Uterine fibroids are the most common tumours among women of reproductive age(Reference Schwartz, Marshall and Baird1). Although the mechanisms are not well understood, clinical and laboratory evidence indicates that oestrogen and progesterone may both be important promoters of myoma growth(Reference Flake, Anderson and Dixon2). Certain dietary components which could affect oestrogen or progesterone may be implicated in the aetiology of fibroids. Dietary fat, soya foods, dietary fibre and alcohol have been associated with endogenous oestrogen levels in some studies(Reference Forman3–Reference Gill5). Previous studies have assessed the association of fibroids with alcohol intake(Reference Chiaffarino, Parazzini and La Vecchia6–Reference Wise, Palmer and Harlow8). However, to our knowledge, only two studies have assessed the relationship between dietary intake other than alcohol and uterine fibroids(Reference Chiaffarino, Parazzini and La Vecchia6, Reference Terry, Missmer and Hankinson9). In a study reported by Chiaffarino et al. (Reference Chiaffarino, Parazzini and La Vecchia6), the consumption of beef and other red meats was positively associated with the risk of fibroids. However, no attempt was made to estimate quantitatively the intake of individual nutrients such as fat. Another study focused on dietary carotenoids in relation to the risk of uterine fibroids and observed no risk reduction(Reference Terry, Missmer and Hankinson9). Both studies did not investigate the relationship between soya intake and fibroids. We previously found that soya intake was inversely associated with the risk of hysterectomy in a population-based cohort study of Japanese women(Reference Nagata, Takatsuka and Kawakami10), suggesting a potentially protective effect of soya against uterine fibroids, the major clinical indication for hysterectomy. In a recent case–control study of fibroids among US women, Atkinson et al. (Reference Atkinson, Lampe and Scholes11) observed that urinary excretion of lignan, a phyto-oestrogen, was significantly lower among cases than that among controls. They found no significant difference in urinary isoflavone excretion between cases and controls. However, they noted that the intake of soya foods, the primary source of isoflavones, was apparently low in the study population. Excretion of lignans and isoflavones is a short-term biomarker that may be unsuitable to capture a long-term exposure, which is the exposure of interest when chronic diseases are investigated. In the present cross-sectional study, we examined the association between uterine fibroids and diet, including fats, soya isoflavones, dietary fibre and alcohol, among Japanese women.
Methods
The present study was part of one designed to assess the relationships among lifestyle, environmental factors and women's health. Study subjects were participants in a medical health check-up programme provided by a general hospital in Gifu, Japan, between October 2003 and March 2006. The purpose of the medical health check-up is to promote public health through early detection of chronic diseases and their risk factors. A medical service of this kind is popular in Japan. A total of 2073 individuals, including return visitors to the programme during the study period, were invited to join the study, and 1545 agreed to participate (the response proportion was 74·5 %). When the response proportion was calculated for only new visitors to the programme during the study period, it was 83·2 % (1103 out of 1325 individuals).
The participants responded to a self-administered questionnaire that included questions on demographic characteristics, smoking and drinking habits, diet, exercise, and medical and reproductive histories. Diet including alcohol intake was assessed with a validated 169-item semi-quantitative FFQ. The participants were asked how often on average they consumed each of the food items listed and what was the usual serving size of each item during the year before the study. Intakes of foods and nutrients were estimated from the frequency of ingestion and portion size using the Japanese Standard Tables of Food Composition, 4th and 5th editions, published by the Science and Technology Agency of Japan. The questions on alcohol use included six different types of liquor, which were sake, beer, light beer, shochu (distilled from sweet potatoes, rice or buckwheat), wine and hard liquor. For each item, the questionnaire included seven frequency categories (from never/less than once per month to once per d or more) and the number of cups, glasses and bottles consumed. Detailed information on the questionnaire including its validity and reproducibility examined in other samples has been described elsewhere(Reference Shimizu, Ohwaki and Kurisu12, Reference Nagata, Takatsuka and Kawakami13). For example, the Spearman correlation coefficients between the questionnaire and twelve daily diet records kept over a 1-year period for intakes of total energy, macronutrients, dietary fibre, soya isoflavones and alcohol ranged from 0·45 to 0·64. Our questionnaire was designed to measure an individual's relative intakes of foods and nutrients rather than absolute values. The means estimated from the FFQ were generally higher than those estimated from twelve daily diet records. Although we presented the mean values of dietary intake, some of them may have been overestimated by our questionnaire.
The present study was limited to female subjects undergoing gynaecological examinations including a Pap smear test, pelvic examination and transvaginal ultrasound screening. A total of 539 women underwent these examinations. As fibroids can shrink after menopause, 122 postmenopausal women were excluded from the study. Women who had been without a menstrual cycle in the past 12 months were classified as postmenopausal. The presence of fibroids was assessed by transvaginal sonogram. Only fibroids with a diameter of 10 mm or more were registered. The size of the largest fibroid was conventionally classified into seven categories (from thumb-size to infant head-size). If a woman had undergone a hysterectomy, self-report of fibroids was accepted. Women who had not responded to the dietary questionnaire (n 108) or those with incomplete or unreliable responses to the dietary questionnaire (criteria shown elsewhere(Reference Shimizu14), for example, staple food five times per d or more) (n 19) were excluded from the present analyses. Additionally, five women who had cancer were excluded. Thus, 285 premenopausal women aged 23–56 years comprised the study population. Informed consent was obtained from each subject. The present study was approved by the ethical board of the Gifu University Graduate School of Medicine.
For statistical analysis, dietary intakes were adjusted for total energy after log-transformation by using the residual method proposed by Willett(Reference Willett and Willett15). Alcohol intake was also log-transformed. OR and 95 % CI of uterine fibroids were computed according to the tertile of energy-adjusted dietary intakes using the unconditional logistic regression model. The linear trends were assessed using continuous values of the dietary variables. The known or suspected risk factors for fibroids, such as age, parity, BMI, smoking status and age at menarche were included in the models as covariates. Some symptoms related to fibroids may make women change their diet. Therefore, we repeated analyses after excluding women who had reported cramps or abnormal bleeding. All the statistical analyses were performed using SAS programs (SAS Institute, Inc., Cary, NC, USA). Dietary intake, parity, BMI, smoking status and age at menarche did not differ between individuals who attended gynaecological examination and those who did not. The percentage of women who had had a prior diagnosis of fibroids did not differ between the two groups of women (15·0 v. 17·4 %).
Results
Fifty-four women were identified as having fibroids (n 50) or having had a hysterectomy due to fibroids (n 4). The distribution of the size of the largest fibroid was nine (18·0 %), eight (16·0 %), eighteen (36·0 %) eleven (22·0 %), three (6·0 %) and one (2·0 %) for thumb-size, walnut-size, small goose egg-size, goose egg-size, large goose-egg size and fist-size, respectively. Distributions of non-dietary factors according to the prevalence of fibroids are shown in Table 1.
Table 1 Characteristics of study subjects according to uterine fibroid status
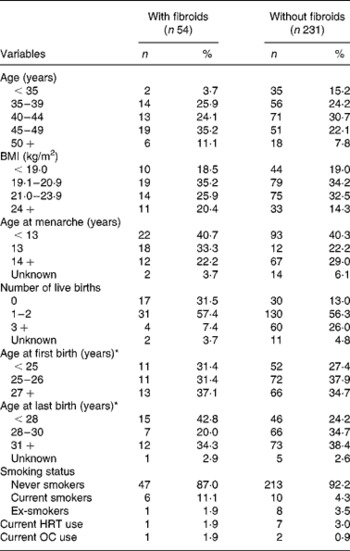
HRT, hormone replacement therapy; OC, oral contraceptive.
* Among parous women.
The OR of uterine fibroids was significantly increased in the highest tertile of alcohol compared with the lowest tertile (non-drinkers) after controlling for covariates (Table 2). There were no significant associations of intakes of fats, dietary fibre and soya isoflavones with the prevalence of fibroids. Additional adjustment for alcohol intake did not alter these associations. Exclusion of women who had undergone a hysterectomy or women who had reported cramps (n 53) or abnormal bleeding (n 16) did not alter the results.
Table 2 Risk of prevalent fibroids by tertile of selected dietary factors
(Odds ratios and 95 % confidence intervals)
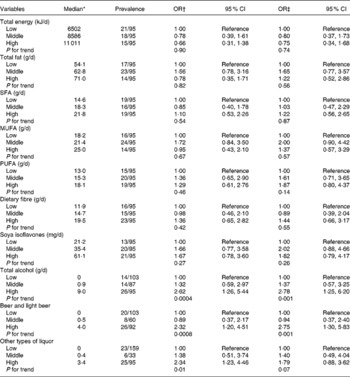
* Adjusted for total energy except for total energy and alcohol intake.
† Age-adjusted.
‡ Adjusted for age (continuous), BMI (continuous), smoking status (never, ex- and current smokers), no. of live births (0, 1–2, 3+ and unknown) and age at menarche ( < 13, 13, 14+ and unknown).
Discussion
Despite the low alcohol intake in this present population, alcohol intake showed a significant positive association with uterine fibroids. Alcohol may have an oestrogenic action on the uterine myometrium. So far, three studies have addressed the association between alcohol intake and fibroids. A significant positive association was reported in two prospective studies, the Nurses' Health Study(Reference Marshall, Spiegelman and Barbieri7) and the Black Women's Health Study(Reference Wise, Palmer and Harlow8). However, a case–control study of Italian women found no association with alcohol intake(Reference Chiaffarino, Parazzini and La Vecchia6).
We expected that isoflavones would be inversely associated with fibroids. However, a somewhat increased OR for the second tertile of intake does not necessarily negate the possibility of a positive association between soya isoflavone intake and the risk of fibroids. We also failed to find significant associations between fibroids and intakes of fats and dietary fibre. Alcohol intake has been associated with increased serum oestrogen levels in women(Reference Gill5). The associations of oestrogen levels with intakes of fats, dietary fibre and soya isoflavones have been inconsistently shown in previous studies(Reference Forman3, Reference Nagata, Takatsuka and Inaba4, Reference Maskarinec, Franke and Williams16). These dietary components may not have sufficient effects to induce an oestrogen-like transcriptional response in the uteri.
The small number of subjects is a limitation of the present study. Although we had no evidence of any association between fibroids and intakes of fats, soya isoflavones and dietary fibre, we had insufficient power to detect a small but significant association. Another limitation is that the present study only identified prevalent, rather than incident, cases. Because the dietary questionnaire referred to habits during the year before the study, and because some proportion of the women with fibroids were those who had a history of hysterectomy, the dietary data for some cases may have reflected a time period after these cases were diagnosed. Some symptoms related to fibroids may make women change their diet. However, the exclusion of women who reported abnormal menstruation or bleeding did not substantially alter the results. In addition, the possible relationship between diet and fibroids was generally unknown to subjects.
Diet is likely to be associated with health behaviours such as participation in a gynaecological examination, which could result in the incidental detection of fibroids. Although studies with a prospective design are generally more efficient for the assessment of a cause–effect relationship, the results can be affected by detection bias unless the follow-up of fibroids status is not self-reported but is based on examinations uniformly conducted among all subjects. In the present study, the transvaginal ultrasound examination was used to determine the existence of fibroids for all subjects. The best practical tool available for epidemiological studies is transvaginal ultrasound, as it yields a much less selective set of cases than would be possible if pathological diagnosis were required(Reference Schwartz, Marshall and Baird1). A high sensitivity (99 %) and specificity (91 %) of ultrasound relative to pathological evidence have been reported(Reference Dueholm, Lunddorf and Hansen17).
The fact that our study subjects were participants in a health check-up programme is a concern. It is possible that, compared with women who eat a healthy diet, women with a less healthy diet are more likely to have a health check-up only when they are experiencing illness, including symptoms of fibroids. The observed positive association between alcohol intake and fibroids may have been affected by such a selection bias. However, diet as well as the percentage of women with a prior diagnosis of fibroids was similar between those who had selected the gynaecological check-up and those who had not.
In conclusion, data from the present study suggested that alcohol intake was associated with the prevalence of uterine fibroids. There was no evidence of a significant association of intakes of fats, soya isoflavones and dietary fibre with uterine fibroids. Further studies on diet, especially phyto-oestrogens, and uterine fibroids are needed, given the limited data currently available.
Acknowledgements
The study was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan. C. N. initiated and organised the study and wrote the manuscript. K. N. was involved in the analyses and interpretation of the study. S. O. helped the design of the analytic strategy. M. H., N. T. and K. Y. helped supervise the field activities and interpret the data. There are no conflicts of interest.




