Hormone-related cancers (HRC) are greatly influenced by hormone levels and generally respond to hormone regulation, which plays an indispensable role in tumour growth(Reference Zadnik and Krajc1). Six cancer types, including breast, ovarian, endometrial, thyroid, prostate and testicular cancer, are usually referred to as HRC since they share the same carcinogenic mechanism(Reference Henderson and Feigelson2). Worldwide, there were estimated 5 million newly diagnosed cases of HRC in 2020, accounting for more than a quarter of new cancer cases(Reference Sung, Ferlay and Siegel3). As the most frequently diagnosed cancer in the great majority of countries, HRC have become the leading cause of cancer death in over 100 countries(Reference Sung, Ferlay and Siegel3).
Considering the side effects of traditional hormone therapy for HRC, the use of phytochemicals with chemoprophylaxis is of great concern(Reference Dandawate, Subramaniam and Jensen4). Phytoestrogens (mainly isoflavones and lignans), abundant in several edible and/or medicinal plants belonging mostly to the Leguminosae family, seem to have hormone-like and anti-hormone effects due to their structural similarity to the natural estrogen estradiol (e.g. 17β-estradiol) as well as other steroid hormones and steroid hormone antagonists(Reference Zand, Jenkins and Diamandis5) and are known to modulate multiple molecular targets in cancer cells(Reference Basu and Maier6,Reference Kuiper, Lemmen and Carlsson7) . Several existing meta-analyses summed up the evidence for the relationship between dietary soy isoflavones and lignans intake and risk of the aforementioned specific HRC among observational studies(Reference Chen, Rao and Zheng8–Reference Velentzis, Cantwell and Cardwell12). Specifically, the present results supported an association of isoflavone intake with a reduced risk of breast cancer in Asians but not in Westerners as well as a more likely link of lignan intake with breast cancer in post-menopausal women but not with prostate cancer(Reference Chen, Rao and Zheng8,Reference Zhang, Feng and Qluwakemi11,Reference Velentzis, Cantwell and Cardwell12) . Soy foods are regularly consumed as part of a normal diet in Asian countries, whereas soy protein is used as a functional ingredient (e.g. to promote water retention or as a bleaching agent) in many Western foods(Reference Messina13). Higher intake (over 35–63 mg/d) of isoflavones in Asians tends to be more than ten times of the higher intake (over 0·03–4 mg/d) in Westerners(Reference Liu, Peng and Qiao14), leading to the extreme difficulty in accurately quantifying isoflavone intake when exposure is so low particularly in the West. Meanwhile, some metabolites from isoflavones, such as O-desmethylangolensin (O-DMA) and equol, vary more markedly in urine excretion and exert more biological activity than the parent compound of soy isoflavones(Reference Frankenfeld15). Of note, Americans have been found to be less capable of producing equol from isoflavone than Asians(Reference Akaza, Miyanaga and Takashima16). However, most studies assessed phytoestrogens intake based on dietary questionnaires, and it is far from sufficient to assess the phytoestrogens intake given the metabolic and absorption levels of these compounds. Urinary phytoestrogens excretion is also correlated to their dietary intake estimated by the FFQ(Reference Arai, Uehara and Sato17–Reference French, Thompson and Hawker19). Therefore, the excretion of phytoestrogens in urine is considered a better measure of bioavailable phytoestrogens than an assessment of dietary soy intake(Reference Arai, Uehara and Sato17). To our knowledge, studies assessing the associations of urinary phytoestrogens with the risk of breast and prostate cancer have yielded inconsistent findings(Reference Ward, Chapelais and Kuhnle20–Reference Park, Wilkens and Franke28). However, there are still no studies that systematically and comprehensively investigate the relationship between urinary phytoestrogens (total isoflavones and total lignans) and HRC.
Studies manifested the role of phytoestrogens on cancer biomarkers were ambiguous. Although individual randomised controlled trials might advocate for the efficacy of phytoestrogens (diets rich in soy and linseed or soy/isoflavones supplements) in reducing prostate-specific antigens (PSA) concentrations, a meta-analysis of randomised controlled trials indicated that the consumption of soy products or isoflavones did not affect the PSA concentrations among men with prostate cancer diagnosis or a clinically identified risk of prostate cancer(Reference Grammatikopoulou, Gkiouras and Papageorgiou29). Further, only a few case reports showed the therapy with over-the-counter phytoestrogens or fermented soy influenced subsequent levels of cancer biomarkers(Reference Cecchi, Lapi and Vannacci30,Reference Klein, He and Roche31) .
Therefore, we aimed to collect cross-sectional data from National Health and Nutrition Examination Survey (NHANES) to estimate the association between urinary phytoestrogens and HRC and concentrations of serum cancer biomarkers among Americans.
Materials and methods
Study design and data source
We selected adult participants (≥ 20 years) from the NHANES, the design and content of which can be obtained from https://wwwn.cdc.gov/nchs/nhanes/default.aspx (Reference Curtin, Mohadjer and Dohrmann32,Reference Curtin, Mohadjer and Dohrmann33) . Each survey participant completed a household interview followed by a standardised physical examination in a mobile examination centre. The NHANES has been approved by the NCHS Ethics Review Board and written informed consent has been obtained from all participants.
In this secondary analysis, data and information on phytoestrogens and history of cancer were both available during 1999–2010 in continuous NHANES(Reference Johnson, Paulose-Ram and Ogden34). From 1999 to 2010, 62 160 participants completed the household interview, of which 59 367 participated in the mobile examination centre. We examined the integrity of variables interested and included 9755 participants aged ≥ 20 years with accessible urinary phytoestrogens and creatinine and history of cancer. After the further exclusion of participants who were pregnant (n 411) and who had cancers other than HRC (n 500), 8844 participants were included finally as investigative samples.
Ascertainment of hormone-related cancers
Participants were questioned, ‘have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any kind?’ in the household interview. If they answered ‘yes’, they would be asked to offer the type of cancer. Up to three types of cancer were registered. When they offer more than 3, enter ‘more than 3 kinds (code 66)’ as the fourth response. HRC were defined as cancers of breast (code 14), ovary (code 28), prostate (code 30), testis (code 36), thyroid (code 37) and corpus uteri (code 38) according to any of the three responses. If they had more than one of these cancers, they were regarded as subjects with multiple HRC.
Measurement of urinary phytoestrogen and creatinine
A minimum of 5 ml of urine specimens were collected from subjects in standard urine collection cups on the morning after a recommended fast at the mobile examination centre, transferred to specimen vials within 4 h of collection and stored frozen in borosilicate glass or polypropylene vials or specimen cups. Teflon-coated stoppers were used to plug vials, and the vials were sealed with an aluminum seal. The specimens were then labelled and frozen immediately to −20°C and stored on dry ice for shipping. Subsequently, spot urine specimens were processed, stored and shipped to the Division of Environmental Health Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention for analysis. The test principle utilises HPLC-atmospheric pressure chemical ionisation-tandem MS (HPLC-APCI-MS/MS) in NHANES 1999–2002(Reference Barnes, Coward and Kirk35), HPLC-electrospry ionisation-tandem MS (HPLC-ESI-MS/MS) in NHANES 2003–2004(Reference Rybak, Parker and Pfeiffer36) and HPLC-atmospheric pressure photoionisation-tandem MS (HPLC-APPI-MS/MS) in NHANES 2005–2010(Reference Li, Hsieh and Korfmacher37) for the quantitative detection of six phytoestrogens, including four isoflavones (genistein, daidzein, equol and O-DMA), as well as two lignans (enterodiol and enterolactone). The comparison of three laboratory methods for testing urinary phytoestrogens was shown in Supplementary Table S1. The detection limits were constant for all of the analytes in the data set (0·2 ng/ml for genistein, 0·4 ng/ml for daidzein, 0·06 ng/ml for equol, 0·2 ng/ml for O-DMA, 0·04 ng/ml for enterodiol and 0·1 ng/ml for enterolactone). Cross-over studies comparing samples analysed by different methods demonstrated high-correlation coefficients (r > 0·99) and regression slopes approximately equal to 1 and intercepts close to 0.
There was a change in the instrumentation and method for urine creatinine from 2006 to 2007. Prior to 2007, the urine creatinine was performed on the Beckman CX3 using a Jaffe reaction, while from 2007 forward, the urine creatinine was performed on the Roche ModP using an enzymatic (creatinase) method(38). Therefore, urine creatinine data from 2005 to 2006 need to be adjusted for comparison with data from 2007 forward. The following equations are recommended:
For urine creatinine < 75:
For urine creatinine 75 to < 250:
For urine creatinine ≥ 250:
Measurement of cancer biomarkers
Considering that cancer biomarker levels are closely related to the onset and progression of cancer, we attempt to explore the relationship between urinary phytoestrogen excretion and cancer biomarkers. Out of 4412 women samples, only 678 serum from women aged over 20 years from NHANES 2001 to 2002 were tested for cancer antigen 125 (CA125, considered as biomarkers for ovarian cancer), cancer antigen 15·3 (CA15.3, considered as biomarkers for breast cancer) and human epididymal secretory protein E4 (HE4, considered as biomarkers for ovarian cancer)(39). Among 4432 men samples, total and free PSA (, considered as biomarkers for prostate cancer) were measured by the Access Hybritech Assay (Beckman Access, Fullerton CA) in 2159 men aged 40 years and older from NHANES 2001–2010(40). PSA ratio is calculated by rounding the percentage of free PSA divided by total PSA. We have used preset cut-off values for the definition of abnormal biomarkers: CA125 ≥ 35 kU/l, CA15.3 ≥ 25 000 mU/ml, HE4 ≥ 70 pM, total PSA > 4 ng/mL and PSA ratio < 15 %. Due to free PSA tending to have no specific diagnosis value, the median value (0·27 ng/ml) was utilised. Ultimately, the associations of biomarkers for males and females with urinary phytoestrogens were analysed.
Covariates
Age, gender, race, education, marital status, poverty income ratio, physical activity intensity and smoking status were self-reported by participants in a household interview. Poverty income ratio was calculated by dividing family income by the poverty guidelines specific to family size, as well as the appropriate year and state(41) and was categorised into poorer (0·00–1·30), average (> 1·30–3·50) and richer (> 3·50 and above) based on Supplemental Nutrition Assistance Program eligibility(Reference Johnson, Paulose-Ram and Ogden34). Different types of physical activity in a typical week were asked before the physical examination, in the home, by trained interviewers using the Computer Assisted Personal Interviewing system. According to the increase in physical exertion, breathing and heart rate, physical activity intensity was categorised as vigorous, moderate and inactive. Based on the participants’ responses to the question of whether they smoked at least 100 cigarettes during their lifetime and whether they were currently smoking, they were categorised as non-smoker, former smokers and current smokers. During the mobile examination centre interview, 24-hour dietary recall was administered to calculate alcohol intake, total energy intake and fat intake. Alcohol intake was defined as no drinking (0 g/d), moderate drinking (0–14 g/d for females and 0–28 g/d for males) and heavy drinking (≥ 14 g/d for females and ≥ 28 g/d for males)(42). Measurements of height, weight and blood pressure were performed following a standardised protocol. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2, < 25, 25 to 29·9, ≥ 30)(Reference Curtin, Mohadjer and Dohrmann32,Reference Curtin, Mohadjer and Dohrmann33) . Hypertension was described as having a diagnosis by a doctor or other health professional with high blood pressure, taking antihypertensive medicines or having systolic blood pressure level of ≥ 130 mm Hg or diastolic blood pressure level of ≥ 80 mm Hg as per the 2017 ACC/AHA Hypertension Guideline(Reference Whelton, Carey and Aronow43). Indicators such as HbA1c, fasting plasma glucose, 2-hour glucose (oral glucose tolerance test), HDL-cholesterol, LDL-cholesterol and TAG were measured in NHANES laboratory(Reference Johnson, Paulose-Ram and Ogden34). Diabetes was described as any participant recognised with diabetes, taking insulin, taking diabetes pills, having a HbA1c level of ≥ 6·5 %, a fasting plasma glucose level of ≥ 126 mg/dl or an oral glucose tolerance test level of ≥ 200 mg/dl(Reference Menke, Casagrande and Geiss44). Dyslipidaemia was characterised as having a physician’s diagnosis, or currently taking lipid-lowering prescribed medicine, or having an abnormal lipid profile that includes an HDL-cholesterol level of < 40 mg/dl, an LDL-cholesterol level of ≥ 110 mg/dl or a TAG level of ≥150 mg/dl based on guidelines from the third report of the National Cholesterol Education Program Adult Treatment Panel III(45).
Statistical analyses
All statistical analyses accounting for the complex, multistage, stratified and cluster-sampling design (including oversampling of certain subgroups) of NHANES were conducted using survey modules of SAS software version 9.4 (SAS Institute). Sample weights were allowed to combine to obtain national estimates that reflect the true relative proportions of particular subgroups in the USA population as a whole.
Urinary phytoestrogens were classified into four isoflavones and two lignans. The isoflavone concentration was the sum of daidzein, genistein, O-DMA and equol concentration. The lignan concentration was the sum of enterodiol and enterolactone concentrations. Six standardised phytoestrogen concentrations (μg/g creatinine) were calculated by dividing each original phytoestrogen concentration by the adjusted creatinine level. The distribution of all urinary phytoestrogens was severely right-skewed with a kurtosis coefficient from 13·9 to 29·1 using Kurtosis test method. Hence, standardised phytoestrogens were log-transformed to accommodate subsequent analyses treating phytoestrogens as continuous variables. Finally, log-transformed data were utilised as categorical variables or continuous variables for subsequent statistical analysis.
Weighted means or geometric means (for urinary phytoestrogens) and proportions of baseline characteristics among participants with and without HRC were compared by using sampling-weighted ANOVA for continuous variables and Rao–Scott chi-square tests for categorical variables. For HRC analysis, sampling-weighted multivariate logistic regression models were performed to calculate the OR and 95 % CI for the overall and gender-stratified association between urinary phytoestrogens in quartile categories and HRC with the adjustment for covariates. P value for linear trend was calculated after treating classified urinary phytoestrogens as a continuous variable. Stratified analyses were further performed by race/ethnicity (White and non-White) and age (≤ 60 years and > 60 years) among females and males, respectively, as well as menopausal status (premenopausal and postmenopausal) only in females. Specifically, we also explored the association of urinary phytoestrogens with major cancer types, primarily female breast and prostate cancer. We calculated the duration of HRC by subtracting the age at HRC diagnosis from age at baseline and performed association analyses of urinary phytoestrogens with HRC based on the duration of HRC (within 5 years or over 5 years). Restricted cubic splines were conducted to assess the non-linear relationship of urinary phytoestrogens to HRC. For biomarker analysis, the weighted geometric means of female cancer biomarkers, CA125, CA15.3 and HE4, and male cancer biomarkers, total PSA, free PSA and PSA ratio were calculated to carry out partial correlation analyses with urinary phytoestrogens. A level of < 0·05 for adjusted two-sided P values was considered statistically significant.
Results
Characteristics of study population
A flow chart of dataset selection and subject screening process is outlined in Supplementary Fig. S1. Among 8844 participants aged 20 years and older in this study, the prevalence rate of HRC was 4·2 %. One hundred and forty-seven had breast cancer and 145 had prostate cancer. The number of subjects with uterine corpus cancer, ovarian cancer, thyroid cancer and testicular cancer was 43, 29, 13, and 5, respectively. Participants with HRC were more likely to be elder, female, non-Hispanic white, divorced/separated/widowed, physically inactive, former smokers, with hypertension, diabetes and dyslipidaemia and with lower total energy intake and lower fat intake, when compared with those without HRC (P < 0·05), as shown in Table 1. But neither female or male cancer biomarkers differed between those with and without HRC (P > 0·05). Compared with subjects with the lowest concentration of total urinary phytoestrogens, subjects with the highest concentration were more likely to be elder, female, non-Hispanic white, well-educated, married/living with partner, high-earning, non-smokers, free of obesity and dyslipidaemia, and with lower total energy intake and lower fat intake (P < 0·05; online Supplementary Table S2).
Table 1. Baseline characteristics of 8844 participants with and without hormone-related cancers from NHANES 1999–2010
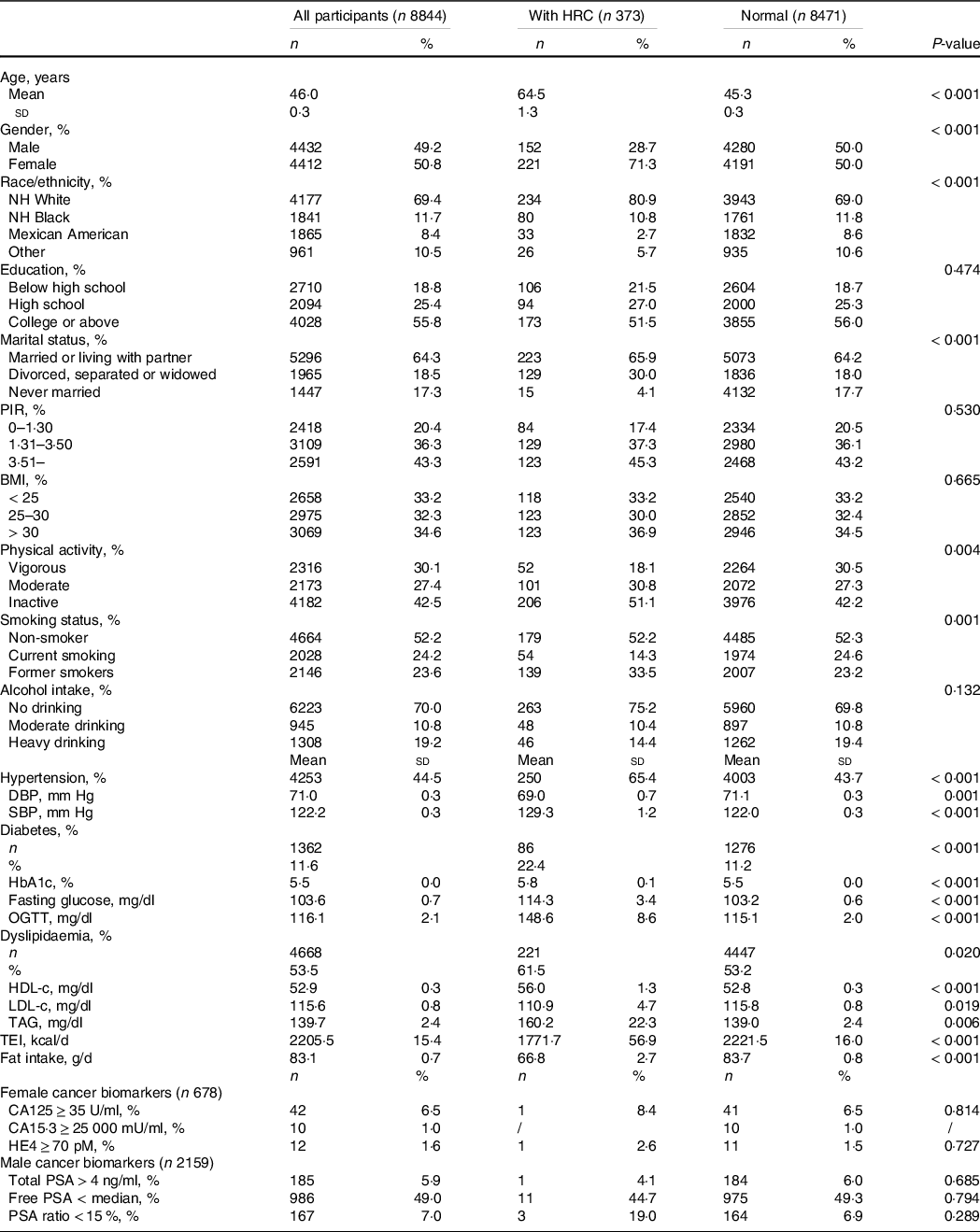
Abbreviations: CA125, cancer antigen 125; CA15.3, cancer antigen 15.3; HRC, hormone-related cancer; NHANES, National Health and Nutrition Examination Survey; NH, non-Hispanic; DBP, diastolic blood pressure; SBP, systolic blood pressure; OGTT, oral glucose tolerance test; ; TEI, total energy intake; HDL-c, HDL-cholesterol; HE4, human epididymal secretory protein E4; LDL-c, LDL-cholesterol; NHANES, National Health and Nutrition Examination Survey; PIR, poverty income ratio; PSA, prostate-specific antigen; sd, standard deviation.
Data were expressed as the weighted mean ± sd or number of participants (weighted proportion). Sampling-weighted Rao-Scott chi-square tests were performed for categorical variables and sampling-weighted ANOVA methods were performed for continuous variables. A level of < 0·05 for two-sided P values was considered statistically significant.
Distribution of urinary phytoestrogens
The median (P25, P75) of total urinary phytoestrogens was 711·2 (314·6, 1520·1) μg/g creatinine in all participants. Among which the excretion of lignan (mainly enterolactone) was approximately four times higher than that of isoflavone. As for isoflavone metabolites, the excretion of O-DMA (median (P25, P75): 3·3 (0·7, 19·4) μg/g creatinine) and equol (median (P25, P75): 7·0 (3·3, 14·5) μg/g creatinine) was much lower than that of maternal compound such as daidzein (median (P25, P75): 50·8 (18·3, 176·9) μg/g creatinine). For urinary phytoestrogens, participants with HRC had higher concentrations of total phytoestrogens, isoflavones (daidzein, genistein, O-DMA, equol) and lignans (enterodiol and enterolactone) than those without HRC (All P < 0·001; Table 2).
Table 2. Distribution of urinary phytoestrogens in participants with and without hormone-related cancers from NHANES 1999–2010
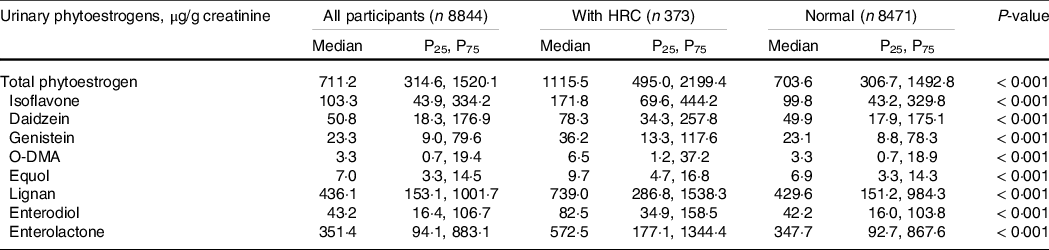
Abbreviations: HRC, hormone-related cancers; NHANES, National Health and Nutrition Examination Survey; O-DMA, O-desmethylangolensin.
Data were expressed as the weighted median (P25, P75). Sampling-weighted ANOVA methods was tested for the distribution difference of urinary phytoestrogens between participants with and without HRC. A level of < 0·05 for two-sided P values was considered statistically significant.
Overall association between urinary phytoestrogens and hormone-related cancers
The multivariable-adjusted OR for HRC by urinary phytoestrogens are shown in Fig. 1. Participants with higher concentration of urinary daidzein and enterodiol had HRC (P for trend < 0·05). Simultaneously, total isoflavones were associated with the augmented prevalence of HRC at Q3 levels (OR = 1·81, 95 % CI: 1·20, 2·73) but showed insignificant effects at Q4 levels. As shown in Supplementary Fig. S2, there was a significantly non-linear trend for the dose–response relationship between urinary lignans (mainly enterodiol) and HRC (P non-linear = 0·033). When the concentration of lignans was above the first quartile (P25, a certain level (ln(Concentration of lignans) = 7·5, i.e., concentration of lignans is approximately 1800 μg/g creatinine)), the higher the concentration, the higher the prevalence of HRC.
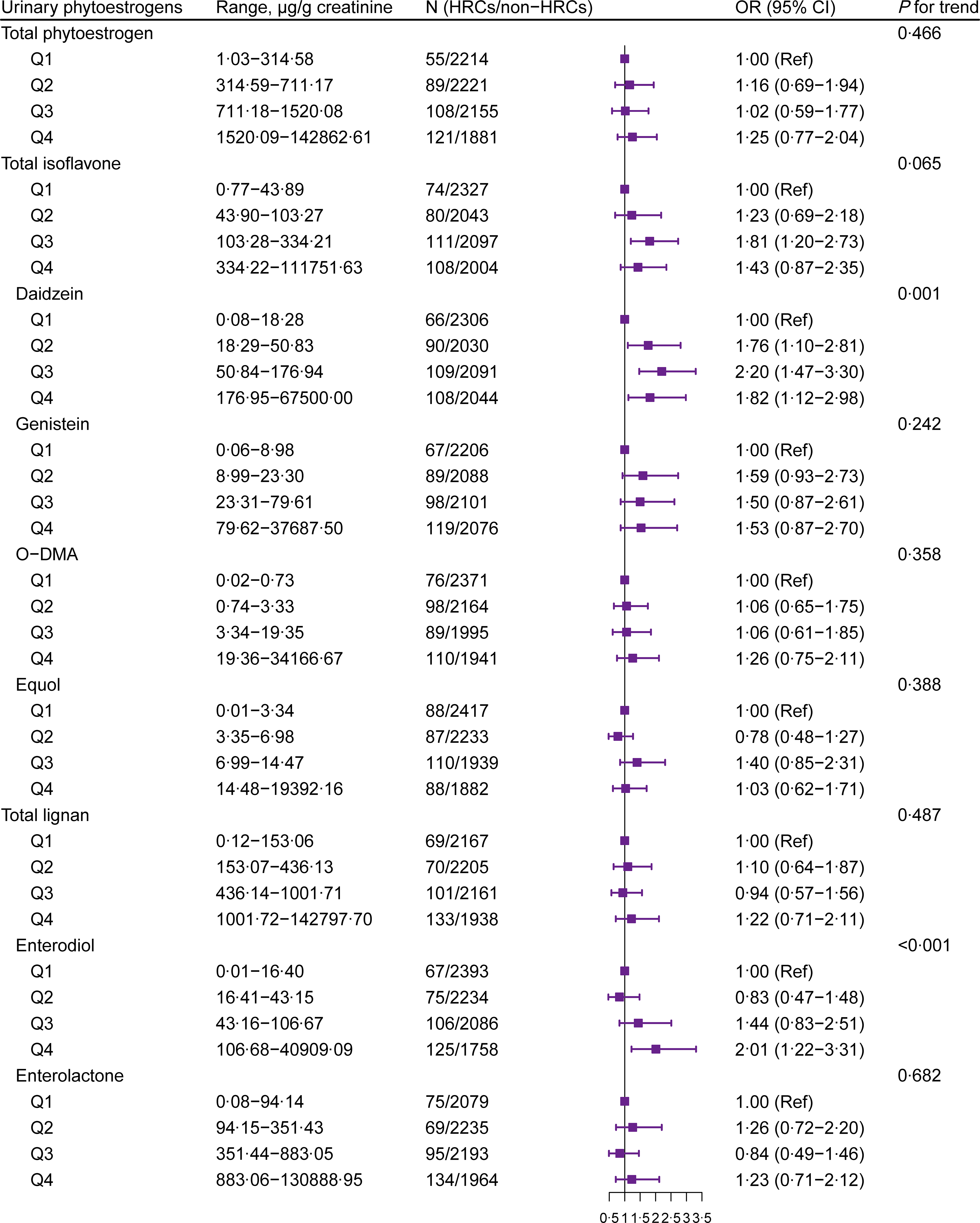
Fig. 1. Forest plot for the association between urinary phytoestrogens and HRC from NHANES 1999–2010. Adjusted for age (continuous), gender (female or male), race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American or other), education level (below high school, high school or college or above), marital status (married/living with partner, divorced/separated/widowed or never married), poverty income ratio (0–1·30, 1·31–3·50 or 3·51–), BMI (< 25, 25–30 or > 30 kg/m2), physical activity (vigorous, moderate or inactive), smoking status (non-smoker, current smoking or former smokers), alcohol intake (no drinking, moderate drinking or heavy drinking), hypertension (yes or no), diabetes (yes or no), dyslipidaemia (yes or no), total energy intake (continuous) and fat intake (continuous). HRC, hormone-related cancer; NHANES, National Health and Nutrition Examination Survey; O-DMA, O-desmethylangolensin.
Association between urinary phytoestrogens and hormone-related cancers by gender
We further conducted analyses on the association between urinary phytoestrogens and HRC among females and males. Higher concentrations of total isoflavone (P for trend = 0·010), daidzein (P for trend = 0·006), equol (P for trend = 0·027) and enterodiol (P for trend = 0·001) were positively associated with HRC among female participants (Fig. 2(a)). Compared with the lowest quartile (Q1) level, Q3 level of equol was positively associated with HRC among female participants, whereas only the highest concentration of equol had an association with a lower prevalence of HRC among males (Fig. 2(b)). For the purpose of focusing on specific subpopulations to investigate further associations, we carried out race/ethnicity- and age-stratified analyses among female and male participants, respectively (Fig. 3 and Fig. 4). We observed a higher prevalence of HRC conferred by a higher concentration of total isoflavone and enterodiol in urine among the White women (P for trend < 0·05) but not among the non-White women (Fig. 3(a)). As for the younger women, the highest concentration of total urinary phytoestrogens was linked with a higher prevalence of HRC (OR = 3·30, 95 % CI: 1·39, 7·81) (Fig. 3(b)). Compared with postmenopausal women, total phytoestrogens, isoflavones and lignans were more positively associated with HRC in premenopausal women (online Supplementary Table S3).
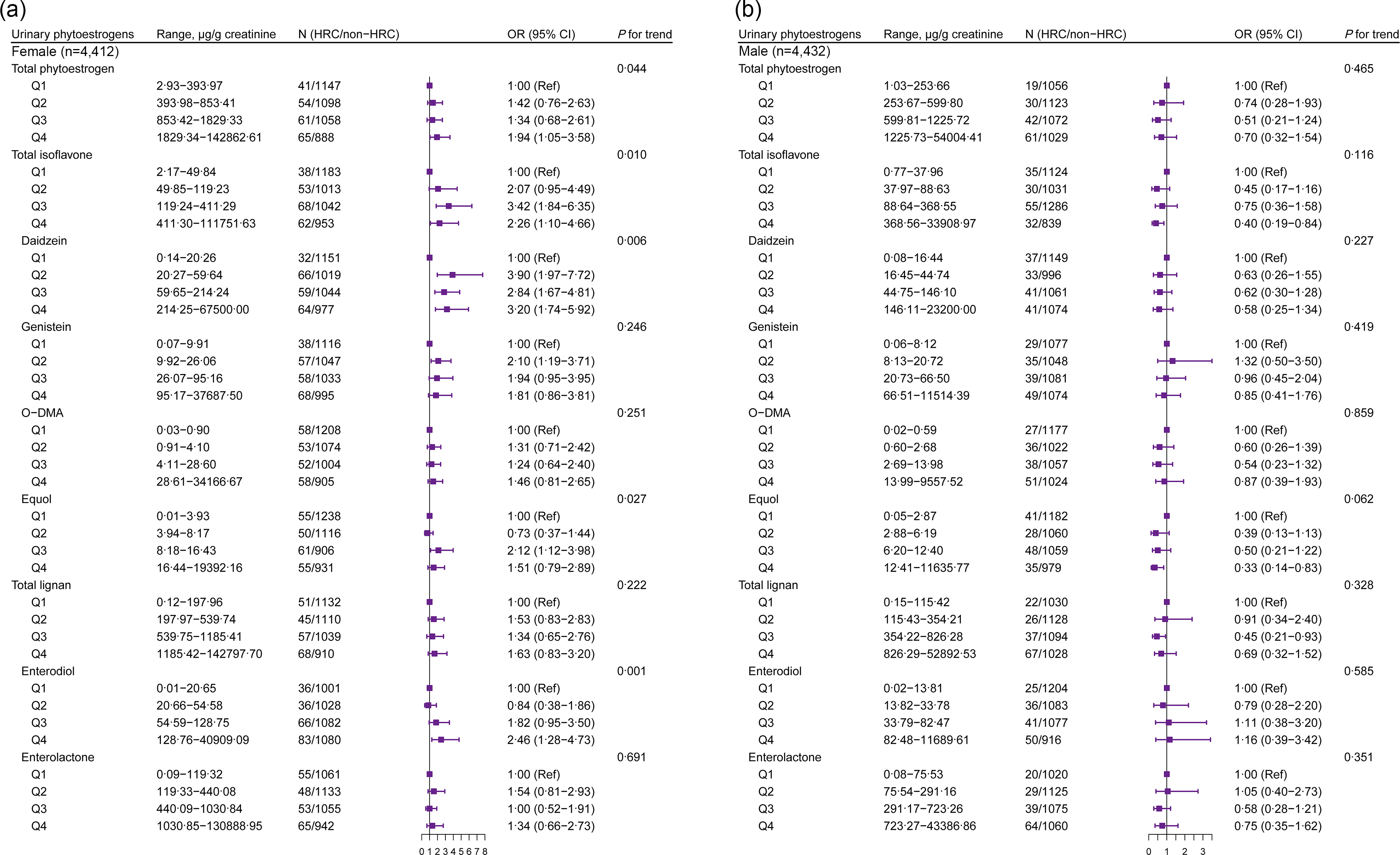
Fig. 2. Gender-stratified association between urinary phytoestrogens and HRC from NHANES 1999–2010. Adjusted for age (continuous), race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American or other), education level (below high school, high school or college or above), marital status (married/living with partner, divorced/separated/widowed or never married), poverty income ratio (0–1·30, 1·31–3·50 or 3·51–), BMI (< 25, 25–30 or > 30 kg/m2), physical activity (vigorous, moderate or inactive), smoking status (non-smoker, current smoking or former smokers), alcohol intake (no drinking, moderate drinking or heavy drinking), hypertension (yes or no), diabetes (yes or no), dyslipidaemia (yes or no), total energy intake (continuous) and fat intake (continuous). HRC, hormone-related cancer; NHANES, National Health and Nutrition Examination Survey; O-DMA, O-desmethylangolensin.
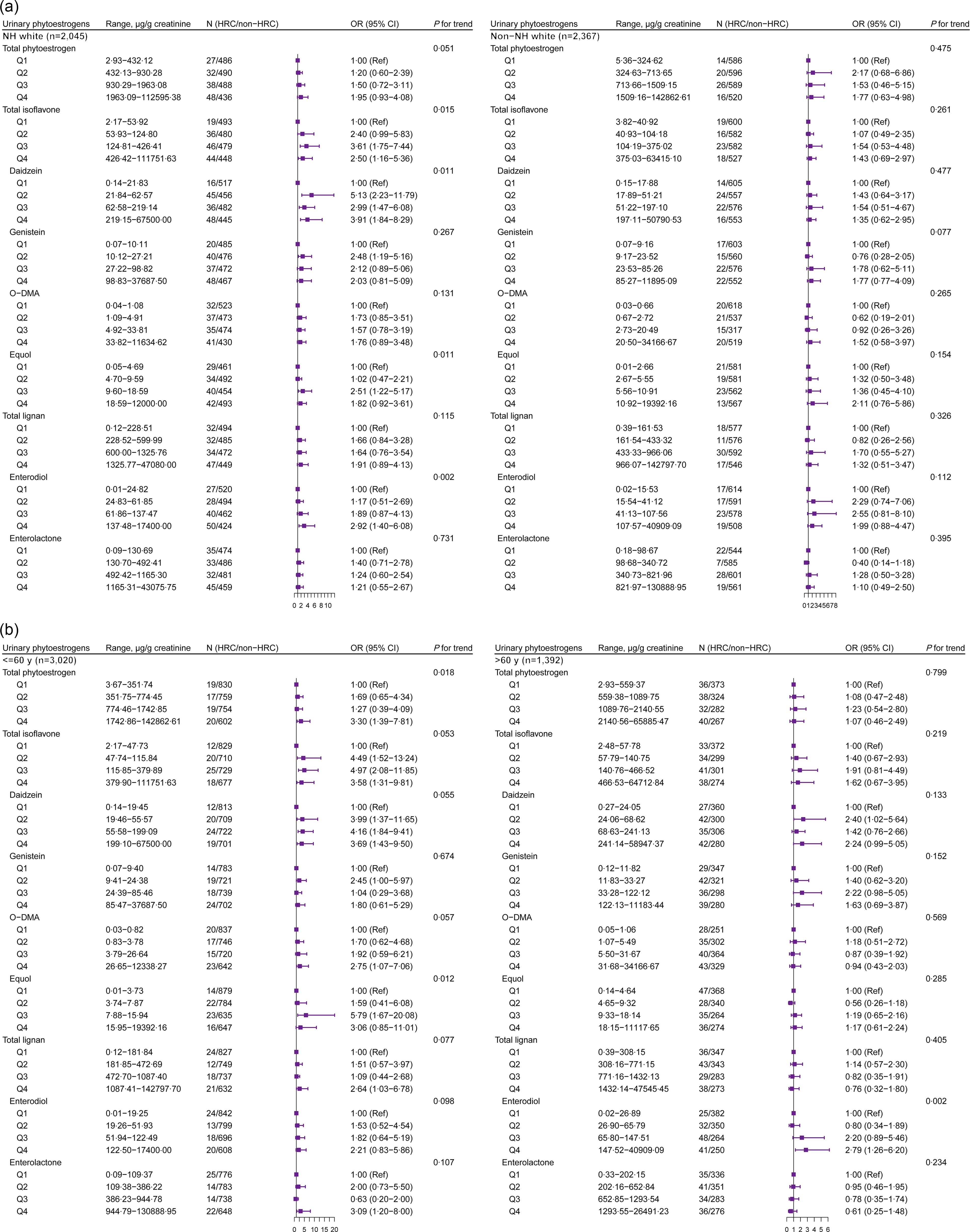
Fig. 3. The stratified analysis between urinary phytoestrogens and HRC among females from NHANES 1999–2010. The analysis was stratified by (a) race/ethnicity (White and non-White) and (b) age group (≤ 60 years and > 60 years). Adjusted for age (continuous, not for age-stratified analysis), race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American or other, not for race/ethnicity-stratified analysis), education level (below high school, high school or college or above), marital status (married/living with partner, divorced/separated/widowed or never married), poverty income ratio (0–1·30, 1·31–3·50, or 3·51–), BMI (< 25, 25–30 or > 30 kg/m2), physical activity (vigorous, moderate or inactive), smoking status (non-smoker, current smoking or former smokers), alcohol intake (no drinking, moderate drinking or heavy drinking), hypertension (yes or no), diabetes (yes or no), dyslipidaemia (yes or no), total energy intake (continuous) and fat intake (continuous). HRC, hormone-related cancer; NHANES, National Health and Nutrition Examination Survey; NH, non-Hispanic; O-DMA, O-desmethylangolensin.
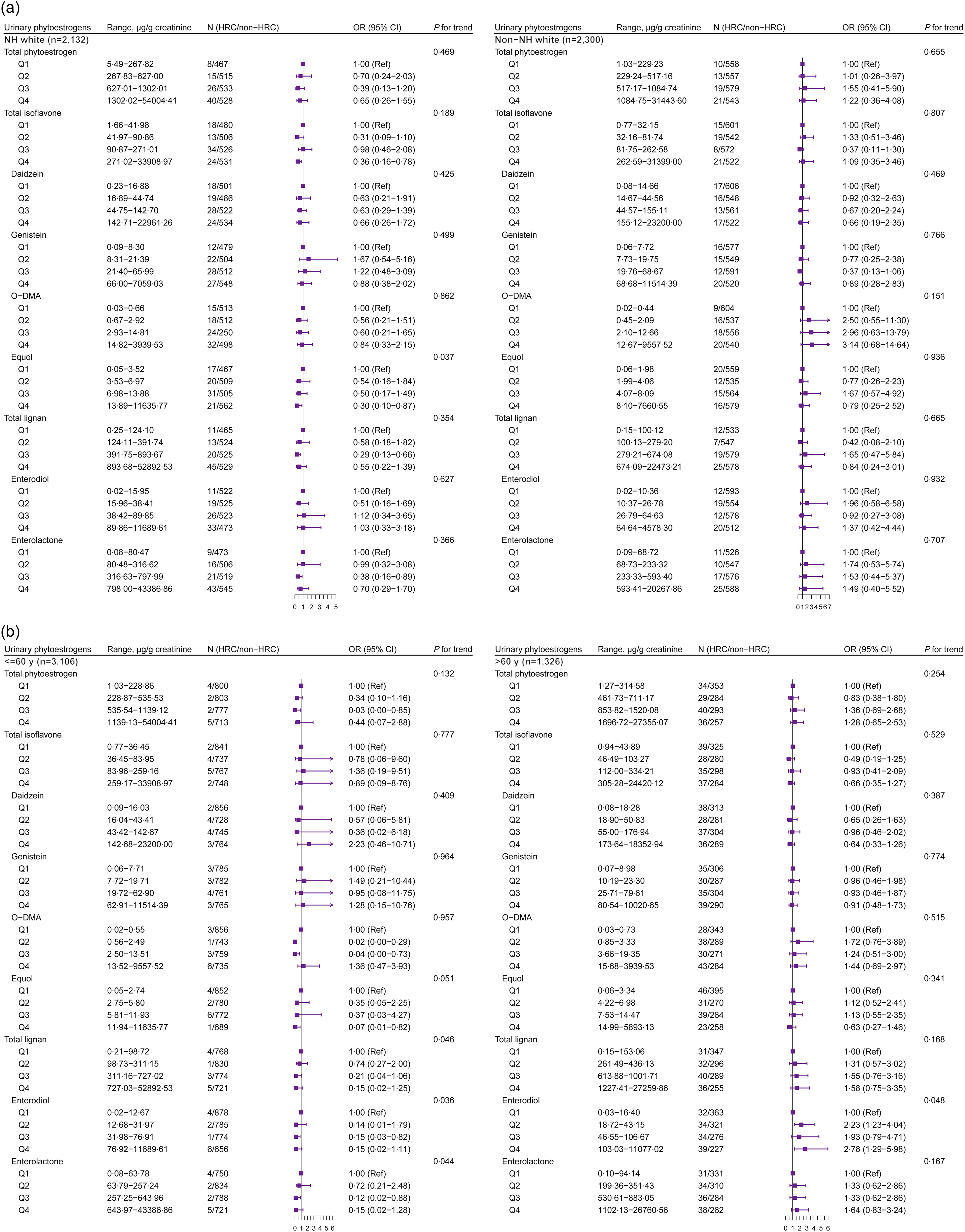
Fig. 4. The stratified analysis between urinary phytoestrogens and HRC among males from NHANES 1999–2010. The analysis was stratified by (a) race/ethnicity (White and non-White) and (b) age group (≤ 60 years and > 60 years). Adjusted for age (continuous, not for age-stratified analysis), race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American or other, not for race/ethnicity-stratified analysis), education level (below high school, high school or college or above), marital status (married/living with partner, divorced/separated/widowed or never married), poverty income ratio (0–1·30, 1·31–3·50, or 3·51–), BMI (< 25, 25–30 or > 30 kg/m2), physical activity (vigorous, moderate or inactive), smoking status (non-smoker, current smoking or former smokers), alcohol intake (no drinking, moderate drinking or heavy drinking), hypertension (yes or no), diabetes (yes or no), dyslipidaemia (yes or no), total energy intake (continuous) and fat intake (continuous). HRC, hormone-related cancer; NHANES, National Health and Nutrition Examination Survey; NH, non-Hispanic; O-DMA, O-desmethylangolensin.
In the analysis limited to male subjects, the excretion of total isoflavones (mainly equol) at Q4 level was negatively associated with HRC among those who were White (Fig. 4(a)). Notably, the highest level of enterodiol (OR = 2·78, 95 % CI: 1·29, 5·98) seemed to be associated with the higher prevalence of HRC in elder males (Fig. 4(b)).
With respect to the main specific cancer type, we observed a similarly positive correlation between daidzein and enterodiol in female breast cancer (online Supplementary Fig. S3). However, the highest concentration of total isoflavones (OR = 0·40, 95 % CI: 0·21, 0·80), mainly equol (OR = 0·38, 95 % CI: 0·16, 0·93), was significantly linked to a reduced prevalence of prostate cancer compared to the lowest concentration (online Supplementary Fig. S4). Also, for HRC with different years of diagnosis, we found that the daidzein and total lignans were more positively associated with HRC over 5 years than HRC within 5 years. The positive association of urinary enterodiol was more pronounced in HRC within 5 years.
Urinary phytoestrogens and cancer biomarkers
For female’s cancer biomarker analysis, firstly, we observed participants with HRC had higher serum HE4 concentrations than those without HRC. The concentration of HE4 was more likely to be correlated with the well-known cancer risk factors (online Supplementary Table S5). Further, we explored the correlation of three female cancer biomarkers with urinary phytoestrogens. Notably, the positive correlation between serum CA15.3 and urinary equol was found among females with HRC in the partial correlation analysis (r = 0·448, P = 0·032) (Table 3).
Table 3. Correlation of urinary phytoestrogens with female cancer biomarkers among 678 participants from NHANES 2001–2002
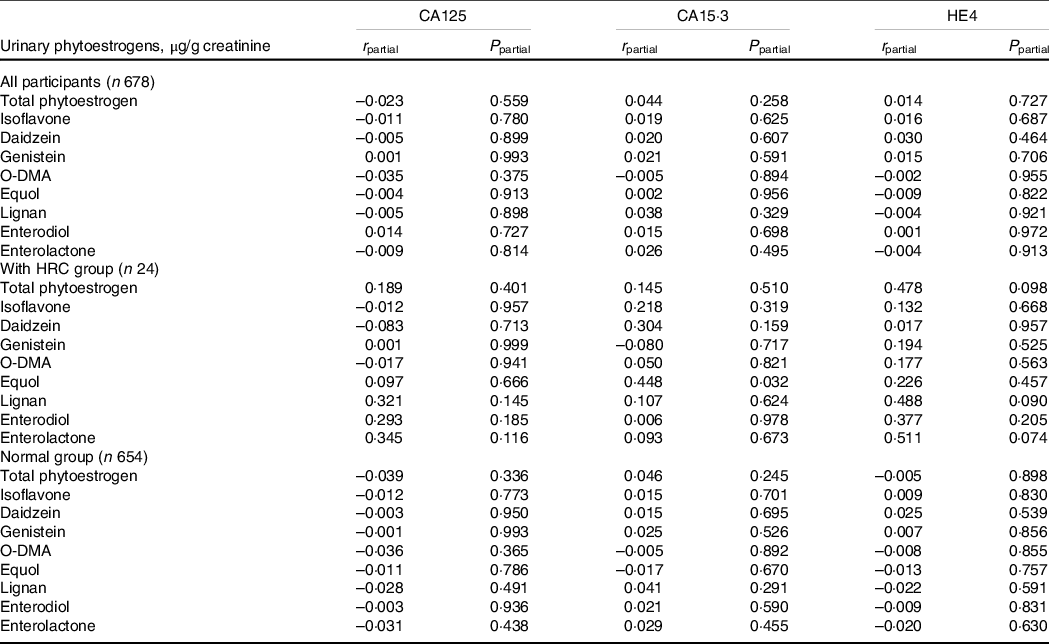
Abbreviations: CA125, cancer antigen 125; CA15.3, cancer antigen 15.3; HE4, human epididymal secretory protein E4; HRC, hormone-related cancers; NHANES, National Health and Nutrition Examination Survey; O-DMA, O-desmethylangolensin.
Data were expressed as the weighted correlation coefficient (r) and adjusted P-value.
For CA125, partial correlation analyses adjusted for age (continuous) and hypertension (yes or no). For CA15.3, partial correlation analyses adjusted for marital status (married/living with partner, divorced/separated/widowed or never married). For HE4, partial correlation analyses adjusted for age (continuous), race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American, or other), education (below high school, high school or college or above), marital status (married/living with partner, divorced/separated/widowed or never married), smoking status (non-smoker, current smoking or former smokers), hypertension (yes or no), diabetes (yes or no), dyslipidaemia (yes or no), total energy intake (continuous) and fat intake (continuous). A level of < 0·05 for two-sided P values was considered statistically significant.
Similarly, 2159 male participants with information on PSA were included in our study. The PSA ratio was correlated with race/ethnicity and marital status and was lower in participants with HRC (online Supplementary Table S6). Generally, no significant correlations between urinary phytoestrogens and serum total/free PSA and PSA ratio were observed in partial correlation analyses (Table 4). However, among patients with prostate cancer, higher concentration of urinary enterolactone was forcefully correlated with decreased total PSA (r = −0·979, P = 0·021) and elevated PSA ratio (r = 0·636, P = 0·036).
Table 4. Correlation of urinary phytoestrogens with male cancer biomarkers among 2159 participants from NHANES 2001–2010
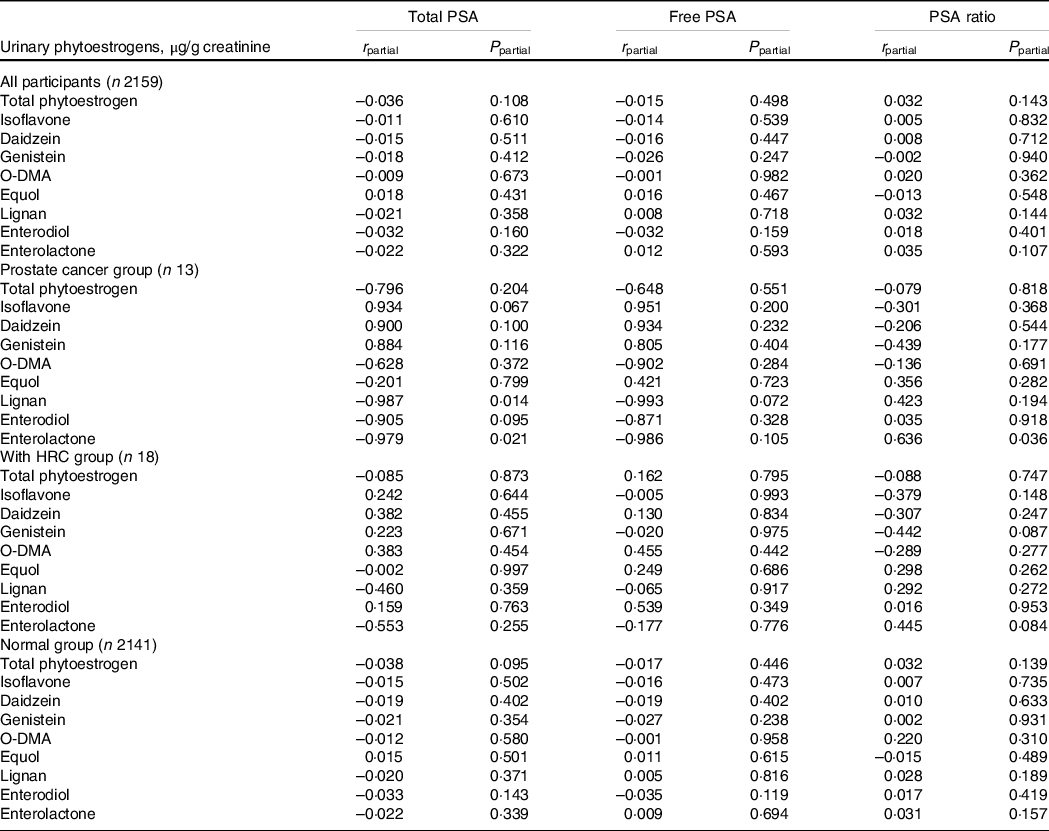
Abbreviations: HRC, hormone-related cancers; NHANES, National Health and Nutrition Examination Survey; O-DMA, O-desmethylangolensin; PSA, prostate-specific antigen.
Data were expressed as the weighted correlation coefficient (r) and adjusted P-value.
For total PSA, partial correlation analyses were adjusted for age (continuous), BMI (< 25, 25–30, or > 30 kg/m2), hypertension (yes or no), total energy intake (continuous) and fat intake (continuous). For free PSA, partial correlation analyses adjusted for age (continuous), BMI (< 25, 25–30, or > 30 kg/m2), smoking status (non-smoker, current smoking or former smokers), hypertension (yes or no), total energy intake (continuous) and fat intake (continuous). For PSA ratio, partial correlation analyses adjusted for race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American or other) and marital status (married/living with partner, divorced/separated/widowed or never married).
Discussion
Although estimates of total phytoestrogens among different studies were not the same (such as isoflavones mainly calculated from the sum of daidzein and genistein or the sum of daidzein, genistein and equol), the study herein firstly comprehensively evaluated the role of total phytoestrogens excretion (the sum of isoflavones including daidzein, genistein, O-DMA and equol and lignans including enterodiol and enterolactone) in HRC. In this study of 8844 participants from the continuous NHANES, we firstly observed higher concentrations of isoflavones (mainly daidzein) and enterodiol were positively associated with female HRC, which appeared even more pronounced in non-Hispanic white and breast cancer. Whereas, the highest concentration of total isoflavones was significantly linked to a reduced prevalence of HRC in white males and of prostate cancer. Among participants with HRC, urinary equol concentration was strikingly positively correlated with serum CA15·3. Inverse correlation of total PSA and positive correlation of the PSA ratio with urinary enterolactone were detected in prostate cancer patients.
To the best of our knowledge, the findings of previous studies in western populations on the relationship between urinary phytoestrogens and risk of breast cancer were conflicting(Reference Ward, Chapelais and Kuhnle20,Reference Grace, Taylor and Low22,Reference Goodman, Shvetsov and Wilkens23,Reference Ingram, Sanders and Kolybaba46) . A nested case–control study within Multiethnic Cohort Study established in America prospectively identified invasive postmenopausal breast cancer cases through linkage to the cancer registries and showed no effects of urinary phytoestrogens excretion on breast cancer risk but the risk varies slightly among different races(Reference Goodman, Shvetsov and Wilkens23), suggesting the existence of a regional and ethnic disparity between different studies was necessitated for consideration. An earlier small-scale case–control study in Australia reported a substantial reduction in breast cancer risk among women with a high excretion of phytoestrogens, particularly the equol and the enterolactone(Reference Ingram, Sanders and Kolybaba46). However, two other nested case–control studies from European Prospective into Cancer-Norfolk (EPIC-Norfolk) study implied that higher concentrations of urinary isoflavones were associated with a marginally increased risk of female breast cancer, but total lignans were not(Reference Ward, Chapelais and Kuhnle20,Reference Grace, Taylor and Low22) , which is consistent with our results, even though the dietary source of isoflavones might be quite different in these two studies. For the EPIC-Norfolk study, approximately 3 % subjects were classified as soya consumers with 38·5 % of their isoflavone intake deriving from vegetable dishes and milks, whereas among non-soya subjects, bread and bread rolls were the main food sources of isoflavones, accounting for 81 %(Reference Mulligan, Welch and McTaggart47). A study on dietary assessment and food sources from NHANES found that 44·8 % of isoflavones were derived from legumes(Reference Kim, Vance and Chun48). Meanwhile, after unit conversion, urinary phytoestrogen concentrations were slightly lower in our data from NHANES than those in the EPIC-Norfolk study (e.g. median for genistein: 2·64 μg/mmol creatinine v. 5·71 μg/mmol creatinine). Phytoestrogens have been suggested to promote carcinogenesis on account of their estrogenic effects and the role in potentially modulating enzymes known to regulate estrogen levels(Reference van Duursen49,Reference Boué, Wiese and Nehls50) , which may explain the results we observed, but it remains to be seen whether this effect will emerge due to such low exposure levels of phytoestrogens in Western women. On the contrary, there were two Chinese studies leading to the suggestion that urinary isoflavones might be linked to a decreased risk of breast cancer(Reference Dai, Franke and Jin21,Reference Dai, Franke and Yu24) . The lower incidence of breast cancer that has been observed in Asian populations may be attributed to a higher intake of phytoestrogen-rich food, higher concentrations of phytoestrogen biomarkers and more production of equol relative to western populations(Reference Ward, Chapelais and Kuhnle20). It is difficult to assess phytoestrogens in western countries, and the limited evidence of an association between urinary isoflavones and breast cancer warrants further study in subsequent examinations of breast cancer risk and phytoestrogen biomarkers. Regarding prostate cancer, a protective effect of urinary daidzein excretion was found in American populations(Reference Park, Wilkens and Franke28). As a metabolite of daidzein formed by intestinal microbiota, equol is considered to be the most bioactive phytoestrogens among soybean isoflavones. The findings of a Jamaican study showed that when compared with males who have no detectable concentrations of equol, equol producers were at a reduced risk of prostate cancer(Reference Jackson, McFarlane-Anderson and Simon27). Consistently, equol seems to possess the potential to reduce the risk of prostate cancer in our study, as well as the total isoflavones. Studies have also observed that equol exerts its inhibitory effect on prostate cancer cells through androgen receptor signaling, Akt/FOXO3a pathway and so on(Reference Lu, Zhou and Kong51,Reference Itsumi, Shiota and Takeuchi52) . Thus, phytoestrogenic metabolites may be more valuable than parent compounds in prostate cancer prevention and treatment. In the future, a more comprehensive evaluation of phytoestrogens and its metabolites is warranted.
Furthermore, it has been suggested that some phytoestrogens like genistein may not be antiestrogens but agonists under the circumstance of relatively lower circulating levels of mammalian estrogen(Reference Ward, Chapelais and Kuhnle20), which may have been the state of the postmenopausal majority. Menopausal status as an effect modifier may have an influence on the association between phytoestrogens and female cancer risk. Ward et al. (Reference Ward, Chapelais and Kuhnle20) stratified by menopausal status and detected a stronger association between total urinary isoflavones and breast cancer risk in premenopausal and perimenopausal women (OR = 1·30), but null findings in postmenopausal women (OR = 1·01). In our study, total urinary isoflavones were positively associated with HRC in both pre- and post-menopausal women, but the OR in pre-menopausal women was quite larger than that in post-menopausal women. For certain quartiles, the OR of Q2 and Q4 for pre-menopausal and Q3 for post-menopausal were significant. Similarly, the effects of lignans seemed more significant in pre-menopausal women. Since phytoestrogens have dual effects, it is necessary to understand the effects of phytoestrogens on HRC in different menopausal states and hormone levels. Nonetheless, the evidence suggests that there may be circumstances where phytoestrogen exposure increases HRC (e.g. breast cancer) risk, and thus the evidence on the role of phytoestrogens modified by menopausal status deserves further study.
Herein, we found that participants with HRC had higher serum HE4 concentrations and lower PSA ratios than those without HRC, which was in line with our expectations. HE4 is a novel tumour marker for ovarian cancer with higher specificity and sensitivity than CA125(Reference Scaletta, Plotti and Luvero53). PSA ratio has proven to be an effective tool in prostate cancer screening, especially in the setting of total PSA in the intermediate range of 4–10 ng/ml and a negative digital rectal examination(Reference Swoboda, Luboldt and Rubben54). Knowledge is still limited about the association of phytoestrogens and cancer biomarkers. A case report showed that the serum CA125 concentration of a post-operative patient with ovarian cancer dropped from 756 μg/ml to 311 μg/ml after an intervention of the fermented soybean product for 6 weeks(Reference Klein, He and Roche31). In our study, we did not find any effect of phytoestrogens on CA125, but a stimulative effect of equol on CA15.3 in twenty-four HRC patients. We further analysed the correlation between CA15.3 and urinary equol in twenty-four HRC patients by cancer type and found that there was a discrepant correlation in the various cancers (for breast cancer: n 16, r = 0·420, P = 0·119; for uterine cancer: n 7, r = 0·843; P = 0·035; data not shown). Studies have suggested that CA15.3 levels significantly correlated with prognostic factors and were a useful prognostic tool for endometrial (uterine) cancer(Reference Tas and Yavuz55). However, whether equol impacts the initiation and development of endometrial cancer by affecting the level of CA15.3 needs further research verification. A randomised, double-blind, placebo-controlled study indicated that a daily diet containing four slices of bread rich in heat-treated soy grits favourably influenced the PSA level and the free/total PSA ratio in patients with prostate cancer, which provides some evidence to support epidemiologic studies claiming that male populations who consume high phytoestrogen diets have a decreased risk of the PSA abnormality and even prostate cancer(Reference Dalais, Meliala and Wattanapenpaiboon56). Additionally, a cross-sectional study examining the relationship between the serum PSA level and urine phytoestrogen concentration from NHANES 2001–2004 revealed that in general healthy US men, 40+ years old without a diagnosis of prostate cancer, urinary isoflavone and lignan concentrations were not associated with serum PSA level(Reference Walser-Domjan, Richard and Eichholzer57). Similar results were found among healthy men without HRC from NHANES 2001–2010 in this study. Of note, among small-scale prostate cancer patients, enterolactone seemed to favourably reduce total PSA levels and increase PSA ratio. The hormone-related activity of lignans may exert anti-hormonal activity and somehow alter physiological hormonal homeostasis in the presence of prostate cancer. Current research is ongoing to better understand the mode of action of phytoestrogens on cancer.
Theoretically, our findings enriched the study of the potential effects of phytoestrogens on HRC and for the first time showed a positive correlation of urinary equol with CA15.3 and a negative and positive correlation of urinary enterolactone with total PSA and PSA ratio in men, respectively. However, there are some limitations and challenges to be settled in our research. First, the cross-sectional data reduces our capacity for evidence. More prospective studies are warranted to explore the causal association. Second, selection bias and recall bias may exist. For instance, information on the history of cancer was recalled and orally reported by the subjects, not confirmed by cancer registries or medical records. And information on cancer treatment or stage of cancer was unavailable. Third, information on dietary phytoestrogens was not included in our analysis and urinary phytoestrogens reflect short term (24–48 h) rather than long-term dietary intake. Meanwhile, since the phytoestrogen data for most of our cancer patients reflect a point in time after diagnosis, it is possible that the eating habits have changed and cannot reflect the typical dietary behaviours before the cancer experience. Such information, if included, would make our analysis more convincing. Besides, even though the potential covariates of interest had been adjusted in the partial correlation analyses, there may be other unknown variables affecting the relationship between phytoestrogens and cancer biomarkers. Therefore, further research is needed to further clarify this issue.
In conclusion, our findings indicated that higher concentrations of total isoflavones and enterodiol were positively associated with HRC among females and equol was negatively linked to HRC among males, differentiated by race/ethnicity and age. Urinary equol excretion was related to serum CA15.3 among participants with HRC, and enterolactone concentration may affect PSA levels in prostate cancer patients. Nonetheless, further prospective studies and a larger number of subjects are warranted to be verified and provide stronger evidence.
Acknowledgements
We gratefully acknowledge the contributions of all staffs who work on the National Health and Nutrition Examination Surveys.
This work was supported by National Key R&D Program of China (2021YFC2500400, 2021YFC2500401), Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009A), the National Natural Science Foundation of China (81974488), and Guangdong Basic and Applied Basic Research Foundation (2022A1515010436).
Conceptualisation, M. Z. and F. S.; Methodology, F. L., M. Z.; Software, F. L. and Y. P.; Formal Analysis, X. W. and C. S.; Writing – Original Draft Preparation, F. L. and Y. P.; Writing – Review & Editing, F. L., Y. Q. and P. W.; Funding acquisition: M. Z. and F. S.; Sulpervision, F. S. All authors have read and approved the final manuscript.
The authors declare no conflicts of interest.
The National Center for Health Statistics Ethics Review Board at the Centers for Disease Control and Prevention approved collection of the NHANES data and posting of the NHANES public-use files, which were required for this investigation. All NHANES participants provided written informed consent.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114522003877











