According to the GLOBOCAN, which is a database providing estimates of the incidence and mortality of major types of cancer in 185 countries, thyroid cancer (TC) ranks ninth with regard to cancer incidence with more than 586 000 new cases worldwide, in 2020(Reference Sung, Ferlay and Siegel1). Notably, Asian countries exhibited the highest incidence among five continents, especially Eastern Asia(Reference Sung, Ferlay and Siegel1). Although TC has been reported to decrease in 2020, South Korea had the highest TC incidence and mortality rates among both sexes globally(Reference Sung, Ferlay and Siegel1). Specifically, TC has been recognised as one of the five most common cancers (thyroid, lung, gastric, colorectal and breast cancers) in South Korea(Reference Kang, Jung and Bang2).
Environment and lifestyle factors have been demonstrated to be associated with TC(Reference Wiltshire, Drake and Uttley3,Reference Kim, Gosnell and Roman4) . Among these factors, nutritional aspects have received significant attention from researchers(Reference Barrea, Pugliese and Frias-Toral5). In detail, the high consumption of fruit and vegetables(Reference Barrea, Pugliese and Frias-Toral5,Reference Fiore, Cristaldi and Okatyeva6) , milk and dairy products(Reference Fiore, Cristaldi and Okatyeva6), and seafood(Reference Fiore, Cristaldi and Okatyeva6) showed an inverse association with TC risk. Additionally, there are several previous studies indicating that micronutrients could affect TC development such as iodine-rich food(Reference Choi and Kim7), β-carotene(Reference D’Avanzo, Ron and La Vecchia8) and vitamin E(Reference D’Avanzo, Ron and La Vecchia8).
Mn is an essential trace element obtained from dietary consumption, with rice, nuts, whole grains and legumes in daily human diets(Reference Chen, Bornhorst and Aschner9). To date, the effect of Mn on autoimmune thyroid diseases and thyroid tumours has been controversial. Mn might disrupt the binding, transport and activities of thyroid hormones at the tissue level(Reference Soldin and Aschner10). For examples, an excess of Mn intake could lead to a reduction of the thyroid hormone system(Reference Wróblewski, Wróblewska and Nuszkiewicz11), while high serum Mn level may trigger an increase in levels of total T4, free T3 and total T3(Reference Obsekov, Ghassabian and Mukhopadhyay12), or a rise in the incidence of TC(Reference Liu, Song and Jiang13). In addition, the association of Mn intake with TC has not been well elucidated and genetic contribution for TC risk could be an important factor.
Inflammation has been well accepted to have certain roles in the aetiology of cancer(Reference Liotti, Visciano and Melillo14), and TC is no exception(Reference Xiong, Sun and Wu15). The possible contribution of polymorphism of IL1 receptor type 1 (IL1R1) gene to TC susceptibility has been indicated in previous studies(Reference Xiong, Sun and Wu15–Reference Tian, Sun and Wu17). IL-1α and IL-1β are two cytokines, and their biological activities are mediated by IL-1 receptor type 1(Reference Chang, Yang and Dai18). Specifically, the combination of IL1R1 with IL1 on the cell surface may influence the nuclear factor kappa-light-chain-enhancer of activated B (NF-κβ) signalling and upregulate inflammation(Reference Chang, Yang and Dai18). Thus, single nucleotide polymorphisms (SNP) in the promoter region of IL1R1 gene may have an impact on the affinity of IL1 to IL1R1, thereby affecting inflammation(Reference Chang, Yang and Dai18). Notably, dietary Mn has been emphasised to have an association with circulating inflammation markers and the NF-κβ pathway(Reference Gong, Lo and Liu19,Reference Kresovich, Bulka and Joyce20) . Based on the inflammatory potential of Mn, we hypothesised an interaction between dietary Mn and IL1R1 rs3917225, a common promoter SNP of IL1R1 gene in the Korean population, and its potential impact on TC development.
To our knowledge, the potential impact of dietary Mn intake on TC has yet to be investigated. Additionally, there may be an interactive effect between Mn and IL1R1 rs3917225, potentially influencing the TC susceptibility. Thus, we aimed to determine whether dietary Mn intake emerges a protective role against TC and whether this preventative effect has an interaction with IL1R1 rs3917225 in South Korea, where a high prevalence of TC has been reported(Reference Sung, Ferlay and Siegel1,Reference Kang, Jung and Bang2) .
Materials and methods
Study population
We recruited a total of 17 754 subjects, who underwent a health check-up at the Center for Cancer Prevention and Detection of the National Cancer Center (NCC) to include in the Korea NCC Cancer Screenee Cohort between October 2007 and December 2020. The information regarding this cohort has been previously conferred(Reference Kim21). Initially, every participant submitted a signed consent form and fulfilled a demographic questionnaire including their sociodemographic, lifestyles and a SQFFQ about their dietary consumption. Subjects were followed until 31 December 2020 to determine incident cancer cases. There are some main exclusion criteria including incomplete responses on the demographic questionnaire or SQFFQ (n 5900), participants with implausible energy intake (<500 kcal or >4000 kcal, n 172) and individuals with a prior cancer diagnosis (n 941). Subsequently, a total of 10 741 eligible participants (108 incident TC cases and 10 633 non-incident TC cases) were included in the analysis of Mn intake in relation to TC risk. Additionally, we excluded participants with missing information on ILR1 rs3917225 for genetic analyses (6212 participants). Of the remaining (fifty-eight incident TC cases and 4471 non-incident TC cases), we performed a propensity score approach to match incident TC cases and non-incident TC based on age and sex with a ratio of 1:1 for genetic analyses due to a considerable variation between TC cases and participants without TC(Reference Nguyen, Gunathilake and Lee22). The ‘MatchIt’ package in R was employed with nearest neighbour method for this procedure, resulting in fifty-eight incident TC cases being matched with fifty-eight non-incident TC cases(Reference Ho, Imai and King23) (Fig. 1). This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were approved by the Institutional Review Board of the National Cancer Center Korea (IRB No. NCCNCS-07-077). Written informed consent was obtained from all subjects/patients.

Fig. 1. Flow chart of the study participants. The figure shows the flow chart of the study participants. 17 754 participants enrolled and are linked to the Korea Central Cancer Registry. In total, 10 741 participants were included in the final analysis after the exclusion of 5900 participants with incomplete questionnaires, 172 participants with implausible energy intake and 941 participants who had a previous diagnosis of any cancer. Additionally, we excluded participants with missing information on ILR1 rs3917225 for genetic analyses (6212 participants). Of the remaining (fifty-eight incident TC cases and 4471 non-incident TC cases), we performed a propensity score approach to match incident TC cases and non-incident TC based on age and sex with a ratio of 1:1 for genetic analyses. After that, fifty-eight incident TC cases being matched with fifty-eight non-incident TC cases. IL1R1, IL1 receptor type 1; TC, thyroid cancer.
Incident thyroid cancer identification
To identify TC incident cases through 31 December 2020, we linked the 2020 Korea National Cancer Incidence Database. For those who could not be linked to the Korea National Cancer Incidence Database, we used Medical Record from NCC Hospital in 2020. Biopsy was considered as the golden standard for diagnosing most patients with TC. We used the International Classification of Disease for Oncology (ICD-O), 10th revision (C73) to identify TC. An event of incident TC was defined as a case who was diagnosed with TC after recruitment for study.
Data collection
All participants were asked about their consumption frequency for each of 106 food items in the SQFFQ during the previous year from nine frequency categories (never or rarely, once a month, 2–3 times per month, once or twice a week, 3–4 times per week, 5–6 times per week, once a day, twice a day and 3 times per day) and usual portion sizes using three portion size categories (small, medium and large). The validity and reproducibility of the SQFFQ have been determined elsewhere(Reference Ahn, Kwon and Shim24). This process is conducted by interviewers who were trained before recruitment. The daily average of nutrient consumption was determined by considering the portion sizes of each meal, consumption frequency and the nutritional composition of these food products. The CAN-PRO 5.0 (Computer Aided Nutritional Analysis Program, Korean Nutrition Society) was employed to compute the daily nutritional consumption for each individual in this study. The total quantity of Mn acquired from all the food ingested throughout the day was regarded as the daily dietary intake of Mn (mg/d).
Sociodemographic information including age (years), sex (male, female), income (10 000 won/month; <200, 200–400, >400), education (high school graduate or less, college or higher), occupation (professional administrative; office, sales, service; labourer, agricultural; others, unemployed), marital status (married or cohabitating, others), smoking status (non-smoker, ex-smoker and current smoker), alcohol consumption (non-drinker, ex-drinker and current drinker), BMI (<23, 23–25, ≥25), first-degree family history of TC (yes, no) and regular exercise (yes, no) were collected by a self-administered questionnaire. The International Physical Activity Questionnaire was used in a short form to assess levels of physical activity. These data were converted into units of metabolic equivalents of tasks (MET) and expressed in minutes per week (MET-minutes/week)(Reference Oh, Yang and Kim25).
Genotype measurement
The extraction of Genomic DNA from the blood samples of participants was performed using MagAttract DNA Blood M48 Kit (Qiagen) and BioRobot M48 automatic extraction equipment (Qiagen). Genotyping was conducted using the Illumina MEGA-Expanded Array (Illumina Inc.), which encompasses 123K variants. A comprehensive explanation of this method has been provided previously(Reference Lu, Kweon and Cai26). Genotype imputation was conducted using the Asian population (n 504) in the 1000 Genomes haplotypes phase III integrated variant set release GRch37/hg19 (https://www.1000genomes.org/) as a reference panel. Genetic markers with deviation from Hardy–Weinberg equilibrium P-values <1 × 10–6, a minor allele frequency <0·05 and a low call rate (<98 %) were discarded. Phasing was achieved using SHAPEIT (v2.r837), and SNP imputation was performed using IMPUTE2 (2.3.2). After filtering for an INFO score exceeding 0·6, the data underwent quality control criteria assessment. Finally, IL1R1 rs3917225 was selected for our analysis.
Statistical analysis
To compare the demographic characteristics between two groups, we used Student’s t test and χ 2 test for continuous variables and categorical variables, respectively. We adjusted Mn intake for total energy intake by using a residual method. We classified Mn consumption into two groups based on median value, namely lower Mn intake (below median) and higher Mn intake (at or above median).
The period time from baseline to the date of TC diagnosis, death or end of follow-up (31 December 2020), whichever was earlier, was considered to calculate person-years (year). The Cox proportional hazards regression model with right censoring was applied to estimate the hazard ratios (HR) and 95 % confidence interval (CI) for TC comparing participants with dietary Mn intake upper and lower the median; among which the lower consumption group served as the reference group. The Cox proportional hazards assumption was assessed with Cox model extended to contain terms involving the time-dependent variables, showing the assumption was satisfied for all covariates. In addition, based on the literature, some confounding variables were considered, including age, sex, income, education, occupation, marital status, BMI, alcohol consumption, first-degree family history of TC, smoking status and physical activity. Regarding stratification, smoking status was categorised as non-smokers and ever-smokers (current smokers and ex-smokers). Similarly, alcohol consumption was grouped as non-drinkers and ever-drinkers (current drinkers and ex-drinkers). Furthermore, a dominant model was considered for genetic association. The interaction analysis was conducted using the likelihood ratio test, comparing models with and without the interaction term (Mn*SNPs). All statistical analyses were conducted by SAS software (version 9.4, SAS Institute, Cary, NC), and two-sided P-values below 0·05 were considered statistically significant.
Results
Baseline information of study participants
After a mean follow-up of 8·07 years, a total of 108 incident TC cases were identified. Among 10 741 participants, the mean age was 52·55 years; 63·9 % were women and 36·1 % were men. In comparison with the lower dietary Mn intake group, participants with a higher intake had lower levels of education and occupation (41·8 % v. 45·0 %, P < 0·001 and 15·9 % v. 16·7 %, P < 0·001, respectively). Similarly, they were more likely to be non-smokers (70·9 % v. 61·7 %, P < 0·001) and non-drinkers (40·3 % v. 34·8 %, P < 0·001). Furthermore, individuals with high Mn intake seemed to have a higher proportion of BMI < 23 (44·6 % v. 41·9 %, P = 0·010) and a higher physical activity level (2484·4 ± 2707·1 MET-min/week v. 2188·1 ± 2633·0 MET-min/week, P < 0·001) (Table 1).
Table 1. The general characteristics by dietary manganese intake among participants

MET, metabolic equivalents of tasks.
* The groups were classified based on median value.
† χ 2 tests and t test were used for categorical variables and continuous variables, respectively.
The significant values are in bold format.
The association between dietary manganese intake and thyroid cancer risk
Table 2 illustrates the association between dietary Mn intake and TC. Our findings indicate that the dietary Mn intake was inversely associated with TC risk. Specifically, participants with higher Mn intake might had 36 % lower hazard of TC than those with lower Mn intake in the adjusted model (HR = 0·64 (95 % CI 0·44, 0·95)). However, the marginal association was found for women, but not for men. A greater dietary Mn intake was in relation to a lower TC risk in the crude model (HR = 0·66 (95 % CI 0·44, 0·99)), but the significant relation tended to disappear after adjusting for additional confounders (HR = 0·68 (95 % CI 0·45, 1·03)) in women.
Table 2. The association between dietary manganese intake and thyroid cancer risk

HR, hazard ratio.
* The groups were classified based on median value.
† The number of valid observations was 10 741.
‡ The number of valid observations was 10 679.
Model 1: crude model; Model 2: adjusted for age, sex, income, education, occupation, marital status, smoking status, alcohol consumption, BMI, first-degree family history of thyroid cancer and physical activity.
Significant values in model 2 are bold.
We then stratified based on selected variables to observe the association between dietary Mn intake and TC risk. Non-smokers who consume over 1·9 mg/d may reduce the TC risk (HR = 0·59 (95 % CI 0·38, 0·91)). A similar pattern was observed in alcohol consumption group where there was a lower risk of TC among non-drinkers who have higher intake of dietary Mn (HR = 0·47 (95 % CI 0·26, 0·83)). When stratified by BMI, a higher dietary Mn intake was found in relation to a decrease in the risk of TC among individuals with BMI of 23–<25 kg/m2 (HR = 0·36 (95 % CI 0·17, 0·80)) (online Supplementary Table 1).
The association between IL1R1 rs3917225 polymorphisms and thyroid cancer risk
There are three genotypes of IL1R1 rs3917225 including AA, GA and GG. A dominant model was used to investigate IL1R1 rs3917225 in relation to TC development with two groups (AA and GA/GG). A minor allele served as a protective factor against TC. Specifically, a significant reduced risk of TC was found in participants carrying the minor allele G of IL1R1 rs3917225 compared with those with homozygous wild-type allele (HR = 0·45 (95 % CI 0·24, 0·84) (Table 3).
Table 3. Associations of IL1R1 rs3917225 genetic polymorphisms with TC risk in the dominant model
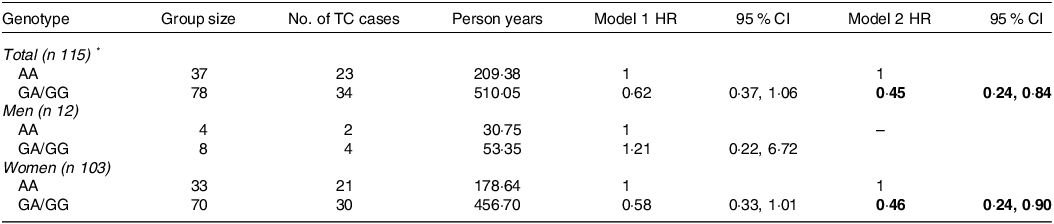
IL1R1, IL1 receptor type 1; TC, thyroid cancer; HR, hazard ratio.
* Excluding one participant with ‘0/0’ genotype of IL1R1 rs3917225.
Model 1: crude model; Model 2: adjusted for age, sex, income, education, occupation, marital status, smoking status, alcohol consumption, BMI, first-degree family history of thyroid cancer, and physical activity.
The significant values are in bold format.
Interaction between IL1R1 rs3917225 genetic polymorphisms and dietary manganese intake with thyroid cancer risk
We then performed stratification by genotypes to identify whether IL1R1 rs3917225 modifies the protective roles of dietary Mn intake against TC. The relationship of Mn with TC was found to be allele-specific with a greater effect in G-allele carriers. Specifically, among participants with GA/GG genotypes, a beneficial effect of Mn was exhibited among individuals who have a high intake (HR = 0·30 (95 % CI 0·11, 0·86). In contrast, a non-significant association of Mn with TC was observed for those in AA genotype group (HR = 2·07 (95 % CI 0·51, 8·40)). Importantly, a significant interaction between IL1R1 rs3917225 and Mn with TC susceptibility was determined (P interaction=0·028) (Table 4).
Table 4. Interaction between IL1R1 rs3917225 genetic polymorphisms and dietary manganese intake with TC risk in the dominant model
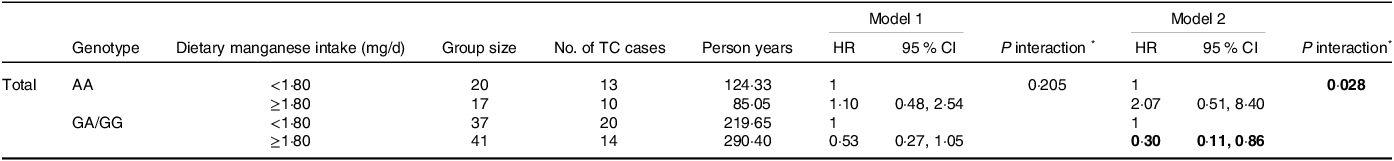
IL1R1, IL1 receptor type 1; TC, thyroid cancer; HR, hazard ratio.
* The interaction term: (High Mn)×(GA/GG).
Model 1: crude model; Model 2: adjusted for age, sex, income, education, occupation, marital status, smoking status, alcohol consumption, BMI, first-degree family history of thyroid cancer and physical activity.
The significant values are in bold format.
Discussion
In our study, a higher Mn intake was found to play a protective role against TC. Additionally, there is an inverse association between dietary Mn intake and TC risk among individuals who were non-drinkers, non-smokers and had a BMI of 23–<25 kg/m2. However, IL1R1 rs3917225 seemed to modify this association with a greater effect in G-allele carriers.
Even though there are several studies in both humans and animals considering the relationship between Mn and TC, the findings are still controversial thus far. A previous case–control study indicated that Mn is a potential risk factor for thyroid tumours and goitre(Reference Liu, Song and Jiang13). Similarly, Mn level was observed to increase in thyroid tissue among TC patients compared with individuals with benign thyroid conditions in a meta-analysis(Reference Van Gerwen, Alerte and Alsen27). In contrast, other studies supported the hypothesis that Mn is a protective trace element against thyroid disorders. As far as we know, the amount of trace elements impacts the regular metabolism and operation of the thyroid. Any alteration in the concentration of trace elements can disrupt not only the endocrine system but also other bodily systems, causing disorders in thyroid function, including cancers(Reference Zhou, Xue and Zhang28). A deficiency in Mn levels could have a negative impact on the functioning of the thyroid hormone system(Reference Wróblewski, Wróblewska and Nuszkiewicz11). Additionally, Mn was considered essential for antitumor immune responses by an experimental study(Reference Lv, Chen and Zhang29). Notably, a higher dietary Mn intake was recognised to be a protective factor against liver cancer(Reference Kasai, Eshak and Tamakoshi30,Reference Ma, Yang and Li31) . Our finding contributes to supporting this notion highlighting its association with TC. To our knowledge, this is the first epidemiological study focusing on the relationship of dietary Mn intake with TC. It indicates that an appropriate level of Mn consumption could potentially lead to a reduced TC risk.
Notably, cigarette smoking and alcohol consumption have been demonstrated to be associated with a reduction in TC susceptibility(Reference Yeo, Shin and Han32–Reference Kitahara, Linet and Beane Freeman34). This implies the contribution of non-smokers or non-drinkers in the aetiology of TC. In contrast, our study found that non-smokers and non-drinkers who consume more Mn dietary may reduce TC risk. A possible reason may be proposed that Mn is an essential trace element for living organisms and could diminish the harmful effects of risk factors by its antioxidative property.
One biologically plausible explanation regarding the inverse association between Mn intake and TC risk could be associated with the potential antioxidative properties of Mn. Mn plays a crucial role as a cofactor for multiple enzymes, including transferases, hydrolases, lyases, isomerases, ligases and oxidoreductases(Reference Anagianni and Tuschl35). Importantly, when Mn levels are inadequate, it can negatively affect the thyroid hormone system(Reference Maouche, Meskine and Alamir36). Consequently, imbalances in thyroid function are closely linked to the harmful effects of reactive oxygen species(Reference Maouche, Meskine and Alamir36). Furthermore, experimental research has revealed that Mn serves as a cofactor for antioxidant enzymes, including MnSOD, a crucial mitochondrial enzyme responsible for scavenging reactive oxygen species(Reference Li and Yang37). Meanwhile, the impact of reactive oxygen species generated within mitochondria has been closely examined in relation to the metabolism of TC. The authors propose that disruptions in mitochondrial oxidative phosphorylation, coupled with an associated increase in glucose and glutamine consumption, establish an environment that fosters the progression of cancer(Reference Coelho, Fortunato and Carvalho38).
Furthermore, inflammation has been widely recognised to be a contributor in the carcinogenesis and its involvement in TC development has been confirmed(Reference Xiong, Sun and Wu15). A variety of cytokines are considered as potential regulators in various activities and emerge anti- or pro-inflammatory effects(Reference Xiong, Sun and Wu15). IL1 is a pleiotropic cytokine family, which is known to have certain functions in inflammation and immunity through activation of gene expressions related to innate and adaptive immune responses(Reference Tian, Sun and Wu17,Reference Chang, Yang and Dai18) . As a result, it is an important factor in the cancer development(Reference Tian, Sun and Wu17). IL1R1 is a receptor of the IL1 family, which plays a crucial role as a mediator of immune and inflammatory responses triggered by various cytokines and have an association with thyroid carcinogenesis(Reference Xiong, Sun and Wu15,Reference Tian, Sun and Wu17) . The IL1R1 promoter region has demonstrated highly polymorphism affecting the level of expression in a wide range of tissues(Reference Park, Kim and Kwon16). The SNP, rs3917225 is located in the promoter of IL1R1 and may impact on recruitment of RNA polymerase for IL1R1 leading to disturb the initiation of transcription and subsequent signal transduction pathways(Reference Xiong, Sun and Wu15). It was indicated to be associated with an increased TC risk with respect to this hypothesis in Chinese Han people. However, significance disappeared after adjustment for age and sex(Reference Xiong, Sun and Wu15). Similarly, the association of rs3917225 with papillary thyroid carcinoma was not determined in the Korean population in a previous study(Reference Park, Kim and Kwon16). Notably, rs3917225 demonstrated the potential to lower TC risk under the dominant model in our study. Although the mechanism is not clear, IL1R1 quantity is not changed by rs3917225, but it may have an influence on some quality. The affinity of IL1 to IL1R1 may be affected by this variant leading to a potential blockage of the up-regulation of inflammation(Reference Chang, Yang and Dai18). However, further studies are needed to elucidate this association.
While adequate intake of Mn has several beneficial effects including lowering oxidative stress(Reference Kresovich, Bulka and Joyce20), the high concentrations could potentially be toxic(Reference Owumi and Dim39), whereas its deficiency may cause inflammation and endothelial dysfunction(Reference Gong, Lo and Liu19). Additionally, Mn constitutes an essential component of MnSOD, which plays a significant role in protecting energy-producing mitochondria against oxidative damage(Reference Owumi and Dim39,Reference Li and Zhou40) . Notably, IL1 cytokines have been implicated to upregulate levels of MnSOD mRNA(Reference Li and Zhou40). Furthermore, a higher dietary Mn was demonstrated to be in relation to a lower level of circulating inflammatory markers. Its potential roles in inflammatory biomarker production were also emphasised in other previous study, which indicates a greater concentration of IL-1β in participants with the highest quartiles compared with those with the lowest quartile(Reference Kresovich, Bulka and Joyce20). Thus, we hypothesised that Mn intake could interact with the IL1R1 gene, which encodes cytokine receptors for IL1, potentially impacting thyroid carcinogenesis. Importantly, our study suggests that the beneficial effect of Mn intake against TC is contingent upon an individual’s genetic characteristic. Specifically, a higher Mn intake tended to emerge a beneficial effect against TC in minor allele carriers. Our finding is in line with other previous studies on the connection between Mn and inflammation. For example, participants with an increased Mn intake showed lower levels of IL 6 and hs-CRP, which partially mediate the relationship of Mn with type 2 diabetes risk(Reference Gong, Lo and Liu19). Additionally, Mn exhibited ability to suppresses chlorpyrifos-induced inflammation in rats(Reference Owumi and Dim39). Furthermore, Mn may contribute to production of inflammatory biomarkers as presented by concentrations of IL-1β, IL-8, IL-6 and NF-κβ when the intake level is slightly above nutritional sufficiency(Reference Kresovich, Bulka and Joyce20). Similarly, Mn was identified as a dominant trace metal in relation to increased IL-1β levels in another previous study(Reference Aung, Meeker and Boss41). Possible mechanisms may be proposed for the link between Mn and IL-1β. First, studies focus on the mechanisms of Mn indicate that upon entering cells, it has the potential to be sequestered within mitochondria leading to Ca ion transport disruption, mitochondrial dysfunction and an imbalance in intracellular redox status(Reference Aung, Meeker and Boss41). Second, Mn may influence danger-associated molecular patterns and stimulate IL-1β production(Reference Aung, Meeker and Boss41). Third, Mn intake was associated with DNA methylation of NF-κβ-regulating genes, thus contributing to IL production(Reference Kresovich, Bulka and Joyce20). Taken together, our study may provide insights into a potential biological interaction between Mn intake and gene encoding cytokine receptors for IL1 (IL1R1 rs3917225) with an emphasis on inflammatory effects of excessive Mn consumption.
This study is the first prospective cohort study to investigate the association between dietary Mn intake and TC risk. Importantly, our finding suggests the potential impact of the interaction between Mn intake and an inflammatory gene (IL1R1 rs3917225) on TC development. Additionally, we accurately identified newly diagnosed cancer cases with a high-quality database by linking to the National Cancer Registry and medical records. Furthermore, information on nutrient intakes was obtained using the validated SQFFQ. By utilising a customised SQFFQ that considers the dietary behaviours of the Korean population, our investigation may present noteworthy results pointing to a potentially favourable effect of Mn in reducing the risk of TC among Koreans. Moreover, we adjusted possible confounders associated with demographic characteristics and lifestyle. However, our study still has some limitations. First, dietary Mn intake was only collected at baseline. Second, several environmental exposures (e.g. ionising radiation, history of benign thyroid nodules/adenoma) and nutritional factors (e.g. iodine intake) were not evaluated in our study, which could potentially introduce bias to the observed relationship. Third, the statistical power may be affected by a small number of TC cases in subgroup stratifications, especially genotype associations. Thus, these associations are needed to clarify in further investigations with a larger sample size.
Conclusion
In conclusion, a higher dietary Mn intake was suggested to be a protective factor against TC. Additionally, a potential biological interaction between Mn intake and IL1R1 rs3917225 was drawn in our study. In detail, this preventative effect seemed to depend on an individual’s genetic characteristic, with a greater impact observed in participants carrying a minor allele. This implies that when considering the cancer-preventive role of Mn, it is important to account for the influence of the involvement of inflammatory gene.
Acknowledgements
This work was supported by the International Cooperation and Education programme (NCCRI NCCI 52210-52211, 2024) of the National Cancer Center, Korea, and grants from National Cancer Center, Korea (22109902).
T. T. T. and H. T. M. N.: conceptualisation, formal analysis and preparation of original draft. M. G.: writing – review and editing. J. L.: data curation, investigation and methodology. J. K.: writing – review and editing, data curation, methodology, funding acquisition, project administration and supervision. All authors have critically reviewed and approved the final version of the manuscript submitted for publication.
The authors declare that they have no conflicts of interests.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711452400206X








