Osteoporosis is a major public health issue worldwide. It results in an increased susceptibility to fractures, which in turn lead to lower quality of life( Reference Gold 1 ), and increased disability and mortality( Reference Ioannidis, Papaioannou and Hopman 2 ). Of note, low BMD is a major risk factor for fracture throughout the lifetime( Reference Rubenstein 4 , Reference Tinetti, Speechley and Ginter 5 ). BMD in adults depends on both peak bone mass that is achieved at around the third decade of life and the rate of bone loss subsequently( Reference Fukagawa, Wolfson and Judge 6 , Reference Puthoff and Nielsen 7 ). Therefore, creating a greater reserve of bone mass at a younger age and slowing bone loss throughout adult life are critical parts of any strategy for preventing future osteoporotic fractures.
Falls are also a major fracture risk factor – more than 90 % of hip fractures are the result of a fall( Reference Grisso, Kelsey and Strom 3 ) and falls cause a high rate of mortality( Reference Rubenstein 4 ). Poor balance is a primary risk factor of falling in older adults( Reference Tinetti, Speechley and Ginter 5 ). In addition, muscle weakness is associated with reduced balance in the older population( Reference Fukagawa, Wolfson and Judge 6 , Reference Puthoff and Nielsen 7 ) and progressive loss of muscle strength (especially of the lower limbs) with aging is also a major risk factor for falls( Reference Sayer, Syddall and Martin 8 , Reference Moreland, Richardson and Goldsmith 9 ). Importantly, both muscle strength and balance decline dramatically between 45 and 55 years of age( Reference El Haber, Erbas and Hill 10 , Reference Choy, Brauer and Nitz 11 ), suggesting there could be potential benefits of early interventions during this age period.
Studies focussing on particular nutrients (e.g. Ca and vitamin D), foods or food groups (e.g. dairy products) have identified associations between individual nutrients and musculoskeletal health outcomes( Reference Palacios 12 , Reference Robinson, Cooper and Aihie Sayer 13 ). In particular, studies have demonstrated beneficial associations of sufficient nutrients (e.g. protein, vitamin D and antioxidant nutrients) with BMD, muscle strength and balance( Reference Robinson, Cooper and Aihie Sayer 13 – Reference Reinders, Murphy and Song 15 ). However, such approaches of using individual nutrients or food items do not consider interactions, intercorrelations and cumulative effects between different nutrients and foods( Reference Kontogianni and Yiannakouris 16 ). Dietary pattern analysis has been used as an alternative to deal with these limitations by studying the overall diet rather than intakes of specific individual nutrients. This is particularly important for disease prevention or treatment because the effect of a single or a few nutrients may be too small to be detectable while dietary pattern analysis considers the joint effects of nutrients and foods based on entire eating pattern/behaviour. The results of dietary patterns studies can also be easy for the public to translate into diets and refine the dietary guidelines in the prevention of diseases.
The links between dietary patterns and bone health have largely been investigated in the elderly( Reference McNaughton, Wattanapenpaiboon and Wark 17 – Reference Hardcastle, Aucott and Fraser 19 ), but the results might not be generalisable to younger adults. Indeed, conflicting conclusions have been drawn in the literature on the relationship between a ‘Western’ dietary pattern and bone health among younger( Reference McNaughton, Wattanapenpaiboon and Wark 17 , Reference Okubo, Sasaki and Horiguchi 20 ) and older adults( Reference McNaughton, Wattanapenpaiboon and Wark 17 – Reference Hardcastle, Aucott and Fraser 19 ), which might be due to the change in diet behaviours and quantity of foods/nutrients for improving musculoskeletal health. In contrast, only a few studies have examined the association of dietary patterns and musculoskeletal health outcomes in younger adults( Reference McNaughton, Wattanapenpaiboon and Wark 17 , Reference Okubo, Sasaki and Horiguchi 20 , Reference Whittle, Woodside and Cardwell 21 ), showing an inconclusive association of various dietary patterns with bone health. However, these studies in younger adults have assessed bone density outcomes( Reference McNaughton, Wattanapenpaiboon and Wark 17 , Reference Okubo, Sasaki and Horiguchi 20 , Reference Whittle, Woodside and Cardwell 21 ) but not muscle strength or balance. Therefore, this study aimed to assess the association of dietary patterns identified by factor analysis with multiple musculoskeletal outcomes, including bone density, lower limb muscle strength and balance in a cohort of Australian women aged 36–57 years.
Methods
Study population
The sample for this cross-sectional study comprised 347 women (aged 36–57 years) who participated in a 10-year additional follow-up of a 2-year osteoporosis randomised controlled trial in Southern Tasmania, Australia, with details reported previously( Reference Winzenberg, Oldenburg and Frendin 22 ). In brief, women aged 25–44 years were randomly selected from the Tasmanian Electoral Roll in 2000. Women were recruited if they were free of the following: previous measurement of BMD (as the intervention involved used bone density to provide feedback to participants of their relative risk of fractures in later life), history of thyroid disease, renal failure, malignancy, rheumatoid arthritis, hysterectomy, hormone replacement therapies, pregnancy or planning pregnancy within 2 years of study entry, or lactating. At baseline, 470 women were randomly assigned to one of two osteoporosis educational interventions: group education using the Osteoporosis Prevention and Self-management course (OPSMC) or an information leaflet. The OPSMC is a chronic disease self-management course developed by the Arthritis Foundation of Victoria and utilised by Osteoporosis Australia. It aims to increase knowledge, improve confidence and awareness and self-management of osteoporosis prevention with an emphasis on promoting appropriate lifestyle changes. The osteoporosis information leaflet, from Osteoporosis Australia ‘Understanding Osteoporosis’, provided a comprehensive description of osteoporosis and a discussion of the role of lifestyle factors including diet, exercise and smoking, and optimal levels of Ca intake and exercise. Participants had their BMD measured at the spine and hip at baseline, 2 and 12 years. At baseline, those with a mean spine and hip T score <0 were informed that they were at a higher risk in later life whereas those with a mean T score of 0 or greater were informed that they were not at higher risk (here termed as fracture risk feedback). At 12 years, all women from the original study were contacted to ask for participation in this cross-sectional study. For both the original randomised controlled trial and the present cross-sectional study, ethics approval was obtained from the Tasmania Health and Medical Human Research Ethics Committee (EC00337) and all participants gave written informed consent. A total of 347 women who attended the 12-year follow-up and had at least one of the outcomes were included in this cross-sectional analysis, and the methods for the measures are described below.
Bone mass
Total body bone mineral content (TB BMC) and BMD at the lumbar spine (LS) and femoral neck (FN) were measured by dual-energy X-ray absorptiometry using fan beam setting on an in-house Hologic Delphi bone densitometer (Hologic QDR2000; Hologic), calibrated daily with CV 1 %.
Balance
We measured four clinical balance tests: the timed up and go test (TUG), the step test (ST), the functional reach test (FRT) and the lateral reach test (LRT). These tests assess balance performance from either a static or dynamic aspect, and are able to differentiate between ‘fallers’ and ‘non-fallers’ in older adults( Reference Isles, Choy and Steer 23 ). All have been validated in older women and have a high reliability, with normative values determined in women of the age in our study( Reference Isles, Choy and Steer 23 – Reference Brauer, Burns and Galley 25 ).
TUG( Reference Podsiadlo and Richardson 26 ) is a test of dynamic steady-state balance and gait. Participants sat in an armchair (45 cm high) with their back against the chair, then stood without using the arms, walked three metres, turned, walked back, and sat down. The average time of two trials was used for analysis.
The ST( Reference Hill, Bernhardt and McGann 27 ) measures speed of performing a dynamic stepping task. Participants stood 5 cm from an 8·5-cm-high block positioned against a wall and placed the whole foot of one leg onto the block and then returned it to the floor repeatedly as fast as possible for 15 s. The number of steps was recorded. Both sides were tested, and the mean number of steps for each side was calculated for analysis.
The FRT measures ability to reach forward with each arm from a bilateral stance position( Reference Duncan, Weiner and Chandler 24 ). Participants stood with feet a comfortable distance apart behind a line perpendicular and adjacent to a wall. The arm closest to the wall was raised to shoulder height and the position of the knuckle of the middle finger marked( Reference Duncan, Weiner and Chandler 24 ). Participants leaned forward as far as possible and distance of the knuckle from the first mark is recorded. The mean of three trials on each side was calculated for analysis.
The LRT measures ability to reach to the side in a bilateral stance( Reference Brauer, Burns and Galley 25 ). Participants stood with their backs near but not touching a wall with the heels 10 cm apart. Participants raised both arms to shoulder height while the tip of the third finger on the side being measured was marked. Participants then lowered the arm not being measured and reached sideways as far as possible with the arm being measured. The position of furthest reach was marked and the difference between the two marks calculated. The mean of three trials on each side was calculated for analysis.
Lower limb muscle strength
Lower limb muscle strength (LMS) was measured to the nearest kilogram using a dynamometer (TTM Muscular Meter)( Reference Dore, Quinn and Ding 28 ) to assess isometric strength, predominantly of the quadriceps and hip extensors. The examiner demonstrated the correct technique to the participant before testing. Participants stood on the back of the dynamometer platform, with back against a wall and knees flexed to an angle of 115°. They held a bar, connected to the dynamometer by a chain, and lifted the bar using maximum force using their legs, with the back and neck straight. Two readings were made, and the mean calculated for analysis. The intra-class correlation coefficient for LMS was 0·94 (95 % CI 0·92, 0·95) in this study (from two-way random-effects model( Reference Shrout and Fleiss 29 )).
Dietary food intakes
Dietary food intakes were measured using the AntiCancer Council of Victoria FFQ (CCV FFQ) and participants were asked to answer questions about their usual eating habits over the past 12 months. The details of the CCV FFQ have been described elsewhere( Reference Keogh, Lange and Syrette 30 , Reference Hodge, Patterson and Brown 31 ). In brief, this FFQ includes questions on 101 food and drink items. Participants were asked about the consumption of foods in four main categories (cereal foods, sweets and snacks; dairy products, meat and fish; fruit; vegetables) with frequency options ranging from never to three or more times per day, and detailed information on alcohol consumption. Also assessed were the number of pieces of fresh fruit and types of vegetables consumed daily, the type and daily amount of milk and bread eaten, type of spread put on bread, amount of sugar consumed daily, eggs eaten per week and type of cheese eaten, and serving sizes of potato, vegetables, steak and meat or vegetable casserole. The nutrients content of food were determined by Australian food composition tables( Reference English and Lewis 32 ).
Other measurements
Strenuous and light physical activity levels were measured by a validated questionnaire( Reference Aaron, Kriska and Dearwater 33 ), which was modified for Tasmanian conditions and has been used previously in women of this age, where physical activity was related to bone mass of the FN( Reference Jones and Scott 34 ). Participants were asked how many days in the last fortnight did they do at least 20 min of strenuous exercise, measured in five categories (1=0 d, 2=1–2 d, 3=3–5 d, 4=6–8 d, 5=≥9 d). Frequency of light physical activity was asked in the same way. Height was measured by a stadiometer (The Leicester height measure, Invicta Plastics Ltd) and weight by a single set of calibrated scales (Heine). BMI was calculated (weight/height (kg/m2)). We used a standardised questionnaire to collect smoking history (current/former/never), breast-feeding history (yes/no), number of children, family history of osteoporosis and/or fracture, and previous fractures, education level, employment status of main financial provider in the household, menopausal status, and marital status.
Statistical analysis
We had a power of 0·8 to detect a correlation of 0·15 at a Type 1 error of 5 %. We are not aware of any published data on the size of correlations to be expected between dietary pattern and bone density, muscle strength and balance, but this effect size is small so we are unlikely to be failing to detect any meaningful effect. For example, it is much smaller than the correlation between vitamin D and bone density (r 0·354 for lumber spine and 0·305 for FN in postmenopausal women)( Reference Mezquita-Raya, Munoz-Torres and Luna 35 ) and muscle strength (r 0·37 in older adults)( Reference Mowe, Haug and Bohmer 36 ), and comparable with the correlation with balance (r 0·17 for gait speed and 0·14 for the Romberg balance test in older women)( Reference Gerdhem, Ringsberg and Obrant 37 ). Participant characteristics were summarised using means and standard deviations, range (continuous variable) or frequency (%). We classified 101 food and drink items into thirty-three pre-defined food groups as had previously been done in an Australian cohort using the same FFQ, in which a western dietary pattern was associated with greater cognitive decline (online Supplementary Table S1)( Reference Gardener, Rainey-Smith and Barnes 38 ). The thirty-three groups were used to derive dietary patterns using exploratory factor analysis, which is commonly used to identify underlying structure (i.e. pattern) of the large number of food groups and is particularly useful when there is no a pre-defined idea about the dietary patterns as compared with other techniques. Factor loadings were extracted using the principal component method with varimax rotation to produce orthogonal factors. Factor loadings are interpreted as correlations between food items and derived factors. We determined the number of main dietary patterns based on eigenvalues>1·25, identification of break-point in the Scree plot and interpretability. Three major dietary patterns were extracted. We used weighted sum scores to calculate dietary pattern scores for each participant, as estimates of the underlying factor values. Items with factor loadings with absolute factor loadings ≥0·20 were considered as significantly contributing to a dietary pattern, and were included in the dietary pattern score calculations. Factor loading cut-points used in the literature are arbitrary, but generally range from 0·2 to 0·3. We used 0·2 as this could include more important food groups in defining the patterns, which would give us a broader picture of the patterns( Reference Slattery and Boucher 39 ). Foods that had factor loadings <0·20 were excluded from the pattern score calculation.
The associations between dietary pattern scores and musculoskeletal outcomes were estimated using multivariable linear regression with adjustment for confounders. We considered adjustment for both education intervention group and for risk feedback group (i.e. of high or normal risk of fracture). The former was considered in our process for selecting confounders, described below. However, the latter was determined by baseline BMD and so was highly correlated with BMD. Therefore, we could not adjust BMD in models for fracture risk feedback group. Also, at baseline, unsurprisingly, being in the high v. normal risk feedback group, that is in the lower half of the BMD distribution was strongly associated with weight, height, BMI and lower limb strength. As we already adjusted for BMI in our models, further adjusting for risk feedback group would be an overadjustment. Furthermore, risk feedback group was not associated with change in lower limb muscle strength at 2 years. Thus we did not adjust for fracture risk feedback group.
We selected other potential confounders (including education intervention) based on the biological plausibility of an association of a factor with both the outcome and the exposure of interest. Thus we considered age, menopausal status, weight, height, educational intervention, total energy intake, Ca and vitamin D supplement use, education level, employment status, marriage status, strenuous physical activity, hours of watching television, current smoking status, current use of oestrogens and oral contraceptive pill, history and total years of taking oral contraceptive, history of fractures and family history of osteoporosis and fractures as potential confounders. Weight, height and total energy intake were included in all models. Other factors were retained in the final model for each outcome when the estimated coefficient of each dietary pattern for that outcome changed by more than 10 %. Standardised dietary pattern scores were used in regressions, so the β-coefficients are interpreted as the change in the predicted value of the outcome for a standard deviation increase in the dietary pattern scores. Partial correlation coefficients between each dietary pattern score and energy-adjusted nutrient intake were calculated with adjustment for age. A two-tailed P<0·05 was considered significant, except the partial correlation between dietary patterns scores and nutrient intake, where a correlation coefficient with an absolute value of >0·2 was considered significant as described in previous literature( Reference Okubo, Sasaki and Horiguchi 20 ). To account for multiple testing, a critical value of 0·006 (0·05/8) for statistical significance was subsequently applied. All analyses were performed in Stata version 12 (Stata Corporation).
Results
Baseline characteristics of participants who did and did not complete the 12-year follow-up have been previously reported( Reference Wu, Laslett and Wills 40 ). In brief, compared with those who remained in the study, women lost to follow-up (26 %) were younger, had lower levels of educational attainment, and were more likely to be current smokers or to have ever smoked, and less likely to be married or in a de facto relationship. Other anthropometric and demographic factors were comparable. Table 1 shows characteristics of the study participants including intakes of nutrients.
Table 1 Characteristics of study participants (women, n 347) (Mean values and standard deviations; numbers and percentages)

TB BMC, total body bone mineral content; BMD, bone mineral density.
Three dietary patterns were identified (Table 2). We labelled dietary pattern 1 as ‘Healthy’ because it was characterised by a more plant-based diet, with high intakes of vegetables (dark-yellow, green leafy, cruciferous, legumes, garlic and others), fruit, tomatoes, nuts, snacks, whole grains. We labelled dietary pattern 2 as ‘high protein, high fat’ because of its high consumption of red meats, poultry, processed meats, potatoes, cruciferous and dark-yellow vegetables, fish, chips, spirits and high-fat dairy products. We labelled dietary pattern 3 as ‘Processed foods’ because it was characterised by high intakes of meat pies, hamburgers, beer, sweets, fruit juice, processed meats, snacks, spirits, pizza and low intakes of cruciferous vegetables. Mean of pattern scores were 194 (sd 122), 147 (sd 81) and 58 (sd 57) for the Healthy, high protein, high fat and processed foods patterns, respectively.
Table 2 Rotated factor loadings for the three dietary patterns identified from exploratory factor analysis (n 347)

Partial correlation coefficients between each dietary pattern score and energy-adjusted nutrient intake are shown in Table 3. Healthy pattern score was positively correlated with vitamin E (r 0·67), vitamin C (r 0·45), Mg (r 0·45), Fe (r 0·33) and carbohydrate (r 0·26) but negatively correlated with fat (r −0·32) and cholesterol (r −0·22). High protein, high fat pattern score was positively correlated with Zn (r 0·49) cholesterol (r 0·45), protein (r 0·41) and fat (r 0·29) but negatively associated with carbohydrate (r −0·47) and vitamin E (r −0·23). Processed foods pattern was negatively correlated with Mg (r −0·29), P (r −0·28), Zn (r −0·22) and protein (r −0·21).
Table 3 Partial Pearson’s correlation coefficients between each of three dietary patterns, serum 25-hydroxyvitamin D concentrations and energy-adjusted daily nutrient intakes
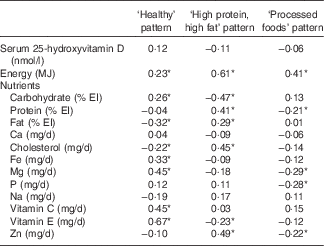
EI, energy intake.
* Statistically significant (P<0·05).
Associations between dietary patterns and musculoskeletal outcomes are shown in Table 4. In univariable analysis healthy pattern score was associated with LMS and FRT. These did not persist after adjustment for our identified confounders, though a borderline significant positively association with LMS remained (β=2·8; 95 % CI −0·04, 5·7; P=0·054). LMS was approximately 3·7 % higher for each standard deviation (122 unit) increase in score. Processed foods pattern score was significantly inversely associated with TB BMC (β=−32·2; 95 % CI −59·0, −5·4) and FRT (β=−0·78; 95 % CI −1·47, −0·08) but no other outcomes. These equate to approximately 1·4 and 1·9 % lower TB BMC and FRT, respectively, for each standard deviation (57 unit) increase in dietary pattern score. There were no associations of high protein, high fat score with any outcome in adjusted models. All associations were not statistically significant when the threshold of significance accounting for multiple testing was applied.
Table 4 Linear regression for associations between three dietary patterns (‘Healthy, ‘high protein, high fat’ and ‘Processed foods’) and multiple musculoskeletal outcomes (β-Coefficients and 95 % confidence intervals)

TB BMC, total body bone mineral content; BMD, bone mineral density.
* P<0·05.
† P=0·054.
‡ β-Coefficients are the change in the outcome for a SD-unit increase in each factor score (i.e. 122, 81 and 57 for dietary pattern 1, 2 and 3, respectively).
§ Adjusted for weight, height, strenuous physical activity, smoking, total energy intake, Ca and vitamin D supplement and menopausal status.
║ Adjusted for age, weight, height, strenuous physical activity, employment status, hours of watching television, total energy intake and Ca and vitamin D supplement.
¶ Adjusted for age, weight, height, strenuous physical activity, educational level, hours of watching television, total energy intake and Ca and vitamin D supplement.
Discussion
Few studies have examined the association of dietary patterns with bone mass in middle-aged women. To our knowledge, none have examined associations with other musculoskeletal outcomes of muscle strength and balance. The ‘Healthy’ dietary pattern, more plant-based and characterised by high consumption of vegetables, legumes, fruit, tomatoes, nuts, snacks, garlic, whole grains, was positively associated with 3·7 % higher LMS for each standard deviation increase in dietary pattern score. In contrast, the ‘Processed foods’ dietary pattern consisting of high intakes of meat pies, hamburgers, beer, sweets, fruit juice, processed meats, snacks, spirits, pizza and low intakes of cruciferous vegetables was significantly and inversely associated with 1·4 and 1·9 % lower TB BMC and FRT for each standard deviation increase in dietary pattern score. These findings suggest that maintaining a healthy diet may be important for TB BMC, muscle strength and balance early in adult life, but do not support the presence of a significant impact of these patterns on BMD in younger women. However, the exploratory nature of the analyses means that confirmation in longitudinal studies and/or trials with pre-specified hypotheses is needed.
The association of our Healthy dietary pattern characterised by high consumption of fruit and vegetables with LMS might be clinically important as age-related loss in muscle strength and balance begins to accelerate between 40s and 50s( Reference Robinson, Jameson and Batelaan 14 ), and estimated annualised rates of loss are 2·2 and 2·5 % for grip strength in women aged 50 and 60 years, respectively( Reference Daly, Rosengren and Alwis 41 ). However, this should be confirmed by further studies with larger sample size as the finding was no longer statistically significant after full adjustment of confounders and multiple testing. Previous to our study, an association with such a dietary pattern has only been reported in older adults. A cross-sectional analysis of the Hertfordshire Cohort Study found that a dietary pattern high in fruit and vegetable consumption was positively associated with grip strength in men and women aged 59–73 years( Reference Robinson, Jameson and Batelaan 14 ). The link between fruit and vegetable intake and muscle strength in older adults has been further confirmed by a recent randomised controlled trial, in eighty-three participants aged 65–85 years comparing habitual diet (≤2 portions of fruit and vegetables per d) with consumption of ≥5 portions of fruit and vegetable per d for 16 weeks( Reference Neville, Young and Gilchrist 42 ). Participants in the 5 portions/d group had improved grip strength compared with those in the 2 portions/d group that approached significance (mean=2·04 (sd 5·16) and 0·11 (sd 3·26) kg, respectively, P=0·06). The benefits of high consumption of fruit and vegetables are biologically plausible as fruit and vegetables contain antioxidants (e.g. vitamins C and E)( Reference Wu, Beecher and Holden 43 ), which may protect against catabolic effects of oxidative stress on skeletal muscle( Reference Doria, Buonocore and Focarelli 44 ). In our study, a ‘Healthy’ dietary pattern was strongly correlated with intake of both vitamin C and E. In older people, low plasma levels of vitamin E and carotenoids and low dietary consumption of vitamin C and β-carotene have been shown to be related to reduced muscle strength( Reference Cesari, Pahor and Bartali 45 ). These data in older adults, together with our findings in middle-aged women, suggest it is likely that the benefits for maintaining a ‘Healthy’ dietary pattern on muscle strength are likely to begin earlier rather than later in adult life.
The inverse relationship between a ‘processed foods’ dietary pattern and FRT was novel and the effect size was of a magnitude that could reach clinical significance, given that annualised rates of deterioration in balance as assessed by Romberg test are 0·3 and 1·6 % in women aged 50 and 60 years, respectively( Reference Daly, Rosengren and Alwis 41 ). However it is not clear why there were no associations of this dietary pattern with other balance measures. Given that this is an isolated finding from a battery of four balance tests, and the lack of data to support a direct link between the FRT in isolation and clinically important outcomes such as falls or fracture, the clinical relevance of this finding is uncertain. Nonetheless, it is biologically feasible that dietary patterns may influence balance, by effects on skeletal muscle( Reference Scott, Blizzard and Fell 46 , Reference Houston, Nicklas and Ding 47 ), visual function( Reference Yao, Qiu and Wu 48 – Reference Renzi, Bovier and Hammond 50 ) and reaction time( Reference Bovier, Renzi and Hammond 51 ). Further studies to confirm links between dietary patterns and balance in middle-aged women are therefore warranted.
In contrast to our study, a study in premenopausal Japanese women reported associations between a dietary pattern high in fruit and vegetable intakes and forearm BMD( Reference Okubo, Sasaki and Horiguchi 20 ). In the Japanese study, the dietary pattern consisted of fish and shellfish, fruit, processed fish, seaweed and soya products in addition to green and white vegetables, mushrooms and fruit. Thus, while this pattern shared fruit and vegetables as major constituents, potential differences include the phyto-oestrogen content from soya (e.g. soya isoflavones)( Reference Patisaul and Jefferson 52 , Reference Greendale, FitzGerald and Huang 53 ), and protein and other nutrients from seafood (such as fatty acids)( Reference Zalloua, Hsu and Terwedow 54 ) in the Japanese study, both of which may be beneficial for bone health. There were also moderate correlations between the dietary pattern and intakes of Ca (r 0·51) and vitamin D (from fish, shellfish, seaweed and soya) (r 0·53) in the Japanese study but not in ours (see Table 4). In fact, in our study high-fat dairy products loaded negatively on the healthy pattern and low-fat dairy products did not contribute to the pattern score resulting in very low correlation with Ca intake. Both Ca and vitamin D are nutrients important for bone health and overall this, suggests that a dietary pattern high solely in fruit and vegetables may not be optimal for bone health.
Conflicting conclusions have been drawn in the literature on the relationship between a ‘Western’ dietary pattern and bone health among younger( Reference McNaughton, Wattanapenpaiboon and Wark 17 , Reference Okubo, Sasaki and Horiguchi 20 ) and older adults( Reference McNaughton, Wattanapenpaiboon and Wark 17 – Reference Hardcastle, Aucott and Fraser 19 ). Our findings for the ‘processed foods’ and ‘high protein, high fat’ pattern (similar to the ‘Western’ pattern in terms of higher in animal sources of protein) are consistent with those in younger but not older participants of a study demonstrating that a pattern high in soft drinks, potato crisps, French fries, processed meats and desserts was not associated with FN BMD in premenopausal women or younger men but had inverse associations in postmenopausal women and older men (>50 years)( Reference Langsetmo, Poliquin and Hanley 18 ). Similarly, McNaughton et al. ( Reference McNaughton, Wattanapenpaiboon and Wark 17 ) identified a dietary pattern high in energy-dense, nutrient-poor foods (refined cereals, soft drinks, fried potatoes, sausages and processed meat, vegetable oils, beer and take-away foods) that was not associated with BMD at LS or total hip in women aged 18–65 years; however, there was an inverse association between this pattern and TB BMC, which is consistent with our findings for the processed pattern. Interestingly, a study of Japanese women aged 40–55 years showed that a ‘Western’ dietary pattern (high in meats, processed meats, fats and oils) was inversely but not statistically significantly associated with forearm BMD, but TB BMC was not examined in this study( Reference Okubo, Sasaki and Horiguchi 20 ). Of note, the negative association of processed foods with bone health might be explained by the finding that protein from other foods, such as low-fat milk, is more beneficial than that from processed foods( Reference Mangano, Sahni and Kiel 55 ). Potential reasons for this might include the different amino acid composition of proteins from different sources and the potential synergies of proteins with other nutrients, though in at least one study, patterns of dietary intake by protein food group were not associated with bone density or muscle measures whereas total protein intake was positively associated with muscle measures but not BMD( Reference Mangano, Sahni and Kiel 56 ). Some of the inconsistency in these findings may be due to the patterns not being directly comparable in terms of the food groups contributing to each pattern, as well as differences in populations and study design including age, ethnicity, other lifestyle factors and site at which BMD was measured. Importantly, longitudinal studies are needed to demonstrate the impacts of those dietary patterns on the progression of musculoskeletal health outcomes.
We did not observe associations between the ‘high protein, high fat’ or ‘processed foods’ dietary patterns and LMS. Studies specifically examining associations between a ‘processed foods’ dietary pattern and muscle strength have not been previously reported, but individual nutrients have been associated with muscle function. For example, intervention studies have shown benefits of increased vitamin D status on muscle strength in older adults, though little research has been conducted in younger adults( Reference McCarthy and Kiely 57 ). Low protein intake is associated with impaired physical function and the US Health, Aging and Body Composition Study found that both men and women (aged 70–79 years) in the highest quintile of protein intake lost approximately 40 % less lean mass and appendicular lean mass compared with those in the lowest quintile over 3 years( Reference Houston, Nicklas and Ding 47 ). However, a prospective study of 2·6 years found no association between nutrient intake and muscle strength of the knee extensors in older adults aged 50–79 years, though a number of nutrients were positive predictors of change in appendicular lean mass (Mg (β=0·07, P=0·02), P (β=0·07, P=0·047) and Zn (β=0·08, P=0·02))( Reference Scott, Blizzard and Fell 46 ). The authors speculated that diet alone may not offset age-related strength declines and factors such as physical activity are more important for maintenance of muscle function in older adults. Arguably, this could also be the case in our study of middle-aged women.
Our study has limitations. This was an exploratory analysis, and if thresholds for significance that accounted for multiple comparisons were applied, the results were not statistically significant. However, the effect sizes are potentially clinically important, and this arguably warrants more emphasis than P values in exploratory studies( Reference Bender and Lange 58 ). Nonetheless, this is a limitation and these results require confirmation in longitudinal studies and/or trials with pre-specified hypotheses. The CCV FFQ does not measure soft drink intake, which may have effects on musculoskeletal health outcomes( Reference Tucker, Morita and Qiao 59 ). In addition, FFQ may be subject to recall bias, though the questionnaire we used has been previously validated( Reference Keogh, Lange and Syrette 30 ) and has been used for dietary pattern analysis in older populations( Reference Gardener, Rainey-Smith and Barnes 38 ). An objective method such as weighed food records or 24 h recalls would provide more accurate information on food intake, although logistically more difficult for large-scale studies. The cross-sectional design cannot infer causal associations between dietary patterns, muscle strength and balance, and longitudinal studies are needed. Women who had a higher value of the ‘Healthy’ dietary pattern might also have a healthier lifestyle as they had a higher level of strenuous physical activity and were more likely to take vitamin D supplements. However, we did a comprehensive selection of confounders and adjusted for physical activity and vitamin D supplement use so the results are independent of these factors. Finally, although the original study( Reference Winzenberg, Oldenburg and Frendin 22 ) had a population-based design, participants were exposed to an osteoporosis behavioural intervention and a dropout rate of 26 % occurred by the end of final follow-up. Some differences in sociodemographic characteristics and smoking behaviour between women retained in the study and those lost to follow-up were observed but the wide spread of education levels at baseline and employment rate at 12 years approximates the overall population figures for these socioeconomic factors and adjustment for potential confounders was made. Therefore our findings are likely to apply to healthy middle-aged women from a range of sociodemographic backgrounds.
In conclusion, we identified specific dietary patterns associated with LMS, FRT and TB BMC but not other measures of balance or BMD in middle-aged women. Maintaining a healthy diet may be important for bone acquisition, muscle strength and possibly balance even in early adult life. Our findings should be confirmed in longitudinal studies of larger sample size with pre-specified hypotheses. Our findings may provide evidence for developing potential dietary strategies, such as improving intake of fruit, vegetables and whole grains, for improving muscle and balance health in middle-aged women.
Acknowledgements
The authors thank all the volunteers and participants involved in the present study.
This study was funded by the National Health and Medical Research Council (NHMRC) (APP1003437) and Royal Australian College of General Practitioners (RACGP)/Osteoporosis Australia Bone Health Research Grant. T. W. was supported by a NHMRC/Primary Health Care Research, Evaluation and Development (PHCRED) Career Development Fellowship (grant no. APP102859) and G. J. is supported by a NHMRC Practitioner Fellowship. L. L. is supported by an Arthritis Foundation Australia – Australian Rheumatology Association Heald Fellowship, funded by the Australian Rheumatology Association and Vincent Fairfax Family Foundation; and a NMHRC Early Career Fellowship (Australian Clinical Research Fellowship) (grant no. APP1070586). They did not have any role in the study concept, design, data analysis, writing of the manuscript or submission of the manuscript for publication. The researchers are totally independent of the funders.
T. W., F. W., G. J., and B. O. were involved in study design. T. W. and G. J. were responsible for data collection and management. F. W. performed data analysis, in consultation with K. W., T. W., L. L. and G. J. F. W. and T. W. drafted the manuscript together. All authors revised manuscript content and approved the final manuscript and had access to the data. T. W. is the guarantor of the study and accepts full responsibility for the finished article, had access to any data and controlled the decision to publish.
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517002483







