristics of breast cancer patients with tumour tissues
(Numbers and percentages)
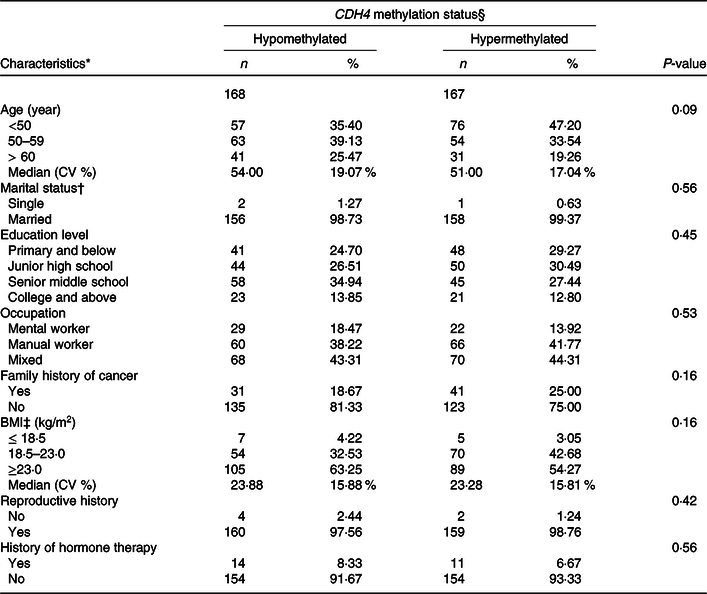
* Missing data: Age 13; Marriage status 18; Educational level 3; Occupation 4; Family history of cancer 5; BMI 5; Reproductive history, 10; History of hormone therapy, 2.
† Marriage status: Single is a person who was unmarried, widowed (not remarried), separated (due to discord or long distance) and divorced (not remarried). Married is a person who is married or cohabitated.
‡ BMI (weight/height2).
§ CDH4 methylation status, the methylation values were tested from tumour tissue specimen, and its cut-off value was 55·5 %, selected according to the 50 % percentile.
Table 5 displays the association of dietary factors and lifestyle exposure with CDH4 methylation in breast tissue DNA. Based on the univariate analysis, physical activity, milk and pork were significantly related to CDH4 methylation (P < 0·05). The multivariate logistic regression was calculated with these three factors. The results suggested that individuals who consumed milk ≥1 times/month (v. <1 times/month) have a lower probability of hypermethylated CDH4 (30·25 %, OR = 0·61 (95 % CI 0·38, 0·99)) in tumour cells. There was no statistical difference between other dietary factors or lifestyle (such as beef and mutton, poultry and fish) and CDH4 methylation.
Table 5. Association of dietary factors and lifestyle exposures and CDH4 methylation in breast tissue DNA
(Numbers and percentages; odd ratios and 95 % confidence intervals)
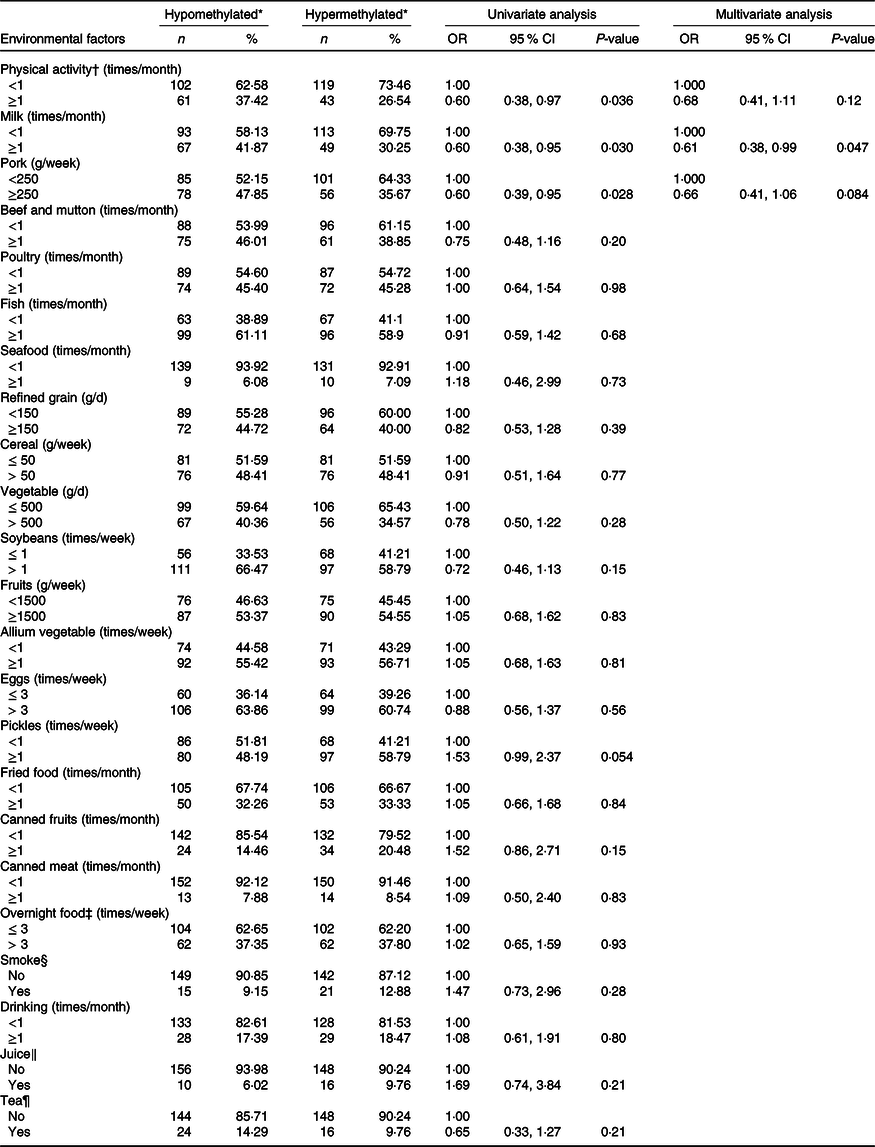
* CDH4 methylation status, the methylation values were tested from tumour tissue specimen and its cut-off value was 55·5 %, selected according to the 50 % percentile.
† Physical activity, Yes/No means exercise ≥ 70 min/<70 min of moderate-intensity aerobic physical activity; or ≥30 min/<30 min of vigorous-intensity aerobic physical activity such as running, swimming and participating in ball games.
‡ Overnight food, the cooked vegetables, eggs or meats that were left overnight either in the fridge or outside of the fridge.
§ Smoke, Yes means smoking at least 1/d for more than 6 weeks.
‖ Juice, Yes means drinking at least 1 cup/week for more than 3 months.
¶ Tea, Yes means drinking at least 2 times/week for more than 3 months.
Correlation between clinicopathological characteristics and Cadherin-4 methylation in peripheral blood leukocyte DNA and breast tissue DNA
The association between clinicopathological characteristics and CDH4 methylation in both PBL DNA and breast tumour tissue DNA is summarised in Supplementary Table 6. The selected clinicopathological characteristics were tumour location, TNM stage (tumour invasion, lymphnodes involved and metastasis status), ER status, PR status, HER2 status, molecular subtype, P53 status, histological type and Ki67 proliferation marker. No statistically significant results were observed between clinicopathological characteristics and CDH4 methylation in PBL DNA and breast tumour tissue DNA.
Discussion
The ‘mission critical’ events of cancer development include sustaining proliferative signalling, evading growth suppressors, resisting cell death and other events. Cell proliferation and apoptosis play a vital role in maintaining cell populations and as a defense mechanism(Reference Elmore10). Natural dietary products could influence the development of cancers by mediating DNA methylation to promote or inhibit cell proliferation and apoptosis(Reference Llanos, Dumitrescu and Brasky25). The key finding in this study is that we determined CDH4 hypermethylation increased BC risk, and its combined effects with low intake frequencies of vegetables, allium vegetables, fish, milk, low frequencies of physical activity and high intake frequencies of overnight food and pork increased BC risk. Moreover, lower intake frequencies of milk were significantly related to CDH4 hypermethylation in BC tissue.
The mechanism of DNA methylation in peripheral blood leukocytes and breast tumourigenesis has not been confirmed. But Marsit et al. (Reference Marsit and Christensen26) reported that tumours do not develop as an isolated phenomenonin their target tissue, other organ systems, including the immune system (such as peripheral blood leukocytes) is also involved in the occurrence of tumours. Moreover, carcinogenic process usually leads to immunological changes related to inflammation, causes changes in white blood cell subpopulations and their numbers, thus results to changes of epigenetic characteristics in peripheral blood leukocytes(Reference Koestler, Marsit and Christensen27). Epidemiologic studies have analysed and suggested that peripheral blood leukocytes DNA can be used as a potential tumour biomarker(Reference Terry, Delgado-Cruzata and Vin-Raviv28). Increasing studies have also reported the association of gene methylation levels in PBL and BC risk, indicating that epigenetic alteration of PBL may be a potential biomarker for BC risk(Reference Choi, James and Link29–Reference Delgado-Cruzata, Wu and Perrin31).
CDH4 has been reported to play anti-proliferation and pro-apoptosis roles in salivary adenoid cystic carcinoma, glioblastoma, etc.(Reference Xie, Feng and Lin17). R-cad, encoded by CDH4, usually expresses in non-transformed breast cells but is downregulated in breast tumour cell lines. R-cad could also inhibit lung metastasis by suppressing critical regulators of metastatic seeding and growth (MMP1, MMP2 and COX2)(Reference Agiostratidou, Li and Suyama19). Du et al. reported that CDH4 can inhibit cell proliferation, colony formation, migration and elicit cell communication. Du et al. also report that CDH4 is hypermethylated in five nasopharyngeal carcinoma cell lines, nasopharyngeal carcinoma cell xenograft lines and 94·3 % of primary tumours but not in any of twelve normal epithelial samples(Reference Du, Huang and Sun32). Elena et al. reported that CDH4 methylation can be detected in 78 % of colorectal and 95 % of gastric carcinomas, and the gene expression can be restored by treatment with demethylating agent 5-aza-2-deoxycytidine(Reference Miotto, Sabbioni and Veronese20). In this study, we demonstrate that CDH4 hypermethylation in PBL DNA was associated with increased BC risk (ORadj = 2·70, (95 % CI 1·90, 3·83), P < 0·001), which indicated that CDH4 hypermethylation is an independent risk factor for BC occurrence. In addition, studies on BC subtype indicated that luminal B BC has a higher expression of proliferation markers and higher histologic grade(Reference Ades, Zardavas and Bozovic-Spasojevic33). Li et al. (Reference Li, Hu and Tu34) found that patients with luminal B BC have a higher proportion of local recurrence and single bone metastasis, and these risks presented during a 2- to 5-year period and after 5 years. In our study, CDH4 hypermethylation was significantly associated with increased luminal B BC risk (ORadj = 2·93, (95 % CI 1·89, 4·56), P < 0·001), which indicated that CDH4 methylation may be a biomarker of luminal B BC.
Vegetables, together with allium vegetables, are essential in Chinese diet and have been shown to relate to decreased BC risk(Reference Li, Meng and Xie35,Reference Zhang, Ho and Chen36) . Vegetables contain a number of nutrients and phytochemicals with cancer chemopreventive properties, including folate, fibre, carotenoids, antioxidants, etc., and allium vegetables are rich in organosulphur compounds, such as diallyl trisulphide(Reference Li, Meng and Xie35,Reference Zhang, Ho and Chen36) . Antioxidants, such as vitamin C and vitamin E, can protect DNA from oxidative damage and help reduce oxidative stress to prevent BC(Reference Zhang, Ho and Chen36). In addition, dietary lycopene can inhibit MCF-7 cell proliferation by decreasing the mitogenic activity of insulin-like growth factor I. Also, all-trans-retinoic acid, a retinoid, can induce a dose-dependent MCF-7 cell death(Reference Karas, Amir and Fishman37,Reference Prakash, Russell and Krinsky38) . Diallyl trisulphide has been reported to inhibit BC stem cell proliferation by suppressing the Wnt/β-catenin pathway activation and thus inhibit the occurrence of BC(Reference Li, Meng and Xie35). The anti-cancer effects of vegetables and allium vegetables are also reflected in inflammation marker inhibition. γ-Tocopherol found in vegetables and quercetin found in onions were shown to decrease the expression of cyclooxygenase-2 (COX-2) by inhibiting the p300 signalling and blocking the binding of multiple transactivators to COX-2 promoter and then inhibit cell proliferation in BC, which are consistent with the mechanism of CDH4 above(Reference Agiostratidou, Li and Suyama19,Reference Smolarek, So and Thomas39,Reference Xiao, Shi and Liu40) . Similarly, this study found eating vegetables ≥500 g/d (v. <500 g/d) and allium vegetables >3 times/week (v. ≤3 times/week) decreased the risk of BC (ORadj are 0·59 and 0·37 (95 % CI are 0·41, 0·84 and 0·26, 0·53)). The low intake frequencies of these two dietary factors were significantly correlated with increased BC risk when combined with CDH4 hypermethylation (ORadj are 4·33 and 7·00 (95 % CI are 2·63, 7·10 and 4·17, 11·77)), which indicated that CDH4 hypermethylation carriers may benefit from consuming more vegetables and allium vegetables to further decrease BC risk.
Fish are rich in n-3 PUFA, while pork contains SFA and cholesterol(Reference Bessaoud, Daurès and Gerber41,Reference Schley, Brindley and Field42) . n-3 PUFA can alter cell membrane phospholipid fatty acid composition and disrupt lipid rafts. This not only reduced the expression of inflammatory genes but also altered the level of epidermal growth factor receptor and induced the apoptosis of human BC cells(Reference Schley, Brindley and Field42,Reference Calder43) . SFA can stimulate the production of endogenous estrogen and induce the proliferation of breast alveolar epithelial cells, thereby promoting BC(Reference Bessaoud, Daurès and Gerber41). In this study, consuming fish ≥3 times/month (v. <3 times/month) decreased BC risk (ORadj = 0·45, (95 % CI 0·25, 0·81)), while consuming pork ≥250 g/week (v. 0 g/week) increased BC risk (ORadj = 1·92, (95 % CI 1·14, 3·24)). However, the low intake frequencies of fish and high intake frequencies of pork combined with CDH4 hypermethylation increased BC risk (ORadj = 7·92and 5·59; (95 % CI 3·79, 16·53 and 2·94, 10·62)). In view of the anti-proliferative and pro-apoptotic effects of CDH4 (Reference Xie, Feng and Lin17), we recommend that CDH4 hypermethylation carriers ingest more fish and decrease the consumption of pork to prevent BC.
Our study indicated consuming milk ≥3 times/week (v. <3 times/week) could decrease the risk of BC (ORadj =0·57, (95 % CI 0·35, 0·93)), and the combined effect of low intake frequencies of milk and CDH4 hypermethylation was correlated with increased risk of BC (ORadj = 6·30, (95 % CI 3·41, 11·66)). These results may be due to the presence of vitamin D. The high level of 25-hydroxyvitamin D, the form of vitamin D in serum, has been reported to decrease the activity of vascular endothelial growth factor and inhibit the migration and invasion of breast tumour tissue(Reference Shao, Klein and Grossbard44). Finally, the activity of vitamin D receptors can induce cell cycle arrest, autophagy and apoptosis, thus decreasing the occurrence of BC(Reference Welsh45). We also found that consuming milk ≥1 times/month (v. <1 time/month) was significantly related to decreased CDH4 methylation (OR = 0·61, (95 % CI 0·38, 0·99)) in BC tissue, which may provide an important inspiration of diet and epigenetic mechanisms in cancer. Vitamin D can induce DNA demethylation, and this demethylation effect has been shown for E-cadherin in triple-negative BC(Reference Lopes, Carvalho and Durães46). Based on the above findings, we propose that consuming milk may decrease CDH4 methylation levels and thus decrease the risk of BC; however, this proposal still needs to be validated via in vitro experiments.
We also found consuming overnight food >3 times/week increased BC risk (v. ≤3 times/week, ORadj = 2·38, (95 % CI 1·44, 3·92)), and the combination of high intake frequencies of overnight food and CDH4 hypermethylation increased BC risk (ORadj = 4·63, (95 % CI 2·69, 7·99)). Previous research has shown that the nitrate content in cooked vegetables would greatly increase when left for 24 h(Reference Raineri and Weisburger47). The nitrate can form N-nitroso compounds with amines and amides in vivo, which are known potent carcinogens. Maki et al. evaluated the interaction of dietary nitrate intake with total folate intake on BC risk and found that the interactive effect of the two dietary factors increased BC risk(Reference Inoue-Choi, Ward and Cerhan48). Therefore, we recommend CDH4 hypermethylation carriers to lower overnight food consumption to minimise BC risk.
Dietary factors are closely related to physical activity in the cancer mechanism. For example, SFA contained in pork can stimulate the production of endogenous estrogen, which can lead to the occurrence of BC, while physical activity can decrease the risk of BC by inhibiting oestrogen levels(Reference Bessaoud, Daurès and Gerber41,Reference Tworoger, Missmer and Eliassen49) . Leptin has been reported to promote cell proliferation and reduce cell apoptosis, while adiponectin has the opposite effect on cells; physical activity can reduce the risk of BC by reducing leptin levels and increasing adiponectin levels(Reference Jardé, Caldefie-Chézet and Goncalves-Mendes50,Reference Bouassida, Chamari and Zaouali51) . In addition to dietary factors, we also analysed the role of physical activity and CDH4 methylation in BC. In this study, a rate of physical activity ≥1 times/month decreased BC risk (v. <1 time/month, ORadj = 0·57, (95 % CI 0·39, 0·83)) and the combination of low frequencies of physical activity and CDH4 hypermethylation increased BC risk (ORadj = 4·72, (95 % CI 2·87, 7·76)). CDH4 methylation is related to low expression and its anti-proliferative and pro-apoptotic effects may be inhibited(Reference Miotto, Sabbioni and Veronese20). Thus, CDH4 hypermethylation carriers are more likely to develop BC if they do not participate in higher frequencies of physical activities.
BC is a complex disease characterised by the accumulation of multiple molecular events, and each tumour phenotype shows special evolutionary potential(Reference Traoré, Koulibaly and Diallo52). Jacob et al. found a high methylation percentage of GSTM2 in ER/PR-negative tumours compared with ER/PR-positive tumours, indicating that GSTM2 hypermethylation may serve as a potential biomarker for aggressive tumour development(Reference Kresovich, Gann and Erdal53). However, no significant association between clinicopathological characteristics and CDH4 methylation in PBL DNA or breast tumour tissue DNA was observed in this study.
This study does posses several limitations. First, the tissue sample from the BC patients is a very mixed population of cell types rather than only tumour cells, which may lead to a bias in estimates of the carcinogenic effects of CDH4 methylation. Second, recall bias as a basic disadvantage of case–control studies is unavoidable. Third, we observed that CDH4 hypermethylation is related to increased BC, but in vitro experiments are still needed. Finally, sample size may limit the statistical power of combination and interaction analysis, thereby larger numbers of subjects are encouraged to verify the presented conclusions.
Conclusion
In summary, we have reported a significant association between CDH4 hypermethylation in PBL DNA and increased BC risk. The combination of CDH4 hypermethylation in PBL DNA and lower intake frequencies of vegetables, allium vegetables, fish, milk, lower frequencies of physical activity, and higher frequencies of overnight food and pork intake may increase BC risk. Moreover, increasing milk intake significantly prevents the risk of BC, especially in CDH4 hypermethylation carriers, and may also improve the prognosis by mediating the hypermethylation status of tumour tissue.
Acknowledgements
This study was funded by the National Nature Science Foundation of China (Nos. 81202262, 81773503), Postdoctoral Science Special Foundation of China (2013T60390) and Dr Wu Lien-teh Science Foundation of Harbin Medical University (WLD-QN1106).
F. W. and H. C. contributed to the study conception and design. Material preparation and data collection were performed by Z. W., Y. C. and K. X. Sample processing was performed by N. Z., S. L., L. L. and H. Y. Analysis were performed by Z. L., J. D. and V. D. The first draft of the manuscript was written by N. Z. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
The authors declare that they have no conflict of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114521002804








