Obesity represents one of the major public health problems worldwide. In Mexico alone, it affects almost 70 % of people between 30 and 60 years old(Reference Shamah-Levy, Villalpando Hernández and Rivera Dommarco1).
Obesity directly contributes to an increase in proinflammatory adipocytokines, such as leptin, TNF-α, IL-6 and IL-1β, and a decrease in adiponectin. Alterations in the concentrations of these cytokines are known to result from greater fat mass (adiposity), causing the chronic inflammation associated with type 2 diabetes and CVD, among other complications(Reference Coppack2–Reference Schmatz, Madan and Marino4).
In pregnant women, obesity may exacerbate the chronic inflammation associated with gestation, particularly at term, increasing the mother's risk of presenting several complications such as gestational diabetes, pre-eclampsia, infection and preterm labour, among others(Reference Madan, Davis and Craig5–Reference Mitchell, Keelan, Peebles and Myatt7).
Furthermore, high concentrations of proinflammatory cytokines can also affect fetal development by causing major alterations in bronchopulmonar and neurological development and predisposing to a number of childhood and adult obesity-related conditions(Reference Schmatz, Madan and Marino4, Reference Viscardi, Muhumuza and Rodriguez8, Reference Chen, McNiff and Madan9).
However, the impact that greater maternal adiposity might have on the concentration of pro-inflammatory cytokines in fetal circulation is still uncertain. Therefore, the purpose of the present pilot study was to identify the possible associations between maternal adiposity and the concentration of various pro-inflammatory markers at term gestation both in maternal and fetal circulations.
Experimental methods
Patient selection
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving patients were approved by the Internal Research and Ethics Committees of the National Institute of Perinatology in Mexico City. A written informed consent was obtained from all patients.
Twenty women with term pregnancies (>37 weeks of gestation) who delivered by elective caesarean section at the National Institute of Perinatology in Mexico City were included in the present study, together with their newborns. Patients were carefully selected to discard labour, infection, metabolic or autoimmune pathologies.
Patients were programmed for elective caesarean section because of a personal or familial history of pregnancy-related or other complications, according to institutional policies. Most of the patients had a history of pre-eclampsia, abortions and preterm deliveries; however, these were all conditions of previous pregnancies and patients presented no complications during the current gestation.
For the present pilot study, women were initially selected using their pregestational BMI to include ten patients diagnosed as ‘normal’ and ten diagnosed as ‘overweight/obese’ according to the BMI classification of the World Health Organization(10).
The main characteristics of participant women and their newborns are presented in Table 1. No differences were found in maternal age, parity or gestational age. Maternal adiposity was significantly different between groups (as expected). No differences were found in newborn characteristics between groups.
Table 1 Characteristics of participant women and their newborns
(Median values and ranges)

PreBMI, pregestational BMI.
* Values were statistically different using Mann–Whitney's test.
† Total body fat calculated with the equation from Villar et al. (Reference Villar, Cogswell and Kestler11).
Anthropometric measurements
Maternal measurements were made at the time of their admission, 12 h before surgery. The present weight of the participants was measured using a Tanita scale model 1631 (Tanita, Arlington Heights, IL, USA). Height was measured with a Seca 206 instrument (Seca Corporation, Hanover, MD, USA). Tricipital, subscapular and leg skinfolds were measured using a Harpender skinfold caliper (Baty International, West Sussex, UK). Resistance was measured with a bioimpedance system (RJL Systems, Clinton Township, MI, USA) according to the manufacturer's instructions. All measurements were made by the same standardised person.
Newborn characteristics, including weight, length, cephalic, thoracic and abdominal perimeters, were obtained from the clinical files.
Pregestational BMI was calculated using pregestational weight referred by the patients.
Estimation of maternal adiposity
Although pregestational BMI was used to initially select the patients, we believe that maternal adiposity (measured at the time of cytokine quantification) would be a much better parameter to relate with the inflammatory environment of term gestation.
Maternal adiposity, defined as total body fat, was estimated for each patient using the equation developed by Villar et al. (Reference Villar, Cogswell and Kestler11), which considers present weight, body surface, subscapular skinfold, leg skinfold and resistance.
Biological samples
Maternal blood (5–10 ml) was drained from the patient's forearm 12 h before surgery and fetal blood was taken from the umbilical cord vein immediately after the placenta was removed. Samples were collected in 10 ml plastic tubes with heparin, and plasma was separated by centrifuging at room temperature for 15 min at 3000 rpm and stored at − 70°C until used.
Hb concentrations were determined to ensure equal blood volumes (Table 1).
Cytokine quantification
Leptin, adiponectin, TNF-α, IL-6 and IL-1β were chosen because of their known implication in the onset of inflammatory processes, which may affect fetal development and gestational outcome(Reference Schmatz, Madan and Marino4). These cytokines were quantified simultaneously using Fluorokine® MAP MultiAnalyte Profiling kit (R&D Systems, Minneapolis, MN, USA) with molecule-specific antibodies in a Bio-Plex system (Bio-Rad, Hercules, CA, USA) following the manufacturer's protocol. Samples were diluted fourfold as recommended by the manufacturer, and the results were multiplied by the dilution factor.
Detection ranges of the assays were 53·8–43 668·9 pg/ml for leptin, 347·4–255722·9 pg/ml for adiponectin, 3·8–2793·5 pg/ml for TNF-α, 4·7–3487·5 pg/ml for IL-6 and 2·2–1596·1 pg/ml for IL-1β. Intra-assay coefficients of variance were < 5 % for all cytokines.
Statistical analyses
Maternal adiposity was arbitrarily categorised in to two groups according to the median value of total body fat (group 1 ‘low adiposity’ = total body fat < 50 percentile; group 2 ‘high adiposity’ = total body fat >50 percentile).
Correlations between maternal adiposity (uncategorised) and cytokine concentrations were evaluated with Spearman's test. Only strong, significant correlations are reported.
Differences in cytokine concentrations were analysed with Mann–Whitney's test. Differences with P ≤ 0·05 were considered significant.
Differences in sex frequencies of newborns between adiposity groups were analysed with a χ2 test, since fetal sex has been correlated with higher amounts of cytokines(Reference Scott, Hodyl and Murphy12, Reference Basu, Laffineuse and Presley13).
All statistical analyses were made with Statistical Package for Social Sciences version 12.0 software (Chicago, IL, USA).
Results
No differences were found in maternal age, gestational age, newborn birth weight and length, and newborn cephalic, thoracic or abdominal perimeters between low-adiposity and high-adiposity groups.
Maternal adiposity showed a significant negative correlation with fetal plasma adiponectin (r − 0·587, P = 0·01) not observed in maternal plasma. Adiponectin concentration was significantly higher in fetal blood than in maternal blood in both groups. Interestingly, this cytokine was significantly lower in fetal blood of newborns from women in the high-adiposity group compared with newborns in the low-adiposity group, a difference not observed in maternal blood (Fig. 1).
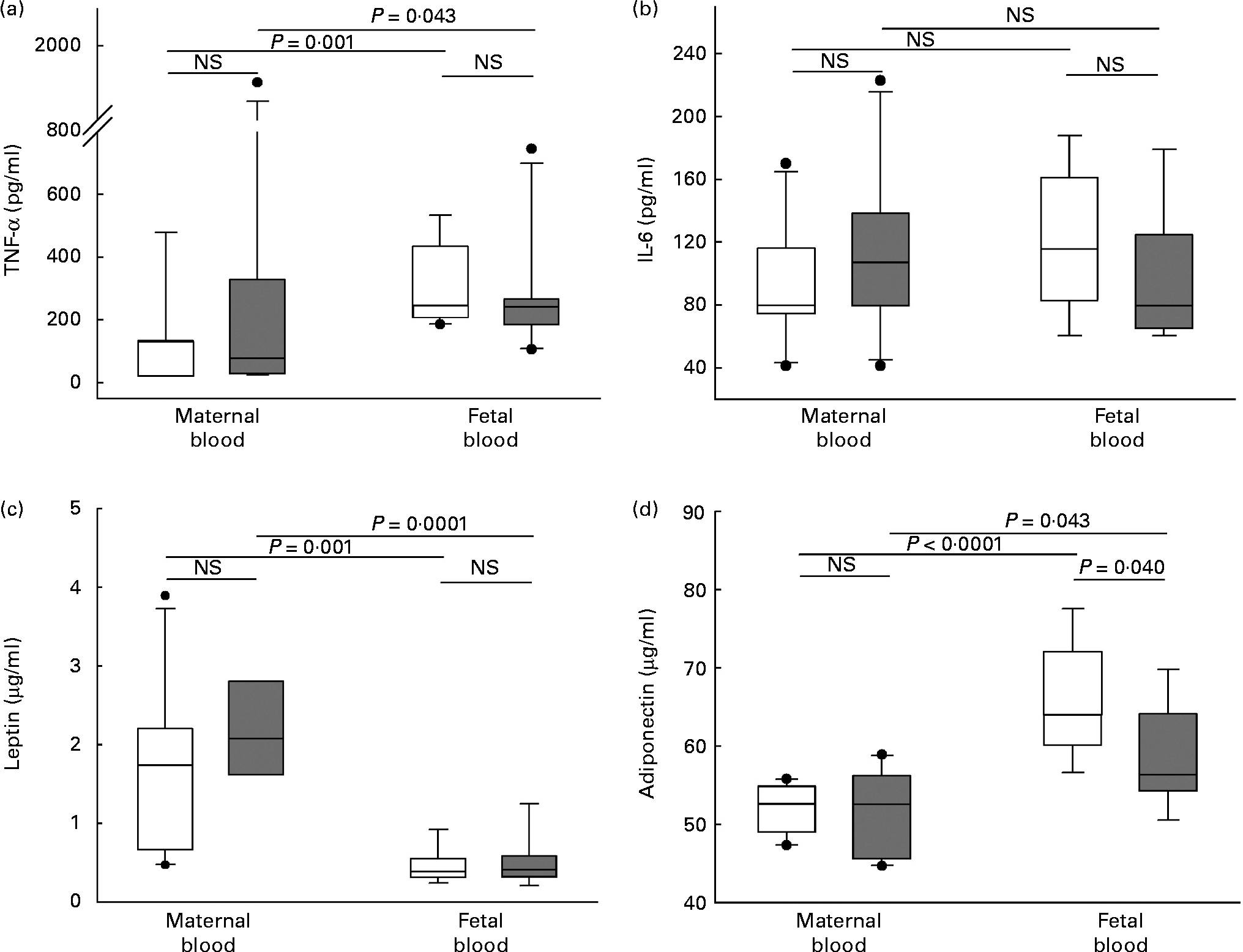
Fig. 1 Plasma concentration of cytokines in maternal and fetal blood. (a) TNF-α, (b) IL-6, (c) leptin and (d) adiponectin. Samples were categorised in to ‘low-adiposity’ group ( < 50 percentile (□) of total body fat) and ‘high-adiposity’ group (>50 percentile (![]() ) of total body fat), according to the median value of maternal adiposity. The values represent median concentrations with interquartile ranges. Outlier values are represented by ●. Differences tested with Mann–Whitney's test. NS (P>0·05).
) of total body fat), according to the median value of maternal adiposity. The values represent median concentrations with interquartile ranges. Outlier values are represented by ●. Differences tested with Mann–Whitney's test. NS (P>0·05).
IL-6 also showed a significant negative correlation with maternal adiposity (r − 0·466, P = 0·05). However, its concentration showed no difference between blood (maternal/fetal) or adiposity (low/high) groups (Fig. 1).
As expected, there was a significant positive correlation between maternal adiposity and leptin plasma concentration in maternal peripheral blood (r 0·527, P = 0·02). This was not observed in fetal blood. Leptin concentrations were not different between the low-adiposity and high-adiposity groups, although they clearly tended to increase in the high-adiposity group. Its concentrations were significantly higher in maternal blood compared with fetal blood (Fig. 1).
Plasma concentration of TNF-α did not correlate with maternal adiposity and was not different between low-adiposity and high-adiposity groups. However, it was significantly higher in fetal blood than in maternal blood (Fig. 1).
IL-1β could not be quantified in any sample, since all of the measurements were below the assay sensitivity limit (0·05 pg/ml).
Discussion
In the present study, we provide evidence that maternal adiposity has an effect on the concentration of adipocytokines in fetal and maternal circulations. The relevance of these findings lies in the fact that a chronic inflammatory environment, such as that characteristic of obesity, may have several adverse effects on pregnancy outcomes and fetal development and predisposition to obesity-related complications(Reference Schmatz, Madan and Marino4).
We found a significant inverse correlation between maternal adiposity and fetal blood concentrations of adiponectin and IL-6. Adiponectin is known to be anti-inflammatory in various cell types, while IL-6 plays a regulatory role in inflammation processes(Reference Xing, Gauldie and Cox14, Reference Ouchi and Walsh15). The present study results also show higher amounts of adiponectin in fetal circulation, agreeing with a previous report(Reference Mazaki-Tovi, Kanety and Pariente16). Since the major source of this hormone is the fetus, this could suggest that there may be some maternal-originated factors that could pass through the placenta and affect adiponectin secretion by fetal tissues.
The fact that newborns from patients with high adiposity had significantly lower amounts of circulating anti-inflammatory cytokines might suggest that their capacity to control inflammation could be diminished, and therefore be at more risk of complications resulting from an exacerbated inflammation (e.g. abortion and preterm labour)(Reference Challis, Lockwood and Myatt17).
Maternal adiposity correlated with leptin concentrations in maternal but not fetal blood, suggesting that its transport to fetal circulation is carefully controlled by the placenta.
Plasma concentrations of TNF-α and IL-1β were not altered by maternal adiposity since the effects of these adipose-derived cytokines are limited to the local environment and not extended systemically(Reference Xu, Hirosumi and Uysal18, Reference Matsuki, Horai and Sudo19).
Even with the small sample size of the present pilot study, some interesting associations between adipocytokines and maternal adiposity could be observed. A larger study, with sample size and power calculated from the results of the present pilot study, would allow us to divide the patients in more groups and further strengthen the associations.
In summary, the present results suggest that, due to lower circulating anti-inflammatory cytokines, fetuses from obese women may be less able to control the inflammatory process (e.g. during intra-uterine infections or even during normal labour), which could limit optimal fetal development or even increase the risk of abortion or preterm labour.
Acknowledgements
We thank Otilia Perichart-Perera for valuable technical and methodological assistance throughout the study. The authors declare no conflict of interests. Funding was obtained through internal funds of the National Institute of Perinatology in Mexico City. R. V.-S. designed the study, analysed the data and wrote the manuscript; H. A. B.-V. recruited the patients, collected and processed the samples and analysed the data; G. R. also analysed the data and wrote the manuscript; A. E.-N. processed the samples; J. B.-M. recruited the patients; F. V.-O. designed the study.




