Betaine (N, N, N-trimethylglycine) that serves as an effective methyl donor and an osmolyte is commonly used in animals and human beings for the purpose of sparing dietary methionine, and helping to treat homocysteinaemia separately( Reference Cholewa, Guimarães-Ferreira and Zanchi 1 , Reference Craig 2 ). In vivo, betaine plays a key role in sulphur-amino acid metabolism, and is metabolised to dimethylglycine and sarcosine( Reference Kim and Kim 3 ). Numerous studies indicate that betaine has important nutritional and physiological functions( Reference Ratriyanto, Mosenthin and Bauer 4 – Reference van Wettere, Herde and Hughes 8 ), such as growth promotion, anti-stress effect, reproductive performance improvement, antioxidant preventive effect as well as osmotic protection. Furthermore, studies on swine and poultry have suggested that betaine supplementation can decrease overall fat deposition, and improve carcass characteristics by stimulating lipolysis( Reference Sales 9 – Reference Wang, Xu and Feng 11 ). Clinical studies have shown that betaine might serve as a safe and promising therapeutic agent for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in human beings( Reference Abdelmalek, Angulo and Jorgensen 12 , Reference Abdelmalek, Sanderson and Angulo 13 ). Lipid accumulation results from an imbalance among the synthesis, oxidation and transportation of fatty acids( Reference Lelliott and Vidal-Puig 14 – Reference Yang, Li and Kong 17 ), and plays a key role in disease initiation, which can be seen in many chronic liver diseases. Therefore, the beneficial effects of betaine in preventing hepatic fat accumulation have received much attention in recent years. In rats and human subjects, high-fat diet, ethanol as well as high sucrose induced hepatic lipid accumulation and liver injury, and these changes could be reversed by the administration of betaine( Reference Zhang, Wang and Wang 18 – Reference Song, Deaciuc and Zhou 20 ). Previous studies indicated that betaine triggers hepatoprotective effect mostly through alleviating impairment of sulphur-amino acid metabolism and oxidative stress( Reference Kwon, Jung and Kim 21 – Reference Alirezaei, Jelodar and Ghayemi 23 ).
However, the effects of betaine on hepatic lipid accumulation and molecular mechanisms behind these alterations were not fully clarified. Therefore, the present study focused on the effects of betaine on hepatic lipid accumulation, and investigated the regulation interrelationship between betaine and related key factors involved in protective effects against abnormal lipid accumulation in Sprague–Dawley rats.
Materials and methods
Animal experimental procedure
The present study was approved by the Institutional Animal Care and Use Committee of Zhejiang University (Hangzhou, China). The experiment was conducted in the Laboratory Animal Center of Zhejiang University, and all necessary precautions were taken to mitigate pain. Sprague–Dawley rats used for the investigation were purchased from Medical Animal Laboratory Center of Zhejiang Chinese Medical University (Hangzhou, China). Thirty-two 3-week-old male Sprague–Dawley rats (initial weight: 100 (sd 2·50) g) were randomly divided into four different groups: basal diet (T1), basal diet with betaine administration (T2), high-fat diet (T3) and high-fat diet with betaine administration (T4). T1 and T2 were given basal diet (Table 1), while T3 and T4 were given high-fat diet (Table 1). Meanwhile, T2 and T4 were administrated intragastrically with 1 ml of betaine (98 % of purity; Sigma-Aldrich) at a concentration of 400 mg/kg at 17.00 hours per d, while T1 and T3 groups were given 1 ml of 0·9 % saline by oral administration. The energy compositions of basal diet were 13·8 % derived from fat, 25·7 % from protein and 60·5 % from carbohydrates (Table 1). In high-fat diet, 40·0 % of its energy derived from fat, 20·0 % from protein and 40·0 % from carbohydrates (Table 1). The diets were formulated according to AIN-93G (American Institute of Nutrition recommendation for laboratory rodents)( Reference Reeves, Nielsen and Fahey 24 ). The basal and high-fat diets were prepared specifically for our study by Slac Experimental Animal LLC (Shanghai, China). A schedule of 12 h light and 12 h darkness was used for the Sprague–Dawley rats. All rats were individually housed in a stainless steel cage and had free access to chow diet and water throughout the entire feeding period. Body weights of rats were recorded weekly. The experiments lasted for 28 d.
Table 1 Nutrition formulation of diet*
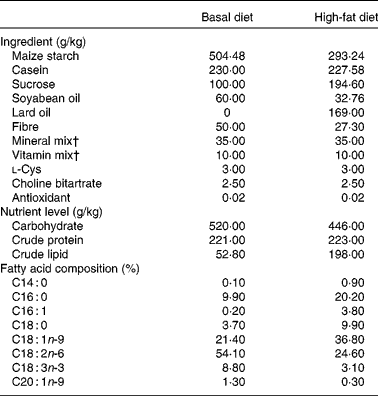
* The data of nutrient level and fatty acid composition were analysed values.
† Vitamin mix and mineral mix are in accordance with the American Institute of Nutrition-93 guidelines.
Sampling
The rats were anesthetised with halothane after the 28 d intragastric administration trial. Orbital blood was collected into coagulation promoting tubes and centrifuged at 3000 g for 10 min at 4°C. Then the serum was extracted and stored at − 80°C. The rats were then killed by cervical dislocation, and liver samples were collected. About 2 cm-wide of fresh liver chop was collected in a CryoTube (Corning) and frozen in liquid N2 for Oil-Red-O histological assessment, another specimen of liver fixed in 10 % formaldehyde for haematoxylin–eosin stain. The remainders were snap-frozen in liquid N2, and stored at − 80°C for subsequent analysis of lipid metabolites, mRNA and protein expression.
Analysis of lipid metabolites in serum
Serum TAG, total cholesterol, NEFA and glucose were measured by using the commercial analysis kits (JianCheng Institute of Biotechnology). The serum levels of lecithin and VLDL were determined by using ELISA kits (A&D Company Limited) according to manufacturer's instructions.
Hepatic histology and hepatic lipid metabolites analysis
After being fixed in 10 % formaldehyde for 24 h and dehydrated, specimens of liver were embedded in paraffin, sliced into sections of 4 μm thickness and stained with haematoxylin–eosin. For Oil-Red-O assessment, 10 μm frozen sections of liver tissue were immersed in isopropanol for 1 min and stained with Oil-Red-O for 10 min. After being washed three times with distilled water, the sections were counterstained in haematoxylin, and washed again in distilled water before microscopic analysis. The positive result of Oil-Red-O staining was bright red. Software of Image pro-plus 6.0 (Media Cybernetics) was used to estimate the positive ratio that described the percentage of the red area in each image. The levels of TAG, total cholesterol, NEFA, lecithin, VLDL and fibroblast growth factor 21 (FGF21) in liver were also measured by using the commercial analysis kits as mentioned above. Hepatic carnitine, carnitine palmitoyltransferase 1 (CPT1) and betaine–homocysteine methyltransferase (BHMT) levels were determined by using ELISA kits (A&D Company Limited) according to manufacturer's instructions.
Analysis of gene expression
Total RNA of liver and cells were extracted by using Trizol (Invitrogen) according to the protocol provided by the manufacturer. Isolated RNA was quantified by using the NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific, USA), and its integrity was confirmed by agarose gel electrophoresis. The complementary DNA (cDNA) synthesis was performed in a 20 μl reaction volume containing 2 μg total RNA, 1 μl random hexamer, 4 μl of five reaction buffer, 2 μl of 10 mmol/l deoxyribonucleotide triphosphate (dNTP) and 10 μl of 200 units/μl Moloney murine leukaemia virus (M-MLV). And the abundance of target gene was measured by quantitative real-time PCR analysis, using the ABI StepOne PlusTM RT-PCR system with the SYBR® Premix Ex TaqTM (Tli RNaseH Plus) RT-PCR kit (TaKaRa), according to the manufacturer's instructions. The primer pairs of specific genes were commercially synthesised by TaKaRa, shown in Table 2. Each sample was analysed in duplicate and the expression of the target genes were standardised by the endogenous housekeeping gene (β-actin). The reaction protocol comprised one cycle of 95°C for 2 min, thirty-five cycles of 94°C for 50 s, 60°C for 30 s and 72°C for 50 s. The gene expression was calculated by using the comparative
![]() $$(2^{ - \Delta \Delta C _{T}}) $$
method(
Reference Livak and Schmittgen
25
,
Reference Duan, Li and Li
26
).
$$(2^{ - \Delta \Delta C _{T}}) $$
method(
Reference Livak and Schmittgen
25
,
Reference Duan, Li and Li
26
).
Table 2 Primer-pairs of target genes used for real-time PCR

BHMT, betaine–homocysteine methyltransferase; FGF21, fibroblast growth factor 21; AMPK, AMP-activated protein kinase; CPT1, carnitine palmitoyltransferase 1.
Western blot analysis
Protein of liver samples was extracted and quantified by using a Bradford protein assay kit (Bio-Rad). Briefly, liver samples were homogenised at 4°C (1:10, w/v) in 50 mm-Tris–HCl buffer (pH = 8·0), containing 1 mmol/l phenylmethylsulfonyl fluoride, 1 mg/ml protease inhibitor cocktail, 0·1 % β-mercaptoethanol, and centrifuged at 12 000 g at 4°C for 5 min. After being heated to 95°C for 5 min, proteins were separated on SDS–PAGE gels, and then transferred onto immobilon-P polyvinylidene difluoride membranes (Millipore). After blocking with 5 % non-fat milk/Tween overnight, the membranes were immunoblotted with the antibodies specifiedanti-FGF-21 antibody (Epitomics), and a horseradish peroxidase-conjugated secondary antibody (KPL) was used in the detection of specific proteins. For examining the equal loading, β-actin antibody (Santa Cruz Biotechnology) was used as control. Finally, enhanced chemiluminescence reagent (Amersham) was used to visualise the protein bands. Band intensities were determined by using AlphaEase FC analysis software (version 4; Alpha Innotech).
Statistical analysis
Data analyses were performed with the SPSS 20.0 statistical software package (IBM). Statistical analysis was performed using one-way ANOVA, and was analysed further by Duncan's multiple range test for differences among groups. Results were presented as means and standard deviations. In all analyses, the level of significant difference was set at P< 0·05.
Results
Assessment of body weight
Changes of body weight were shown in Table 3. The body weights of rats in high-fat group were numerically lower than those in other groups since the 14th day, but no significant difference (P>0·05) was found in body weight among experimental groups at the end of the feeding period.
Table 3 Changes of body weight during 4 weeks (g) (Mean values and standard deviations, n 7)
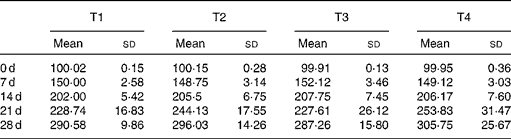
T1, basal diet; T2, basal diet with betaine administration; T3, high-fat diet; T4, high-fat diet with betaine administration.
Effects of betaine on serum lipid metabolites
As shown in Table 4, compared with rats fed with the basal diet (T1), 4 weeks of high-fat diet (T3) feeding significantly elevated (P< 0·05) the concentration of serum TAG, glucose, lecithin and VLDL. Compared with the rats fed with the high-fat diet (T3), the levels of serum TAG, glucose and lecithin were increased (P< 0·05) further in the high-fat diet group with betaine administration (T4). Furthermore, betaine administration in basal diet-fed rats (T2) significantly increased (P< 0·05) the levels of glucose and lecithin compared with the rats fed with the basal diet (T1). Moreover, betaine administration groups (T2 and T4) had numerically higher levels of VLDL. No significant differences were found in total cholesterol and NEFA levels among experimental groups.
Table 4 Effects of betaine on serum lipid metabolism (Mean values and standard deviations, n 7)
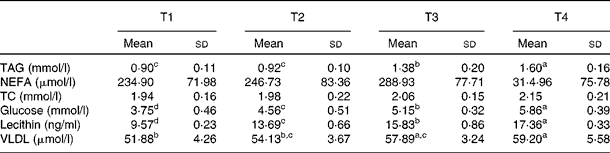
T1, basal diet; T2, basal diet with betaine administration; T3, high-fat diet; T4, high-fat diet with betaine administration; TC, total cholesterol.
a,b,c,dMean values with unlike superscript letters were significantly different (P< 0·05).
Oral administration of betaine effectively alleviated the excessive accumulation of fat in the liver
The high-fat diet feeding for 4 weeks caused hepatic lipid accumulation in rats (Figs. 1 and 2), which was illustrated by severe vacuolar degeneration in haematoxylin–eosin-stained sections, and intensive bright red staining in Oil-Red-O-stained sections respectively (Figs. 1(C) and 2(C)). Oral administration of betaine in rats fed with the high-fat diet (T4) reduced (P< 0·05) hepatic accumulation of lipids (Figs. 1(D) and 2(D)). The differences in vesicular fat accumulations between histological sections staining with Oil-Red-O were confirmed by image analysis. Consistent with the results of hepatic histology, high-fat diet administration (T3) increased (P< 0·05) hepatic levels of TAG and NEFA (Table 5). Oral administration of betaine did not affect TAG concentration in rats fed with the basal diet, but a significant reduction (P< 0·05) in hepatic TAG content was observed in betaine-administered rats fed with the high-fat diet (T4) (Table 5).

Fig. 1 Haematoxylin–eosin-stained liver sections of Sprague–Dawley rats from: (A) basal diet; (B) basal diet with betaine administration; (C) high-fat diet; (D) high-fat diet with betaine administration. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn

Fig. 2 Oil-Red-O-stained liver sections from: (A) basal diet; (B) basal diet with betaine administration; (C) high-fat diet; (D) high-fat diet with betaine administration. Values are means (n 5), with standard deviations. a,b,cMean values with unlike superscript letters were significantly different (P< 0·05). Groups: T1, basal diet; T2, basal diet with betaine administration; T3, high-fat diet; T4, high-fat diet with betaine administration. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Table 5 Effects of betaine on hepatic lipid metabolism (Mean values and standard deviations, n 7)
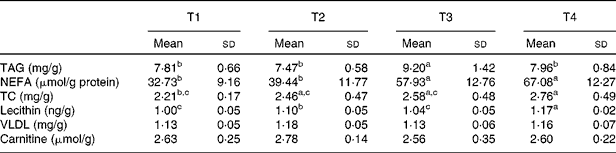
T1, basal diet; T2, basal diet with betaine administration; T3, high-fat diet; T4, high-fat diet with betaine administration; TC, total cholesterol.
a,b,cMean values with unlike superscript letters were significantly different (P< 0·05).
Betaine elevated the activity and the mRNA expression of betaine–homocysteine methyltransferase in the liver
Compared with groups without betaine administration (T1 and T3), betaine supplementation groups (T2 and T4) exhibited a significant increase (P< 0·05) in the activity of BHMT in the liver (Fig. 3(A)). High-fat diet also elevated (P< 0·05) the activity of BHMT when compared with rats fed with basal diet. Furthermore, the gene expression of BHMT in high-fat diet-fed rats was elevated (P< 0·05) by betaine administration whereas the changes of BHMT gene expression in basal diet rats were not observed (Fig. 3(B)).

Fig. 3 (A) Effect of betaine on the activity of betaine–homocysteine methyltransferase (BHMT) in the liver. (B) Effect of betaine on the mRNA abundance of BHMT in the liver. Values are means (n 7), with standard deviations represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05). Groups: T1, basal diet; T2, basal diet with betaine administration; T3, high-fat diet; T4, high-fat diet with betaine administration.
Betaine increased PPARα gene expression and reversed the inhibition of carnitine palmitoyltransferase 1 gene expression induced by high-fat diet in the liver
Effects of betaine administration on the activity of CPT1 and the gene expressions of PPARα and CPT1 were shown in Fig. 4. Betaine administration in rats fed with a high-fat diet exhibited a higher activity of CPT1 (P< 0·05) compared with that of high-fat diet-fed rats (Fig. 4(A)). Compared with rats fed with basal diet, high-fat diet supplementation significantly suppressed the gene expression of PPARα and CPT1 in the liver (P< 0·05). In high-fat group with betaine administration, the inhibition induced by high-fat diet was reversed, oral administration of betaine significantly increasing (P< 0·05) the gene expression of PPARα and CPT1 in the liver. Moreover, betaine administration rats fed with basal diet also showed elevated (P< 0·05) PPARα mRNA abundance, but it had no influence on the mRNA abundance of CPT1 (Fig. 4(B) and (C)).

Fig. 4 (A) Effect of betaine on the activity of carnitine palmitoyltransferase 1 (CPT1) in the liver. (B) Effect of betaine on the gene expression of PPARα in the liver. (C) Effect of betaine on the gene expression of CPT1 in the liver. Values are means (n 7), with standard deviations represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P< 0·05). Groups: T1, basal diet; T2, basal diet with betaine administration; T3, high-fat diet; T4, high-fat diet with betaine administration.
Betaine increased the activity, gene and protein expression of fibroblast growth factor 21, and elevated the gene expression of AMP-activated protein kinase in the liver
The changes of betaine administration on the activity, gene and protein expression of FGF21 in the liver are shown in Fig. 5(A)–(C). Compared with rats fed the basal diet (T1), the high-fat diet (T3) elevated (P< 0·05) the gene expression of hepatic FGF21. Betaine administration significantly increased (P< 0·05) the concentration and gene expression of hepatic FGF21 in both the basal diet (T1) and high-fat diet feeding (T3) groups. Additionally, compared with rats fed with a high-fat diet (T3), oral administration of betaine in the high-fat group (T4) significantly increased (P< 0·05) the protein expression of FGF21. Regarding AMP-activated protein kinase (AMPK), compared with rats fed with the basal diet (T1), the high-fat (T3) diet increased (P< 0·05) the mRNA expression of AMPK (Fig. 5(D)). Oral administration of betaine in the high-fat diet group (T4) significantly increased (P< 0·05) the gene expression of AMPK compared with high-fat diet-fed rats (T3). The gene expression of AMPK in the basal diet-treated group (T1) was not affected by betaine administration.

Fig. 5 (A) Effects of betaine on the content of fibroblast growth factor 21 (FGF21) in the liver. (B) Effects of betaine on the FGF21 mRNA abundance in the liver. (C) Effects of betaine on relative protein expression of FGF21 in the liver. (D) Effects of betaine on the mRNA abundance of AMP-activated protein kinase (AMPK). Band intensities were determined by using AlphaEase FC analysis software (Alpha Innotech). Values are means (n 7), with standard deviations represented by vertical bars. a,b,c,dMean values with unlike letters were significantly different (P< 0·05). Groups: T1, basal diet; T2, basal diet with betaine administration; T3, high-fat diet; T4, high-fat diet with betaine administration.
Discussion
The present study indicated that betaine did not affect body weight in rats fed with either basal diet or high-fat diet. Variable effects of betaine on body weight were previously reported( Reference Kwon, Jung and Kim 21 , Reference Wang, Yao and Pini 27 , Reference Wray-Cahen, Fernandez-Fıgares and Virtanen 28 ). These differences might be attributed to the diversity of animals, dietary nutrients, and trial periods, etc. TAG, cholesterol, lecithin and NEFA were the major components of plasma lipids. In this investigation, treatments of high-fat diet significantly increased the serum concentrations of TAG, lecithin and glucose, which suggested that high-fat diet might result in hyperlipidaemia. It is well-established that the intake of SFA is associated with hyperlipidaemia, diabetes and CVD( Reference Ros and Mataix 29 ). In the present study, compared with basal diet, the high-fat diet supplemented with lard oil increased the energy content as well as the SFA content in high-fat diet. Therefore, the increase in serum lipid maybe associated with the difference of fatty acid composition in diets. The hyperlipidaemia caused by high-fat diet was not relieved by betaine administration in our experiment, which increased the levels of serum TAG, lecithin and glucose further compared with those of high-fat diet-fed rats. This is in line with a previous research( Reference Martins, Neves and Freitas 30 ), which suggested that betaine-supplemented pigs exhibited significantly higher plasma concentrations of TAG, phospholipids and lipoprotein cholesterol. Therefore, the effect of betaine in these parameters suggested that betaine might enhance lipids transportation accompanied with dyslipidaemia risk( Reference Olthof and Verhoef 31 ). The glucose increase induced by betaine was observed in basal diet-fed rats and high-fat diet-fed rats. Accordingly, it is suspected that the increase in serum glucose was due to betaine, which improved lipid and energy metabolism accompanied with decreasing uptake of serum glucose by peripheral tissues; this needs to be confirmed by further research. Our data also showed that treatment of high-fat diet significantly increased hepatic vesicular fat accumulation and Oil-Red-O-stained area, respectively. Moreover, the concentrations of TAG and NEFA in the liver were significantly increased in the group that was given the high-fat diet, indicating that high-fat diet treatment for 4 weeks led to hepatic lipid accumulation in rats. In the present study, oral administration of betaine reduced the Oil-Red-O-stained area, and normalised the concentration of hepatic TAG in rats fed with the high-fat diet, suggesting that hepatic lipid accumulation induced by high-fat diet was reversed by betaine administration.
The present study also investigated the effect of betaine on the factors playing a vital role in protecting the liver against lipid accumulation, which were altered by betaine administration. BHMT plays a critical role in the hepatic one-carbon metabolism, and converts homocysteine to methionine using betaine as a methyl donor( Reference Teng, Mehedint and Garrow 32 ). Significant increase in BHMT activity was observed in betaine administration rats given either basal diet or high-fat diet, which was accompanied with the notable increase of BHMT mRNA abundance in the high-fat group. Similarly, Kharbanda et al. ( Reference Kharbanda, Mailliard and Baldwin 33 ) reported a significant increase in enzyme activity and mRNA abundance of BHMT in rats fed diets supplemented with 1 % betaine for 4 weeks. Feng et al. ( Reference Feng, Liu and Wang 34 ) also reported that BHMT activity was significantly increased when pigs were offered 0·125 % betaine. It has been well-documented that betaine supplementation enhances recycling of homocysteine for the generation of methionine and S-adenosylmethionine, and the increase of hepatic S-adenosylmethionine results in attenuation of fatty liver, by activating a cascade of events including phosphatidylcholine synthesis, formation of VLDL and transportation of hepatic lipid( Reference Kharbanda, Mailliard and Baldwin 33 , Reference Zhu, Song and Mar 35 ). Our results showed that betaine administration elevated the levels of hepatic lecithin and serum lecithin and VLDL in high-fat diet-fed rats, which suggested that betaine reversed hepatic lipid accumulation by elevated lecithin and VLDL levels via increasing the expression and activity of BHMT, thus enhancing hepatic lipid export. Besides, high-fat diet also increased the activity of BHMT in the liver. This is in agreement with Deminice et al. ( Reference Deminice, da Silva and Lamarre 36 ), who reported that high-fat diet treatment for 3 weeks elevated the mRNA level of BHMT. Therefore, high-fat diet might induce the enhancement of hepatic lipid export.
Owing to the specific property, CPT1 is a key rate-limiting enzyme of β-oxidation( Reference Korman, Waterham and Gutman 37 ), and is reported to be a target of PPARα( Reference Ferré 38 ). PPARα, a nuclear receptor, is activated by fatty acids, and it regulates the transcription of numerous genes encoding enzymes in fatty acid oxidation and transportation( Reference Yang, Li and Xiong 39 , Reference Wang, Chen and Tan 40 ). Enzyme analysis showed that betaine administration had no apparent effect on the activity of CPT1 in rats fed with basal diet. This is in agreement with Huang et al. ( Reference Huang, Han and Xu 41 ), who reported that an effect of betaine on the activity of CPT1 in liver was not observed. In the present study, down-regulations of PPARα and its target (CPT1) were observed in the treatments of high-fat diet, which is consistent with the study of Deminice et al. ( Reference Deminice, da Silva and Lamarre 36 ), who reported that high-fat diet reduced mRNA levels for PPARα as well as its downstream targets CPT1. Oral administration of betaine in the high-fat diet rats obviously elevated the gene expression of PPARα and normalised the gene expression of CPT1 in the present study. Wang et al. ( Reference Wang, Chen and Tan 40 ) reported a similar result, which demonstrated that betaine supplementation (2 % betaine/100 g diet) increased both PPARα and CPT1 expressions of apoE− / − mice. The gene expression of CPT1 in liver was not altered by betaine supplement, as reported by Huang et al. ( Reference Huang, Han and Xu 41 ), but, interestingly, betaine reversed the inhibition of CPT1 induced by high-fat diet in the present study. Therefore, betaine may induce the alteration of CPT1 gene expression in specific conditions, as in apoE− / − mice and high-fat feeding rats as mentioned above, which needs the support of further research. Moreover, it is well-established that AMPK indirectly increased CPT1 activity by inhibiting acetyl-CoA carboxylase activity( Reference Assifi, Suchankova and Constant 42 , Reference Xue and Kahn 43 ). Our data indicated that betaine significantly elevated hepatic AMPK gene expression (Fig. 5(D)), and there was a consistent response to betaine between gene expressions of AMPK and CPT1. In the present study, betaine reversed the inhibition of CPT1 gene expression induced by high-fat diet treatment, which may be due to the activation of AMPK. It can be presumed that betaine normalised fatty acid oxidation in rats fed with a high-fat diet via activating AMPK and up-regulating PPARα and CPT1 gene expression, thus preventing lipid accumulation in the liver.
FGF21, a novel metabolic regulator, is produced predominantly in the liver, and involved in the regulation of lipid metabolism, including lipolysis, fatty acid oxidation and ketogenesis( Reference Badman, Pissios and Kennedy 44 – Reference Coskun, Bina and Schneider 48 ). In the present study, FGF21 concentration as well as its gene and protein expressions were significantly increased in betaine administration groups. FGF21 can be tightly regulated by nutrition in animal models, and previous research indicated that 2 weeks of 5 % betaine feeding increased FGF21 expression 7-fold in the liver of mice( Reference Teng, Ellis and Coleman 49 ), which suggested that betaine elevated indeed the expression of FGF21. However, the relationship between BHMT and FGF21 is not clear, since the results of the present study showed that betaine administration resulted in elevation of BHMT and FGF21, whereas a previous study indicated that BHMT− / − mice increased FGF21 expression( Reference Uebanso, Taketani and Fukaya 46 ). Therefore, it can be speculated that BHMT− / − mice may increase FGF21 expression via the elevation of betaine concentration instead of the direct regulation by BHMT. Chau et al. ( Reference Chau, Gao and Yang 50 ) demonstrated that FGF21 regulates energy homeostasis through an AMPK–SIRT1 (sirtuin 1)–PGC1α (peroxisome proliferator-activated receptor-γ co-activator 1α)-dependent mechanism in adipocytes. In the present study, expressions of FGF21 and AMPK were observed to be positively interrelated in response to betaine treatment. AMPK is a heterotrimeric protein that serves as a sensor of cellular energy levels, and it plays a critical role in the regulation of lipid metabolism by stimulating fatty acids oxidation, and inhibiting their synthesis( Reference Lee, Kim and Park 51 , Reference Li, Xu and Mihaylova 52 ). Therefore, it can be speculated that betaine may activate AMPK system through up-regulating FGF21 expression, thus enhancing lipolysis in the liver. Additionally, FGF21 was reported to be regulated by PPARα( Reference Badman, Pissios and Kennedy 44 ), expressions of FGF21 and PPARα were observed to be positively interrelated in response to betaine treatment as well. Therefore, betaine might increase the expression of FGF21 via up-regulating PPARα in the high-fat group.
In summary, the present study indicated that betaine could alleviate hepatic lipid accumulation induced by a high-fat diet via enhancing hepatic lipid export and fatty acid oxidation. In high-fat diet-fed rats, betaine could elevate the lecithin and VLDL levels via enhancing the expression of BHMT, thus improving hepatic lipid export; meanwhile, betaine may increase the expression of CPT1 via activating PPARα, FGF21 and AMPK, thus enhancing fatty acids oxidation. Betaine could induce changes in the key factors involved in alleviating hepatic lipid accumulation, and the mechanism by which betaine regulates these key factors needs to be confirmed by further research studies.
Acknowledgements
This project was funded and supported by the National Basic Research Program of China (2012CB124705). The funding agency had no role in the design, analysis or writing of this article. The present research received no specific grant from any funding agency of commercial or not-for-profit sectors.
The authors' contributions are as follows: J. F. and Y. W. were in charge of the whole trial; L. X., D. H., Q. H., J. W. and J. F. conducted the research; L. X., D. H., and J. W. analysed the data and wrote the manuscript.
The authors have no conflicts of interest to declare.













