Puberty is a milestone in life, accompanied by dramatic physical, psychological and emotional changes, and its effects on health and wellbeing are profound(Reference Patton and Viner1). A secular trend towards an earlier age of puberty onset has been observed in recent years(Reference Chow, Chou and Tung2,Reference Eckert-Lind, Busch and Petersen3,Reference Brix, Ernst and Lauridsen4) . Early puberty onset had implications for the physiology and psychology of children, and for the risk of later adult disease(Reference Golub, Collman and Foster5,Reference Mendle, Ryan and McKone6) , which raised public health concerns. Studies showed that earlier puberty was related to depression, unhealthy and antisocial behaviours, a higher risk of obesity, hypertension, diabetes, CVD or even cancer later in life(Reference Mendle, Ryan and McKone6,Reference Day, Elks and Murray7) . Many factors contributed to the early onset of puberty, and although it was strongly heritable, striking secular trends of early onset of puberty in global childhood suggested that lifestyle and environmental factors warranted attention(Reference Almstrup, Frederiksen and Andersson8). As a modifiable factor, lifestyle is increasingly regarded as an important and cost-effective intervention strategy for disease prevention and control(Reference Tamimi, Spiegelman and Smith-Warner9,Reference King, Meader and Wright10) .
Previous studies found that some lifestyles were related to early puberty, but with inconsistent conclusions. A review indicated that dietary intakes and patterns were important lifestyle factors that influenced the timing of puberty(Reference Cheng, Buyken and Shi11). The relationship between physical activity and puberty timing remained controversial, since most studies were conducted in elite athletes, and little evidence was seen from general population samples(Reference Calthorpe, Brage and Ong12). Later age of menarche seemed to be associated with longer sleep duration(Reference Wang, Kwok and Au Yeung13), but sleep duration had a limited connection with the age of sexual development in another study(Reference Foley, Ram and Susman14). The onset of puberty occurred significantly earlier among people with smoking(Reference Lall, Singhi and Gurnani15), without drinking alcohol before puberty(Reference Peck, Peck and Skaggs16). However, the results were not consistent. Existing literature on lifestyles and pubertal development was mostly examined based on cross-sectional studies without considering chronological order. Moreover, menarche, a retrospective indicator of late puberty, was mostly used in previous studies, which may not precisely define the stage of puberty. Few studies on the association between lifestyle and early onset of puberty undertake clinical examination to evaluate pubertal development.
Based on the existing knowledge, lifestyle factors were found to be clustered in childhood and adolescence, a comprehensive lifestyle index comprising excessive alcohol use, drug use, unprotected sexual behaviours and sleep duration predicted the disease burden outcomes characterised by major depressive disorder, psychological distress, self-harm and suicide attempt with a large precision(Reference Mewton, Champion and Kay-Lambkin17). Lifestyle factors may interrelate or act synergistically, and thus a healthy lifestyle pattern has a stronger preventive effect on diseases than a single factor(Reference Dhana, Haines and Liu18,Reference Yannakoulia, Kontogianni and Scarmeas19) . The strategy of promoting a comprehensive healthy lifestyle was well recognised and common to many guidelines and recommendations in weight loss and CVD prevention(20,Reference Martinez-Gomez, Guallar-Castillon and Higueras-Fresnillo21) . However, previous researches focused on lifestyle and puberty timing mostly studied a single lifestyle factor. Therefore, the purpose of this study was to examine the association between healthy lifestyle patterns before puberty and the risk of early onset of puberty in children based on a cohort study, and to provide support and guidance for early prevention of early puberty onset.
Methods
Study population
In 2017, the pubertal cohort study was established in Xiamen City, Fujian Province. Random cluster sampling was conducted in four schools covering the primary and secondary stages (grades 1–9), and all girls in grades 2–3 and boys in grades 3–4 who did not enter puberty were invited to participate in the project. The subjects were followed up every 6 months for a total of three and a half years up to October 2020. The selection process of participants and follow-up flow chart were shown in online Supplementary Fig. 1. Finally, a total of 1294 children with complete lifestyle information was included in this study. This study adhered to the Declaration of Helsinki for ethical standards and had been approved by the Medical Ethical Committee of Peking University (Number: IRB00001052-17026), and all the written informed consents were obtained from both parents of each child.
Data collection and measurements
Physical examination
Height and weight measurements were conducted by trained medical school graduates using standard procedures and calibrated instruments during each survey. Height was measured to the nearest 0·1 cm using a portable stadiometer with neither shoes, hats nor hair clips. Weight was measured to the nearest 0·1 kg using a weight scale in light clothing. BMI was weight divided by height squared.
According to Tanner stages, pubertal development was assessed individually by two trained graduates in a separate room(Reference Marshall and Tanner22,Reference Marshall and Tanner23) . Breast development in girls and testicular volume and genital development in boys were evaluated by visual inspection and palpation based on the rating scales of Tanner and Prader orchidometer. In case the breast and testicular development of the two sides were not equal, the larger side measurement was used(Reference Dai, Fu and Liang24).
Questionnaire investigation
Children’s pre-pubertal lifestyle, early life factors and parental demographic characteristics were collected by self-administrated questionnaires. The questionnaire had been validated and used in the National Survey on Students Constitution and Health and multi-centre healthy lifestyles interventions study(Reference Yang, Li and Hu25,Reference Chen, Ma and Ma26) . The lifestyle pattern was formed based on previously reported factors that were associated with pubertal timing(Reference Cheng, Buyken and Shi11,Reference Calthorpe, Brage and Ong12,Reference Wang, Kwok and Au Yeung13,Reference Peck, Peck and Skaggs16,Reference Barbaresko, Rienks and Nöthlings27) , including five components such as dietary behaviour, physical activity, sleep duration, smoking and drinking behaviour, and sedentary behaviour. Information was collected through student questionnaires with the assistance of parents. Dietary behaviour included the frequency of having breakfast, milk, high-energy food, eating out, and consumptions of fruit, vegetable, sugar-sweetened beverages, and meat product. Physical activity was assessed by calculating the average time per d when children spent in any of the moderate or vigorous physical activities during the past 7 d. Participants were asked to report their average daily sleep duration for the past 7 d, and their smoking and drinking behaviour (yes/no) in the past month. The quality of sedentary behaviour was categorised by the total time per d when children spent on sitting, reading or doing homework, watching electronic devices and playing electronic games. Children’s early life factors (birth weight, feeding patterns and type of delivery) and parental characteristics (parental BMI and education levels) were collected by parent questionnaires.
Definition of indicators
The age of puberty onset was defined as the age when breast development first reached stage 2 in girls and testicular volume first reached 4 ml in boys by the time of the examination(Reference Marshall and Tanner22,Reference Marshall and Tanner23,Reference Tanner and Whitehouse28) . A child would be defined as early onset of puberty if his age of puberty onset was earlier than the first quartile age of puberty onset for the same-sex population(Reference He, Guan and Liu29).
Each favourable lifestyle component was determined according to the recommendations of the Dietary Guidelines for Chinese school-age children and Canadian Guidelines for children and youth(Reference Tremblay, Carson and Chaput30,31) , as shown in the online Supplementary Table 1. Based on the American Heart Association’s recommendation for ideal cardiovascular health and the contents of this study questionnaire(Reference Lloyd-Jones, Hong and Labarthe32), dietary behaviour included eight dietary factors. The cut-offs of ideal dietary factors were defined according to the Dietary Guidelines for Chinese school-age children(31). Meeting 6–8 ideal dietary factors were considered as good dietary behaviour. Active physical activity was defined as 1 h or more of moderate and vigorous intensity physical activity every day(Reference Tremblay, Carson and Chaput30,31,Reference Lloyd-Jones, Hong and Labarthe32) . For children, adequate sleep duration meant at least 9 h/d(Reference Tremblay, Carson and Chaput30,31) . Smoking and drinking were harmful to children’s health, so not smoking and drinking were recommended(31). Sedentary behaviour, independent of physical activity, had different effects on a variety of health outcomes(Reference Barbaresko, Rienks and Nöthlings27). However, there was no unified standard to classify ideal status. Considering the recommendation for screen time was ≤2 h and doing homework was ≤2 h; thus, the total time of sedentary behaviour ≤6 h was regarded as low sedentary behaviour in this study.
The lifestyle components were calculated on a scale of 0–5 points, with 1 point awarded for meeting favourable criteria and 0 point for the contrary. The healthy lifestyle pattern of individuals was categorised into four groups based on comprehensive lifestyle scores: a healthy lifestyle (those who met 4–5 points), an intermediate healthy lifestyle (3 points), an unfavourable lifestyle (2 points) and a poor lifestyle (0–1 point).
Nutritional status was determined according to sex-age-specific BMI cut-offs recommended by the International Obesity Task Force(Reference Cole, Bellizzi and Flegal33).
Statistical analysis
Descriptive statistics were used to evaluate the basic information of study population. Continuous variables were expressed by mean values and standard deviations, and t test was used for comparisons between groups. Categorical variables were expressed by numbers and percentages, and χ 2 was used for comparison between groups. Multivariate linear regression and log-binomial regression were applied to analyse the association between lifestyle in pre-puberty and age of puberty onset, and risk of early onset of puberty. The β-coefficients (coef) and risk ratios and 95 % CI were calculated. Children’s baseline BMI, early life factors, parental nutritional status and education levels were adjusted as covariates in models. Multiple classifications of lifestyle scores were included as continuous variables in the regression model for the trend test. Population attributable risk was used to estimate the difference between the baseline scenario proportion and the fantasy scenario proportion, which reflected the intervention effects on early onset of puberty if maintaining a healthy lifestyle theoretically. All statistical tests were two-sided and were considered statistically significant at P < 0·05. All analyses were conducted by Stata version 15.0.
Results
Characteristics of the study population
There were no statistically significant differences in basic information and age of puberty onset between children included and excluded in the study (online Supplementary Table 2). Table 1 presented the characteristics of the 1294 children included in our analysis. The average age of children was 8·4 years old at baseline, and girls were younger than boys. There were differences in the age of puberty onset and parental BMI between boys and girls. Most children had an unfavourable lifestyle (36·2 %), and only 12·2 % of children met a healthy lifestyle. Boys and girls had different lifestyles, and boys had more favourable lifestyles, especially for physical activity status.
Table 1. Characteristics of the study population
(Mean values and standard deviations)
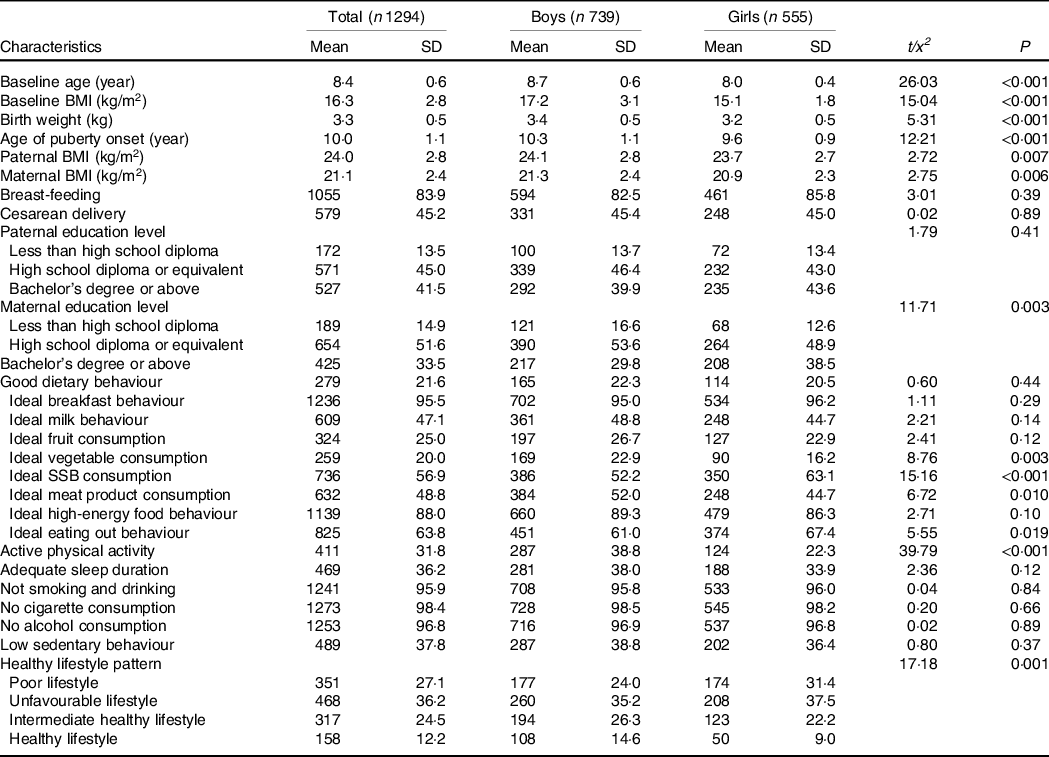
SSB, sugar-sweetened beverages.
Continuous variables were expressed by mean values and standard deviations, and categorical variables were expressed by numbers and percentages.
Association between lifestyles in pre-puberty and early onset of puberty
Table 2 presented the results of multivariate linear regression analysis of the association between pre-pubertal lifestyle and age of puberty onset. After adjusting for confounding factors, active physical activity and low sedentary behaviour could delay the age of puberty onset by 0·19 (coef = 0·19, 95 % CI (0·00, 0·37)) and 0·28 (coef = 0·28, 95 % CI (0·10, 0·46)) years in boys. The healthy lifestyle pattern could significantly delay the age of puberty onset in boys (P for trend = 0·02). Compared with boys who had a poor lifestyle, the age of puberty onset in boys with healthy lifestyle was delayed by 0·36 years (coef = 0·36, 95 % CI (0·08, 0·65)). The healthy lifestyle pattern also seemed to delay the age of puberty onset in girls, but the differences were not statistically significant.
Table 2. Multivariate linear regression analysis of the association between pre-pubertal lifestyle and age of puberty onset
(Coefficient values and 95% confidence intervals; number and percentages)

Model 1 did not adjust for any variables; Model 2 adjusted for baseline BMI, birth weight, feeding patterns, type of delivery, parental BMI and education levels.
Boys with active physical activity and low sedentary behaviour had a low incidence of early onset of puberty (17·4 %). The results of log-binomial regression were consistent with that of linear regression. Boys with active physical activity and low sedentary behaviour had a low risk of early onset of puberty (online Supplementary Table 3). Healthy lifestyle pattern was related to a lower risk of the early onset of puberty in boys, and as the number of satisfied favourable lifestyle components increased, the risk of early onset of puberty decreased (P for trend = 0·01). Compared with boys who had a poor lifestyle, children who adhered to a healthy lifestyle had a 53 % lower risk of early onset of puberty (risk ratio = 0·47, 95 % CI (0·27, 0·80)).
Since nutritional status played a role in the early onset of puberty, stratified by nutritional status in pre-puberty, healthy lifestyle pattern was associated with a delayed age of puberty onset and a lower risk of early onset of puberty in the normal-weight group, and the difference was statistically significant in boys. But in overweight and obese children, the association between healthy lifestyle pattern and the age of puberty onset was not significant (Table 3 and online Supplementary Table 4). Stratified by healthy lifestyle pattern, the adverse effect of baseline BMI on early onset of puberty was observed among girls with an unhealthy lifestyle rather than a healthy lifestyle (online Supplementary Table 5).
Table 3. The multivariate linear regression analysis of the association between pre-pubertal lifestyle and age of puberty onset, stratified by baseline nutritional status

Fig. 1 presented the PAR and incidence of early puberty onset if people maintained a healthy lifestyle. The incidence of early onset of puberty could theoretically be reduced by 8·54 % for all boys and 15·18 % for boys with a poor lifestyle if they adopted a healthy lifestyle, and the differences were statistically significant (P < 0·01). The incidence of early onset of puberty could theoretically be reduced by 8·80 % for all girls and 12·01 % for girls with a poor lifestyle if they adopted a healthy lifestyle, but the differences were not statistically significant (P > 0·05) (online Supplementary Table 6).

Fig. 1. The population attributable risk and incidence of early onset of puberty if people maintained a healthy lifestyle adjusting for baseline BMI, birth weight, feeding patterns, type of delivery, parental BMI and education levels.
Early onset of puberty and different lifestyle combinations
The incidences of early onset of puberty with different lifestyle combinations were different (Fig. 2, online Supplementary Table 7). In any combination of two favourable lifestyle components, it was easier for boys to follow an active physical activity and not smoking and drinking, low sedentary behaviour and not smoking and drinking to reduce the incidence of early onset of puberty because of the relatively high prevalence of these two combinations. Boys who adhered to active physical activity and low sedentary behaviour had a relatively low incidence of early onset of puberty (11·5 %) and 0·49 years delayed age of puberty onset (Table 4). Therefore, future comprehensive interventions targeting this combination will have great potential to reduce the incidence of early onset of puberty.
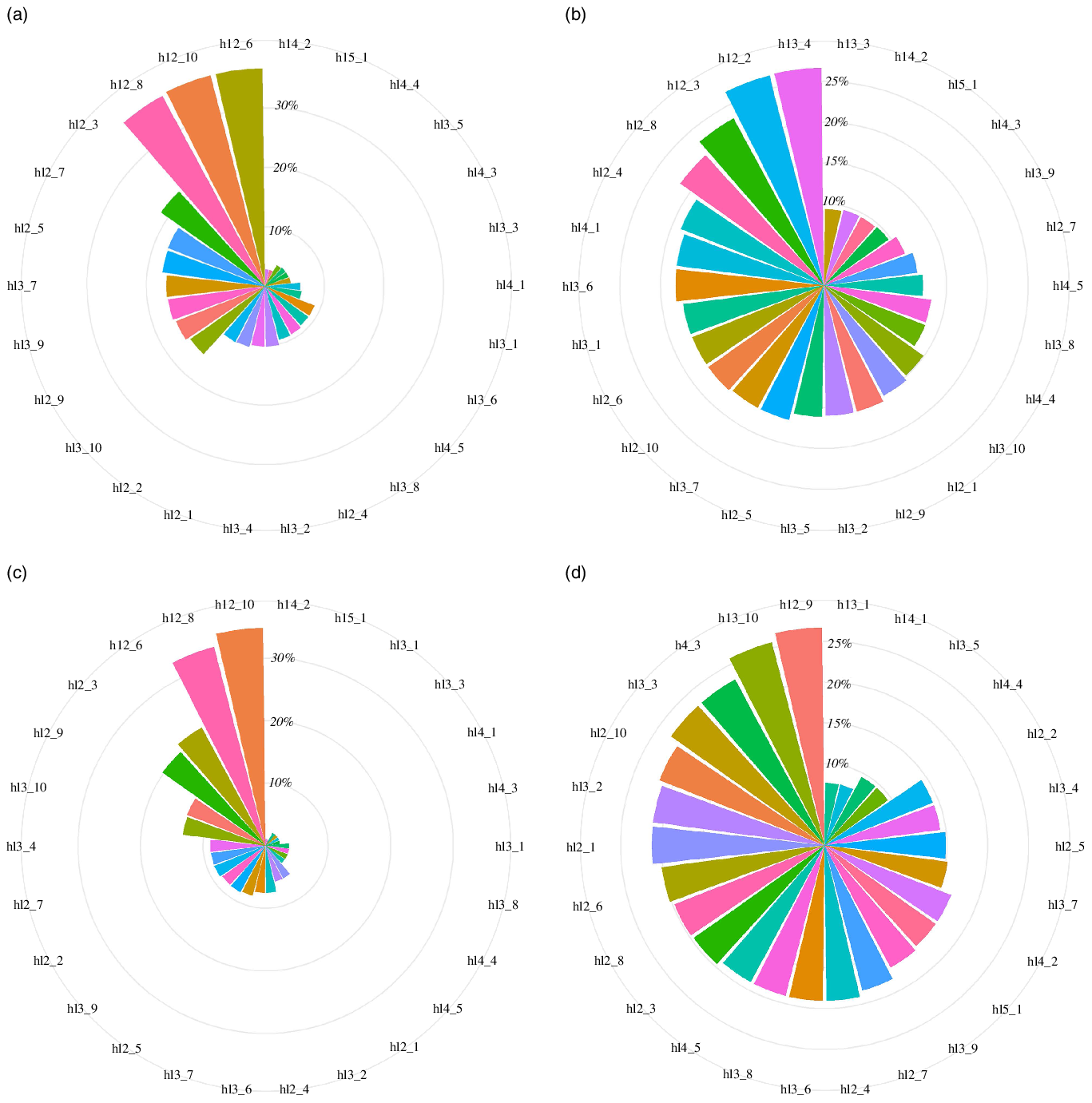
Fig. 2. Incidence of early onset of puberty with different lifestyle combinations. (a) The prevalence of different lifestyle combinations in boys; (b) the incidence of early onset of puberty with different lifestyle combinations in boys; (c) the prevalence of different lifestyle combinations in girls; (d) the incidence of early onset of puberty with different lifestyle combinations in girls. hl2_1, having good dietary behaviour and active physical activity; hl2_2, having good dietary behaviour and adequate sleep duration; hl2_3, having good dietary behaviour and not smoking and drinking; hl2_4, having good dietary behaviour and low sedentary behaviour; hl2_5, having active physical activity and adequate sleep duration; hl2_6, having active physical activity and not smoking and drinking; hl2_7, having active physical activity and low sedentary behaviour; hl2_8, having adequate sleep duration and not smoking and drinking; hl2_9, having adequate sleep duration and low sedentary behaviour; hl2_10, having not smoking and drinking and low sedentary behaviour; hl3_1, having good dietary behaviour, active physical activity and adequate sleep duration; hl3_2, having good dietary behaviour, active physical activity and not smoking and drinking; hl3_3, having good dietary behaviour, active physical activity and low sedentary behaviour; hl3_4, having good dietary behaviour, adequate sleep duration and not smoking and drinking; hl3_5, having good dietary behaviour, adequate sleep duration and low sedentary behaviour; hl3_6, having good dietary behaviour, not smoking and drinking and low sedentary behaviour; hl3_7, having active physical activity, adequate sleep duration and not smoking and drinking; hl3_8, having active physical activity, adequate sleep duration and low sedentary behaviour; hl3_9, having active physical activity, not smoking and drinking and low sedentary behaviour; hl3_10, having adequate sleep duration, not smoking and drinking and low sedentary behaviour; hl4_1, having good dietary behaviour, active physical activity, adequate sleep duration and not smoking and drinking; hl4_2, having good dietary behaviour, active physical activity, adequate sleep duration and low sedentary behaviour; hl4_3, having good dietary behaviour, active physical activity, not smoking and drinking and low sedentary behaviour; hl4_4, having good dietary behaviour, adequate sleep duration, not smoking and drinking and low sedentary behaviour; hl4_5, having active physical activity, adequate sleep duration, not smoking and drinking and low sedentary behaviour; hl5_1, having good dietary behaviour, active physical activity, adequate sleep duration, not smoking and drinking and low sedentary behaviour.
Table 4. Multivariate linear regression analysis of the association between different lifestyle combinations and age of puberty onset
(Coefficient values and 95% confidence intervals)

hl2_1, having good dietary behaviour and active physical activity; hl2_2, having good dietary behaviour and adequate sleep duration; hl2_3, having good dietary behaviour and not smoking and drinking; hl2_4, having good dietary behaviour and low sedentary behaviour; hl2_5, having active physical activity and adequate sleep duration; hl2_6, having active physical activity and not smoking and drinking; hl2_7, having active physical activity and low sedentary behaviour; hl2_8, having adequate sleep duration and not smoking and drinking; hl2_9, having adequate sleep duration and low sedentary behaviour; hl2_10, having not smoking and drinking and low sedentary behaviour; hl3_1, having good dietary behaviour, active physical activity and adequate sleep duration; hl3_2, having good dietary behaviour, active physical activity and not smoking and drinking; hl3_3, having good dietary behaviour, active physical activity and low sedentary behaviour; hl3_4, having good dietary behaviour, adequate sleep duration and not smoking and drinking; hl3_5, having good dietary behaviour, adequate sleep duration and low sedentary behaviour; hl3_6, having good dietary behaviour, not smoking and drinking and low sedentary behaviour; hl3_7, having active physical activity, adequate sleep duration and not smoking and drinking; hl3_8, having active physical activity, adequate sleep duration and low sedentary behaviour; hl3_9, having active physical activity, not smoking and drinking and low sedentary behaviour; hl3_10, having adequate sleep duration, not smoking and drinking and low sedentary behaviour; hl4_1, having good dietary behaviour, active physical activity, adequate sleep duration and not smoking and drinking; hl4_2, having good dietary behaviour, active physical activity, adequate sleep duration and low sedentary behaviour; hl4_3, having good dietary behaviour, active physical activity, not smoking and drinking and low sedentary behaviour; hl4_4, having good dietary behaviour, adequate sleep duration, not smoking and drinking and low sedentary behaviour; hl4_5, having active physical activity, adequate sleep duration, not smoking and drinking and low sedentary behaviour; hl5_1, having good dietary behaviour, active physical activity, adequate sleep duration, not smoking and drinking and low sedentary behaviour.
Discussion
We found that healthy lifestyle patterns had a cumulative effect and were associated with a substantially lower risk of early onset of puberty. Boys who adhered to more favourable lifestyle components had a lower risk of early onset of puberty than those who had less favourable lifestyle components. However, the beneficial effect of a healthy lifestyle pattern was found only in normal-weight boys. The incidence of early onset of puberty could theoretically be reduced by 8·54 % for all boys and 15·18 % for boys with a poor lifestyle if they adhered to a healthy lifestyle. It is important for boys to have at least active physical activity and low sedentary behaviour to prevent early onset of puberty.
In this study, we found that active physical activity was strongly associated with a low risk of early puberty onset. Previous studies mainly focused on menarche and spermarche, in which they explored the association between physical activity and puberty timing based on cross-sectional studies, but did not have consistent results(Reference Calthorpe, Brage and Ong12,Reference Lee, Pabayo and Kawachi34) . The conclusion that childhood physical activity was associated with later age of menarche based on athletes may be biased due to potential selection bias associated with dropout, persistence and selectivity in specific sports and confounding of diet and other factors(Reference Calthorpe, Brage and Ong12,Reference Malina, Rogol and Cumming35) . Although the literature was limited regarding sedentary behaviours, we found that low sedentary behaviour was related to a lower risk of early onset of puberty, consistent with the beneficial effects of active physical activity.
There was no association between the diet and the early onset of puberty. This finding was in line with the result of a previously published study conducted in Belgium(Reference Vandeloo, Bruckers and Janssens36). However, a recent prospective study suggested that, during pre-puberty, children with higher diet quality entered puberty at a later age(Reference Duan, Qiao and Chen37). The differences between studies may be due to not take into account the correlation structure of foods and nutrient intakes, and there may be interactions between different food groups(Reference Villamor and Jansen38). The relationship between diet and early puberty onset still needs to be further explored. One potential explanation for our observation of a null association between smoking, drinking and early onset of puberty was that the children in this study were young and had a low prevalence of smoking and drinking. Our study did not find the association between sleep duration and early onset of puberty, which was consistent with a previous cohort study in the USA(Reference Foley, Ram and Susman14). One of the most plausible reasons was that sleep was related to different endocrine systems, and sleep may affect the adrenal axis rather than the gonadal axis and lead to more relations between sleep and pubic hair development than breast or genital development(Reference Foley, Ram and Susman14).
This was the first time to observe that a healthy lifestyle pattern had a substantial protective effect on the risk of early onset of puberty. And as the number of satisfied favourable lifestyle components increased, the risk of early onset of puberty decreased. This was consistent with the previously found beneficial cumulative effect of simultaneously meeting multiple favourable lifestyle factors(Reference Loewen, Maximova and Ekwaru39,Reference Freisling, Viallon and Lennon40) . Unexpectedly, the association between lifestyle and early onset of puberty was not observed in girls in this study. It may be that nutritional status in pre-puberty have a greater impact on girls and the relatively little effect of healthy lifestyle pattern was obscured. Stratified by healthy lifestyle patterns, the adverse effect of baseline BMI on early onset of puberty was observed among girls with an unhealthy lifestyle rather than a healthy lifestyle, which indicated that healthy lifestyle may also have a protective role in girls to some extent. Research found that different sex might respond differently to the influence of environmental or peripheral signals(Reference Cheng, Buyken and Shi11). Biological origins and psychosocial factors may play a role in sex differences(Reference Foley, Ram and Susman14).
The mechanisms underlying our observations were not completely understood, and the possible explanation for the effect of lifestyle on early pubertal timing could be that lifestyle influenced endocrine, which further regulated the puberty onset. On the one hand, the effect of lifestyle on puberty timing was mediated by body composition, specifically adipose tissue(Reference Calthorpe, Brage and Ong12). Intensive lifestyle intervention had been shown to be useful for weight loss(20). Moreover, children’s BMI was inversely related to the risk of early puberty onset, especially in girls. Overweight and obesity increased availability of metabolic fuels and fat reserves and opened the puberty ‘gate’ earlier in adolescents(Reference Ebling41). On the other hand, the effect of lifestyle on puberty timing was mediated by hormone levels. Dietary and physical activity had been proposed to influence puberty timing by direct action on the maturation or secretion of the hypothalamus–pituitary–gonad system and regulation of sex hormone level(Reference Cheng, Buyken and Shi11,Reference Dorgan, Hunsberger and McMahon42) . The analysis of sex hormones included in future studies will help elucidate the relevant mechanisms.
In the current study, we provided new evidence suggesting that overall lifestyle was important and might influence puberty timing. These findings highlight the crucial role of a healthy lifestyle pattern before puberty in preventing the early onset of puberty. This is an area of particular public health interest, given that lifestyle is amenable to change. In addition, the appropriate lifestyle combination presented in this study can provide effective guidance in practical work. A greater understanding of the benefits of healthy lifestyle patterns may help researchers and policymakers to develop health promotion strategies for children and adolescents during these formative years to prevent early onset of puberty.
Based on a prospective study design, the method of clinical examination of the stages of sexual maturation for assessing pubertal development was more accurate. In the current study, we extended the knowledge beyond the role of individual lifestyle factors to the healthy lifestyle pattern to analyse their relationships to the risk of early onset of puberty, which was more in line with the actual exposure. Moreover, analyses were adjusted for a substantial number of confounders that affected the early onset of puberty, which could more truly reflect the role of lifestyle.
However, there were several limitations to be acknowledged. Firstly, the determination of early onset of puberty is not consistent, and different definitions may lead to different results. However, linear regression analysis using the age of puberty onset as the dependent variable yielded consistent conclusions, supporting the robustness of the relationship between lifestyle and early onset of puberty. Secondly, we excluded children who had already entered puberty at baseline, which could introduce selection bias, underestimating the association between lifestyle and early onset of puberty. Thirdly, information on lifestyle behaviours was only obtained at baseline, leading to different time intervals between the lifestyle surveys and the onset of puberty in children, which may underestimate the association between lifestyle in pre-puberty and early onset of puberty. Fourthly, the lifestyle factors were self-reported by questionnaire, which had been noted as a weakness compared with direct or objective measures, and could not reflect the exact levels in children. However, after half a year, the lifestyle was widely consistent with the previous surveys, indicating that the results of this questionnaire survey had strong reliability (online Supplementary Table 8). Previous studies also showed that there was a moderate to strong correlation between questionnaire and objective measurement. When objective measurement was not feasible, questionnaire survey was still a valid and reliable evaluation instrument(Reference Macfarlane, Lee and Ho43). Fifth, the division of dietary factors was not precise enough to distinguish the different effects of high-fat and high-carbohydrate foods, animal protein and vegetable protein. Future research should attempt to conduct multiple surveys to explore the effect of lifestyle pattern and its changes on the early onset of puberty. More detailed and varies lifestyle factors should be considered, and molecular indicators such as sex hormones, insulin and leptin should be included for analysis to increase the understanding of the underlying mechanisms in the future.
Conclusion
Our study showed that a healthy lifestyle pattern was associated with a substantially lower risk of early onset of puberty, especially in boys with normal weight. Importantly, adhering to a healthy lifestyle is shown to be effective in preventing early onset of puberty in children. Our findings highlight the potentially critical role of favourable lifestyles and provide an optimal combination of different lifestyle factors, which supports health promotion strategies and targeted interventions for preventing early onset of puberty.
Acknowledgements
The authors would like to acknowledge the support from all the team members and the participated students, parents, teachers and local education and health staffs. Thanks to Dr Jun Jiang and Dr Mengjiao Liu for her support in revising the language of this article.
The present study was supported by the National Natural Science Foundation (82103865 to Y. D.), and Beijing Natural Science Foundation (7222244 to Y. D.) and project funded by the China Postdoctoral Science Foundation (BX20200019 and 2020M680266 to Y. D.).
Y. L. and Y. D. designed research, Y. L. and D. G. conducted research, Y. L., Y. D. and D. G. analysed data and drafted the initial manuscript, M. C., Y. M. and L. C. reviewed and revised the manuscript, and J. M. obtained the funding support, coordinated resources and supervised data collection. All authors have read and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The authors have no conflicts of interests to disclose.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114522000563









