Fetuin-A, also referred to as α2-Heremans–Schmid glycoprotein (AHSG), is a protein synthesised and secreted by the liver, particularly in hepatic steatosis( Reference Stefan, Hennige and Staiger 1 ). To a lesser degree, fetuin-A is also secreted by placenta and tongue, and recent findings suggest that it is also expressed and secreted by adipose tissue( Reference Stefan, Hennige and Staiger 1 , Reference Chatterjee, Seal and Mukherjee 2 ). Fetuin-A is related to hepatic insulin resistance and subclinical inflammation, and it has been suggested as a novel marker for hepatic fat accumulation( Reference Stefan, Hennige and Staiger 1 , Reference Ix, Shlipak and Brandenburg 3 , Reference Stefan, Fritsche and Weikert 4 ). Mice deficient for the AHSG gene are resistant to weight gain upon a high-fat diet( Reference Auberger, Falquerho and Contreres 5 , Reference Mathews, Singh and Ranalletta 6 ). Observational studies have shown a positive association between fetuin-A and obesity( Reference Ix, Shlipak and Brandenburg 3 , Reference Weikert, Stefan and Schulze 7 ), and a recent bidirectional Mendelian Randomisation study suggests that fetuin-A is causally associated with higher BMI( Reference Thakkinstian, Chailurkit and Warodomwichit 8 ). In addition, there is growing evidence from prospective studies that high plasma fetuin-A concentrations are associated with a higher risk of type 2 diabetes( Reference Stefan, Fritsche and Weikert 4 , Reference Ix, Biggs and Mukamal 9 ) and cardiovascular diseases( Reference Weikert, Stefan and Schulze 7 ). Taken together, fetuin-A has a role in a number of metabolic conditions and chronic diseases. Therefore, knowledge of modifiable determinants of circulating fetuin-A has direct public health relevance. However, so far little is known about dietary determinants of fetuin-A concentrations in the general population. In a randomised clinical trial among 76 overweight diabetic women, calorie restriction resulted in a decrease in fetuin-A concentrations( Reference Choi, Han and Ahn 10 ). In a cross-sectional analysis within a subcohort from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study, dietary intake of red meat or whole grain was not associated with fetuin-A concentrations( Reference Montonen, Boeing and Fritsche 11 ). In a recent analysis of women from the Nurses’ Health Study, an inverse association between alcohol consumption and fetuin-A concentrations was observed, and fetuin-A explained a substantial proportion of the inverse association between alcohol consumption and risk of type 2 diabetes( Reference Ley, Sun and Jimenez 12 ). In both the EPIC-Potsdam Study and the Nurses’ Health Study, dietary intake was assessed using FFQ.
With this study, we aimed to investigate the association between energy intake, energy-providing nutrients, alcohol consumption and major food groups and plasma fetuin-A concentrations in the Bavarian Food Consumption Survey II (BVS II), a population-based survey in which dietary intake was assessed by three 24-h dietary recalls.
Methods
Study design and population
The BVS II is a cross-sectional study designed to be representative for the Bavarian population. The aim of the study was to investigate dietary and lifestyle habits in Bavaria. The study population comprises 1050 German-speaking participants aged 13–80 years who were recruited in a three-stage random route sampling procedure between September 2002 and June 2003. Participants’ characteristics, lifestyle factors and medical history were collected during a computer-aided personal interview. Within 2 weeks after recruitment, trained interviewers contacted participants by telephone three times (2 weekdays and 1 weekend day) to assess dietary intake by 24-h dietary recalls. In addition to dietary information, physical activity on the previous day was assessed at the end of each telephone interview using standardised questions on type and duration of physical activity, as well as duration of television/personal computer time and sleeping hours. The overall participation rate was 71 %. Within 6 weeks after recruitment, all adults (≥18 years) who had completed the baseline interview and at least one dietary recall were invited to their nearest public health office for blood sampling and anthropometrical measurements. Out of 879 invited subjects, 568 persons followed the invitation (65 % of eligible persons). The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the local ethics committee (Bavarian Ministry of Health)( Reference Himmerich, Gedrich and Karg 13 ). Written informed consent was obtained from all study participants.
Dietary intake assessment
The 24-h dietary recalls were conducted using the EPIC-Soft software( Reference Slimani, Deharveng and Charrondiere 14 , Reference Slimani, Ferrari and Ocke 15 ). The data from the three recalls per person were weighted for weekday and weekend day to calculate the average daily food intake. The different foods reported during the 24-h dietary recalls were grouped into seventeen food groups. Nutrient intakes were calculated using food content data from the German food composition database ‘Bundeslebensmittelschluessel’ (version II.3)( Reference Dehne, Klemm and Henseler 16 ).
Blood sampling and laboratory measurements
Venous blood was drawn into EDTA tubes or serum tubes, chilled at 4°C and processed subsequently within 3 h. Serum was separated from blood cells by centrifugation, and samples were divided into aliquots. Samples were cooled for a maximum of 1 d until they were stored at –80°C. Plasma concentrations of fetuin-A were measured by ELISA (BioVendor Human Fetuin-A ELISA; intra-assay CV 3·9–6·5 %, inter-assay CV 2·6–5·1 % according to the manufacturer) in the laboratory of Professor Pischon, Molecular Epidemiology Group, Max Delbrück Center for Molecular Medicine (MDC). On the basis of internal quality control samples (two on each of the eight analysis plates), the inter-assay CV was 7·7 %.
Statistical analysis
After exclusion of participants with missing information on diet (n 7) or fetuin-A (n 3), 558 participants (235 men and 323 women) were included in the statistical analysis. Waist circumference was missing for a few (n 8) study participants. For statistical analysis, these missing values were replaced with sex-specific median values. In descriptive statistics, we compared participants’ characteristics across sex-specific quintiles of fetuin-A concentrations. The association between total energy intake, energy-providing nutrients (dietary fat, carbohydrates, protein), alcohol or major food groups (vegetables, fruit, milk/dairy products, unprocessed red meat, processed meat, poultry and fish) and fetuin-A concentrations was investigated using multivariable linear regression models with robust variance( Reference White 17 ). Results are presented as mean fetuin-A concentrations with corresponding 95 % confidence intervals in quintiles or categories of dietary intake. In addition, continuous estimates showing the increase or decrease in fetuin-A associated with a prespecified increment in dietary intake are presented. Multivariable models were adjusted for age (continuous in years), sex, smoking status (never, former, current), social status (five categories), physical activity (sex-specific quintiles of total activity in MET-h/d), alcohol intake (except for models investigating alcohol intake; non-drinker or continuous in g/d) and non-alcohol energy intake (continuous in kcal/d with and without additional adjustment for BMI (continuous, kg/m2) and residuals of BMI-adjusted waist circumference (to avoid multicollinearity; continuous). Fasting status was not included in the multivariable models because it was not related to fetuin-A concentrations, and inclusion of fasting status into the models did not alter parameter estimates of dietary variables substantially. For the analysis of dietary fat, carbohydrates or protein intake, we created multivariable energy-density models with nutrient intake expressed in percentage of total energy intake and mutual adjustment for energy intake. The continuous estimates from these models estimate the change in circulating fetuin-A associated with a 5 % higher energy intake provided by the nutrient under study in substitution with 5 % of energy provided by carbohydrates (or fat). Because of the relatively large proportion of non-drinkers (10 % in men and 19 % in women), for the analysis of the association between alcohol consumption and fetuin-A we present adjusted mean fetuin-A concentrations in established categories for alcohol intake( Reference Pai, Hankinson and Thadhani 18 ). Similarly, because of the low consumption of poultry and fish, mean fetuin-A concentrations are presented by categories of these two variables (non-consumers, </≥40 g/d, which corresponds to the approximate median cut-offs). Tests for linear trends across dietary intake quintiles or categories were performed by modelling the median values in each quintile/category and evaluating this variable’s statistical significance using the Wald’s test. To correct for multiple hypothesis testing, we took the false-discovery rate into account, counting each dietary factor under investigation as an independent hypothesis test( Reference Benjamini, Drai and Elmer 19 ) (n 12). In prespecified subgroup analyses, we tested for statistical interaction in the association between dietary factors and fetuin-A by sex using cross-product terms. Because no statistically significant interactions by sex were observed (all P-values>0·2), only combined associations in the whole study population are presented. However, because under-reporting of energy intake has been shown to depend on sex and BMI( Reference Ferrari, Slimani and Ciampi 20 , Reference Heerstrass, Ocke and Bueno-de-Mesquita 21 ), we also describe the association between energy intake and fetuin-A concentrations stratified by sex and BMI (</≥25 kg/m2). In addition, to investigate the robustness of observed associations, we conducted sensitivity analyses. First, we excluded participants who were suspected to be under-reporters of dietary intake based on a low ratio of energy intake (EI) to estimated basal metabolic rate (BMR) (EI/BMR<0·8, n 18 men, n 28 women)( 22 ). Second, we excluded study participants with metabolic diseases (n 203) – that is, participants who were obese (BMI≥30 kg/m2, n 110) and/or had reported prevalent diabetes (n 37) or hypertension (n 138).
Results
Mean fetuin-A concentrations were 303·7 µg/ml in men, ranging from 177·6 to 462·7 µg/ml, and in women the mean was 314·7 µg/ml, with a range from 182·4 to 531·9 µg/ml. The mean age of study participants decreased across quintiles of fetuin-A concentrations (Table 1). Waist circumference and BMI slightly increased across fetuin-A quintiles. Mean dietary intakes of energy, alcohol, energy-providing nutrients and major food groups in male and female BVS II participants are shown in online Supplementary Table S1.
Table 1 Basic characteristics by quintilesFootnote * of circulating fetuin-A in 558 men and women who participated in the Bavarian Food Consumption Survey II (Mean values and standard deviations; numbers and percentages)

* Quintile cut-offs were 264, 291, 314 and 344 µg/ml in men and 265, 299, 325 and 358 µg/ml in women.
There was no clear association between energy intake or intake of energy-providing nutrients with fetuin-A concentrations (Table 2). We observed a slight suggestion of a positive association between total energy intake and circulating fetuin-A (per 2092 kJ/d (500 kcal/d) 3·7 µg/ml, 95 % CI –0·5, 7·8 µg/ml in the multivariable model including body fatness), but the adjusted mean values across quintiles were not suggestive of a linear trend (P trend 0·16). The continuous estimate was slightly higher but still statistically nonsignificant (4·5 µg/ml, 95 % CI –0·3, 9·2 µg/ml) when under-reporters of energy intake were excluded (sample size after exclusion n 512). Although a statistically significant association between energy intake and fetuin-A concentrations was observed in men (continuous estimate 6·4 µg/ml, 95 % CI 1·2, 11·5 µg/ml, P trend 0·02), there was no association in women (2·2 µg/ml, 95 % CI –4·6, 9·1 µg/ml, P trend 0·52; P interaction 0·84). After stratification by BMI, we observed a statistically significant continuous estimate (7·9 µg/ml, 95 % CI 0·1, 15·6 µg/ml) and trend across quintiles (P trend 0·02) in lean study participants (BMI<25 kg/m2), but not in overweight participants (BMI≥25 kg/m2, continuous estimate 2·0 µg/ml, 95 % CI –2·8, 6·7 µg/ml, P trend 0·87), although no multiplicative interaction was observed (P interaction 0·40). Mean fetuin-A concentrations increased slightly across quintiles of fat intake and decreased slightly across quintiles of carbohydrate or protein intake. However, there was no suggestion of any important association between energy-providing nutrient intake and fetuin-A concentrations.
Table 2 Multivariable-adjusted mean fetuin-A concentrations (95 % CI) by quintiles of energy intake or energy-providing nutrient intake in 558 men and women who participated in the Bavarian Food Consumption Survey II

* Increments are 2092 kJ/d (500 kcal/d for total energy); 5 % of energy for fat, carbohydrates and protein.
† Multivariable adjusted for age, sex, smoking status, social status, physical activity, alcohol intake (non-drinker or g/d).
‡ Multivariable adjusted for age, sex, smoking status, social status, physical activity, energy intake, alcohol intake (non-drinker or % of energy) and protein intake (% of energy).
§ Multivariable adjusted for age, sex, smoking status, social status, physical activity, energy intake, alcohol intake (non-drinker or % of energy), fat intake (% of energy).
|| Energy intake refers to non-alcohol energy intake.
¶ Plus body fatness refers to additional adjustment for BMI and BMI-adjusted waist circumference residuals.
We observed an inverse association between alcohol consumption and fetuin-A concentrations (Table 3). In the multivariable model including body fatness, the mean fetuin-A concentrations were 324 (95 % CI 313, 335) µg/ml in non-drinkers, 311 (95 % CI 302, 320) µg/ml in individuals with low alcohol consumption (<5 g/d), 314 (95 % CI 304, 323) µg/ml in individuals with low-moderate alcohol consumption (5–14·9 g/d), 303 (95 % CI 292, 314) µg/ml in moderate drinkers (15–29·9 g/d) and 293 (95 % CI 281, 306) µg/ml in individuals in the highest alcohol consumption category (≥30 g/d); a statistically significant trend across alcohol intake categories was observed (P trend 0·003). The inverse association remained statistically significant after taking the false-discovery rate into account (adjusted P trend 0·04). The adjusted mean fetuin-A values across alcohol intake categories (data not shown) were similar in men (P trend 0·04) and in women (P trend 0·04), in lean participants (BMI<25 kg/m2, P trend 0·66) and in overweight (BMI≥25 kg/m2, P trend 0·05) participants, and after exclusion of participants with metabolic diseases (P trend 0·003).
Table 3 Multivariable-adjusted mean fetuin-A concentrations (95 % CI) by categories of alcohol consumption in 558 men and women who participated in the Bavarian Food Consumption Survey II
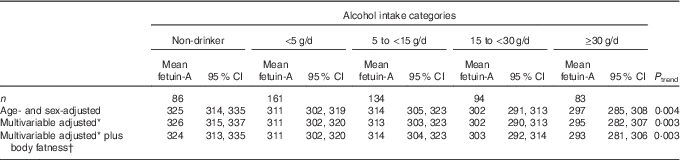
* Multivariable adjusted for age, sex, smoking status, social status, physical activity and energy intake (excluding energy from alcoholic beverages).
† Plus body fatness refers to additional adjustment for BMI and BMI-adjusted waist circumference residuals.
The associations between dietary intake of major food groups and circulating fetuin-A are shown in Table 4: We observed a statistically significant inverse association between milk and dairy product intake and circulating fetuin-A: mean fetuin-A concentrations decreased across quintiles of milk and dairy products (P trend 0·02), and each 150-g increment in milk/dairy products per d was associated with 5·6 (95 % CI –9·6, –1·5) µg/ml lower fetuin-A. This inverse association was slightly attenuated after additional adjustment for protein (–4·9 µg/ml, 95 % CI –9·1, –0·7 µg/ml), but it was not substantially altered after additional adjustment for fat (–5·4 µg/ml, 95 % CI –9·4, –1·4 µg/ml) or Ca intake (–5·4 µg/ml, 95 % CI –11·2, 0·4 µg/ml). Milk, yogurt and cheese were also individually inversely associated with fetuin-A (milk per 100 g/d –3·3 µg/ml, 95 % CI –6·5, –0·2 µg/ml; yogurt per 50 g/d –2·0 µg/ml, 95 % CI –5·6, 1·7 µg/ml; cheese per 30 g/d –2·4 µg/ml, 95 % CI –7·5, 2·8 µg/ml). However, the inverse association between milk and dairy product intake and fetuin-A concentrations was statistically nonsignificant after accounting for the false-discovery rate (adjusted P trend 0·18). Fetuin-A concentrations were not associated with dietary intake of vegetables, fruit, unprocessed red meat, processed meat, poultry or fish.
Table 4 Multivariable-adjusted mean fetuin-A concentrations (95 % CI) by quintiles or categories of major food groups in 558 men and women who participated in the Bavarian Food Consumption Survey II

* Statistically significant results (P<0·05).
† Multivariable adjusted for age, sex, smoking status, social status, physical activity, alcohol intake (non-drinker or g/d) and non-alcohol energy intake.
‡ Plus body fatness refers to additional adjustment for BMI and BMI-adjusted waist circumference residuals.
§ Continuous estimates were calculated per portion. Portion sizes based on approximate standard deviations: vegetables, 100 g/d; fruit, 150 g/d; dairy products, 150 g/d; unprocessed red meat, 50 g/d; processed meat, 50 g/d; poultry, 30 g/d; fish, 30 g/d.
Discussion
To our knowledge, this is the first observational study comprehensively investigating dietary determinants of fetuin-A. Total energy intake and energy-providing nutrients were not clearly associated with fetuin-A concentrations. We observed that alcohol intake was associated with lower fetuin-A concentrations. Among the major food groups, higher dietary intake of milk/dairy products was associated with lower circulating fetuin-A, but this association was not statistically significant after correction for multiple hypothesis testing. Dietary intakes of fruit, vegetables, meat or fish were not associated with fetuin-A concentrations. Because of the described association between fetuin-A and obesity, insulin resistance, diabetes and coronary heart disease, modulation of fetuin-A concentration by dietary guidance may be of public health relevance.
The here observed inverse association between alcohol intake and fetuin-A is largely supported by the existing literature: Univariable inverse associations between alcohol intake and fetuin-A have been observed in several epidemiological studies( Reference Stefan, Fritsche and Weikert 4 , Reference Weikert, Stefan and Schulze 7 , Reference Ix, Biggs and Mukamal 9 , Reference Jensen, Bartz and Mukamal 23 – Reference Obuchi, Adachi and Enomoto 25 ). Similar to our investigation, higher alcohol consumption was associated with lower fetuin-A concentrations in women participating in the Nurses’ Health Study after adjustment for demographic information and lifestyle variables including BMI( Reference Ley, Sun and Jimenez 12 ). Furthermore, post hoc analyses of three randomised cross-over trials on alcohol intake revealed that moderate alcohol consumption decreased fetuin-A in men, although no significant association was observed in women( Reference Joosten, Schrieks and Hendriks 26 ). To date, the physiological mechanisms that may explain the inverse association between alcohol intake and fetuin-A remain unclear.
We also observed that higher milk/dairy product intake was statistically significantly associated with lower fetuin-A, although statistical significance was lost after accounting for the false-discovery rate. The inverse association was slightly attenuated after additional adjustment for protein, suggesting that protein may partly explain the association. However, adjustment for other nutrients found in dairy products, such as fat or Ca, did not alter the associations remarkably. Several epidemiological studies have observed an inverse association between dairy product consumption and presence of the metabolic syndrome( Reference Elwood, Pickering and Fehily 27 , Reference Van Meijl, Vrolix and Mensink 28 ). In a prospective study among young adults, inverse associations between dairy product consumption and the development of obesity and insulin resistance were observed( Reference Pereira, Jacobs and Van Horn 29 ). Furthermore, a few intervention studies have shown that dairy product consumption is associated with improved insulin sensitivity( Reference Turner, Keogh and Clifton 30 ). Considering the role of fetuin-A in the insulin signalling pathway( Reference Srinivas, Wagner and Reddy 31 ) – that is, induction of insulin resistance through inhibition of insulin-receptor tyrosine kinase( Reference Hennige, Staiger and Wicke 32 ) – it is conceivable that fetuin-A may have a mediating role in the association between dairy product consumption and improved insulin sensitivity. This warrants further exploration in prospective studies.
Several limitations of this study should be noted. First of all, because of the cross-sectional study design in which the dietary exposure and the biomarker outcome both were assessed within a short time period, it is difficult to determine the direction of observed associations, and we cannot make any causal inferences. We hypothesised that dietary factors would influence fetuin-A concentrations. It appears unlikely that fetuin-A concentrations directly influence dietary habits, but we cannot exclude that, for example, existing fatty liver disease reflected by high fetuin-A concentrations may have led to a change in dietary habits, because of dietary recommendations given by general practitioners. In addition, although through multivariable adjustment we tried to control for potential confounding as completely as possible, residual confounding cannot be excluded. We also cannot exclude the possibility that storage of plasma samples at –80°C for approximately 10 years may have affected fetuin-A concentrations. However, any such impact on fetuin-A measurement is unlikely to be differential according to participant’s dietary intake, and thus it is unlikely to have introduced systematic bias. Furthermore, the absolute fetuin-A concentrations in our study were comparable to concentrations that have been observed in other observational studies in Germany( Reference Stefan, Hennige and Staiger 1 , Reference Weikert, Stefan and Schulze 7 ). In our study, habitual dietary intake was assessed with three 24-h dietary recalls involving detailed quantification of consumed foods including composition of mixed meals. As with all self-reported methods, the dietary assessment by three 24-h recalls is prone to measurement error, as it depends on the participants’ memory and ability to recall their diet. In addition, because the 24-h recall is an open-ended instrument, the interviewer is also a potential source of bias. However, the training of interviewers and the high standardisation of the 24-h recalls conducted with EPIC-soft limits this source of bias( Reference Slimani, Ferrari and Ocke 15 ). The here applied number of three 24-h recalls has been shown to be sufficient for estimating total energy intake with energy intake assessment using the doubly labelled water (DLW) as the reference method( Reference Ma, Olendzki and Pagoto 33 ). However, misclassification of diet, in particular under-reporting of energy intake, remains a concern when relating self-reported dietary intake to health outcomes. Under-reporting of energy intake has been reported to be more prevalent in individuals with a high BMI and in women( Reference Ferrari, Slimani and Ciampi 20 , Reference Heerstrass, Ocke and Bueno-de-Mesquita 21 ). A recent pooled analysis of five DLW validation studies demonstrated that under-reporting of energy intake was approximately 10 % with three averaged 24-h recalls compared with 30 % with FFQ( Reference Freedman, Commins and Moler 34 ). Although dietary intake is reported more accurately with multiple 24-h recalls than with FFQ, under-reporting of energy intake had a role also in the present study: by comparing the reported energy intake with the estimated basal metabolic rate, we identified a proportion of under-reporters of energy intake (n 46, 8 %) in our sample. In the analysis of energy intake in relation to fetuin-A concentrations, continuous estimates were slightly stronger after exclusion of under-reporters, and statistically significant only in lean study participants and in men. These observations point to the problem of misreporting. Thus, the analysis of energy intake in relation to fetuin-A should be interpreted with caution. Overall, we expect misreporting of diet in our study to be non-differential – that is, misclassification independent of fetuin-A concentrations – which may have biased observed associations towards a null association. A further limitation of our study is related to the generalisability of our findings. Although the BVS II was designed as a representative study, the findings observed here can be generalised to the adult Bavarian population only with caution, as the overall participation of adult study participants who also provided blood samples was 46 % (71 %×65 %), thus compromising the representativeness of our study sample. Whether our findings may be generalised to other populations warrants further investigation, but we expect that the associations found in our study should be comparable in populations with similar characteristics as in our study.
In conclusion, in this comprehensive investigation of dietary determinants of fetuin-A, we observed that higher consumption of alcohol and dietary intake of milk/dairy products were associated with lower fetuin-A concentrations. These observations warrant confirmation by further observational studies or controlled feeding intervention studies. Nevertheless, our findings provide a first suggestion that fetuin-A concentrations may be influenced by targeted dietary interventions. Considering the role of fetuin-A in the development of obesity, insulin resistance, diabetes and coronary heart disease, this may be of direct public health importance. Whether previously observed associations between dietary intake and health outcomes are mediated by fetuin-A requires exploration in prospective cohort studies.
Acknowledgements
The authors acknowledge the cooperation of all study participants. They also thank Georg Karg, Kurt Gedrich and Stefanie Himmerich for their major contribution in the setup and conduct of the study.
This work was supported by funds of the Bavarian Ministry of Environment, Health and Consumer Protection and the Kurt-Eberhard-Bode-Stiftung.
The authors’ contributions were as follows: K. N., T. P. and J. L. contributed to the conception and design of the research; K. N. performed statistical analyses, interpreted the data and wrote the paper; J. L. was responsible for the concept and design of the BVS II, as well as the acquisition of data; J. J. was responsible for the measurement of fetuin-A in plasma samples; all authors critically appraised the manuscript and approved the final version.
There are no conflicts of interest.
Supplementary material
For Supplementary material/s referred to in this article, please visit http://dx.doi.org/10.1017/S0007114515002639







