Potato tubers (Solanum tuberosum) are a staple food in many countries worldwide, because they contain an excellent variety of nutrients such as carbohydrates, protein, lipids, dietary fibre, minerals (Zn, Fe, Mg, Ca, K and Na) and vitamins (vitamin C (VC), vitamin B1, vitamin B2, vitamin B6, niacin, pantothenic acid and folic acid)(Reference Camire, Kubow and Donnelly1, 2). Especially notable is the potato's abundant VC content (35 mg/100 g wet weight)(2). VC is a potent soluble antioxidant and cofactor for several hydroxylases involved in catecholamine biosynthesis and collagen polymerisation(Reference Linster and Van Schaftingen3). Since the human body cannot synthesise VC, our VC intake most often comes from foods. Historically, the ingestion of potatoes has contributed to the prevention of VC-deficiency disease, that is, scurvy. Recently, VC has been associated with numerous health benefits including decreased risk of CVD(Reference Joshipura, Hu and Manson4), stroke(Reference Kurl, Tuomainen and Laukkanen5–Reference Joshipura, Ascherio and Manson8), age-related cataracts(Reference Jacques, Taylor and Hankinson9, Reference Yoshida, Takashima and Inoue10) and type 2 diabetes mellitus and(Reference Harding, Wareham and Bingham11, Reference Afkhami-Ardekani and Shojaoddiny-Ardekani12) has also decreased the frequency of common colds(Reference Sasazuki, Sasaki and Tsubono13).
Potatoes are usually prepared for eating by baking, boiling, steaming, dehydrating or frying. However, each of these cooking methods causes a lesser or greater loss of VC(Reference Camire, Kubow and Donnelly1, Reference Han, Kozukue and Young14, Reference Burg and Fraile15). However, we previously found that the VC content per wet weight of commercial potato chips was about twice that of unprocessed raw potatoes(Reference Miyoshi, Ishihara and Nakamura16), because rapid frying and complete dehydration during the frying process minimised VC loss. Similarly, potatoes processed by boiling and mashing retain VC. Thus, mashed potatoes and potato chips seem to be a superior dietary source of VC to promote human health.
The effect of all dietary compounds depends on the retention of nutrients in blood and tissues. Therefore, actual bioavailability represents a fundamental issue. Orally administered VC is first absorbed in the small intestine, delivered to the liver, and then circulates in the blood followed by uptake into various tissues(Reference Wilson17–Reference Reidling, Subramanian and Dahhan19). In the kidney, proximal tubules absorb VC in primitive urine and release it into the plasma. When VC concentration in primitive urine exceeds the reabsorption capacity in proximal tubules, excess VC is excreted in urine. The bioavailability of VC ingested in water is well understood in human subjects(Reference Levine, Conry-Cantilena and Wang20–Reference Johnston and Luo24). Surprisingly, though, no studies have documented the bioavailability of VC from potatoes prepared by mashing or frying as chips. Therefore, we measured VC concentrations in the plasma and urine of human subjects for 8 h after orally administering mashed potatoes and potato chips.
Subjects and methods
Subjects
A total of five healthy male volunteers aged 22–27 years (24 (sem 1) years) were enrolled in the present study. Their mean BMI was 20·3 (sem 2·8) kg/m2 and did not change significantly during the present study. These subjects took no dietary supplements or medications, did not smoke and were not habitual drinkers of alcoholic beverages. None of the subjects had a chronic illness or food allergy. All study participants were in good health on the basis of a medical history, a physical examination and normal results on clinical laboratory tests, including measurement of total protein (75 (sem 6) g/l), albumin (57·5 (sem 4·8) g/l), glucose (4·8 (sem 0·2) mmol/l), aspartate aminotransferase (15 (sem 2) U/l), alanine aminotransferase (15 (sem 6) U/l), TAG (0·26 (sem 0·12) mmol/l), total cholesterol (4·18 (sem 0·61) mmol/l), urea N (1·94 (sem 0·31) mmol/l) and creatinine (72 (sem 5) μmol/l). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Clinical Research Ethics Committee of the Tokyo Metropolitan Institute of Gerontology, Tokyo, Japan. Written informed consent was obtained from all subjects.
Study design
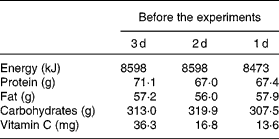
A cross-over study at 4-week intervals was conducted as illustrated in Fig. 1. Subjects enrolled in the study received a ‘standard’ daily meal during the 3 d before the day of VC testing. The standard menu was as follows: (1) breakfast, consisting of bread, cheese and milk; (2) lunch of a commercial frozen meal (Nichirei Foods Direct, Inc., Tokyo, Japan) and rice (Sato Foods Industries Company Limited, Niigata, Japan); (3) dinner of a commercial frozen meal, rice and miso soup with pork and vegetables (Hanamaruki Foods, Inc., Tokyo, Japan). The standard meal was controlled for the content of VC and other nutrients (Table 1). The amounts of energy, protein, fat, carbohydrates and VC delivered were calculated by using the values provided by the manufacturers and listed in the Standard Tables of Food Composition in Japan(2). VC concentration in these standard meals, in part, was measured by using HPLC and an electrochemical detector (ECD). For the VC bioavailability study, subjects were fasted >12 h after their last meal. Their urine was thoroughly excreted at 08.00 hours, after which the subjects immediately drank 50 ml mineral water (Coca-Cola (Japan) Company, Limited, Tokyo, Japan). Blood and urine samples were then collected at 09.00 hours, followed by the subjects' ingestion of a test sample consisting of 282 g mashed potatoes including 50 mg VC and 100 ml mineral water (food group 1), 87 g potato chips including 50 mg VC and 100 ml mineral water (food group 2), 50 mg VC (DSM Nutrition Japan K.K., Tokyo, Japan) freshly dissolved in 100 ml mineral water (food group 3) and 100 ml mineral water (food group 4) at 4-week intervals. Their blood was then sampled every 30 min for up to 4 h and every 1 h from 4 to 8 h. Urine was sampled every 1 h up to 8 h. To provide enough urine samples, the subjects drank 50 ml of mineral water every 1 h until 8 h after collection of the final plasma and urine samples.

Fig. 1 Scheme of the study in which the same five volunteers were fed at 4-week intervals the following vitamin C (VC) intake: 282 g mashed potatoes containing 50 mg VC with 100 ml mineral water (food group 1), 87 g potato chips containing 50 mg VC with 100 ml mineral water (food group 2), 50 mg VC in 100 ml mineral water (food group 3) and 100 ml mineral water (food group 4). Each food group was orally administered at 08.00 hours on the day of the loading experiment.
Table 1 Composition of the standard meal
Collection of blood and urine samples
For plasma VC analysis, each blood sample was drawn into a VENOJECT® collection tube (Terumo Corporation, Tokyo, Japan) containing EDTA-2K as an anticoagulant. For plasma glucose analysis, each VENOJECT® collection tube contained EDTA-2Na and heparin-Na as an anticoagulant and NaF as an inhibitor of glucose degradation. For clinical laboratory tests of serum, each VENOJECT® collection tube contained procoagulant. Plasma and sera were obtained by centrifugation at 1700 g for 15 min at 4°C. After plasma was collected, 100 μl of the supernatant were immediately mixed with 450 μl of cold 3 % (w/v) metaphosphoric acid (MPA; Wako Pure Chemical Industries Limited, Osaka, Japan) containing 1 mmol/l of the metal ion chelator EDTA (Dojindo Laboratories, Kumamoto, Japan) for VC analysis. Urine samples from each subject were first measured to record their volume; then 600 μl of urine were immediately mixed with an equal volume of cold 10 % MPA and 1 mm-EDTA for VC analysis. Remaining aliquots of urine were used for creatinine analysis. All samples were stored at − 80°C until use.
Preparation of mashed potatoes and potato chips
Potato (S. tuberosum cultivar Toyoshiro) tubers of similar size and appearance were obtained for the experiment from a field in Ibaraki, Japan, in July 2009 and stored for a few days. The unpeeled potatoes were washed in tap water and air-dried on filter paper. In preparation for mashing, potatoes were steamed for 50 min in a steam cooker, peeled, mashed with a potato masher and mixed well with 1 % (w/w) salt. The steamed samples were stored at − 80°C until use. Potato chips were prepared as the commercial product manufactured by CALBEE, Inc., Tochigi, Japan. That is, peeled potatoes were sliced and immersed in tap water, then fried in rice and palm olein mixing oil for a few minutes. After the frying process, to avoid degradation of VC, potato chips were stored in a N2 gas-filled pouch with an Al vapour-deposited film at room temperature.
Measurement of vitamin C
VC was measured as the sum of a reduced and oxidised form by using HPLC and ECD as described previously(Reference Sato, Uchiki and Iwama25). For analysis of plasma VC, 100 μl of the subjects' plasma were mixed with 450 μl of cold 3 % MPA and 1 mm-EDTA and centrifuged at 21 000 g for 15 min at 4°C. For analysis of VC content in mashed potatoes and potato chips, 0·4 g of samples were homogenised with 14 volumes of cold 5·4 % MPA and 1 mm-EDTA by using a high-speed homogeniser (Polytron, Kinematica AG, Lucerne, Switzerland) and centrifuged at 21 000 g for 15 min at 4°C. For determination of VC after the resulting reduction of an oxidised form to a reduced form, 90 μl of the supernatant were incubated with 10 μl of 350 mm-Tris(2-carboxyethyl)phosphine hydrochloride for 2 h at 4°C. Then, the samples were diluted with 965 μl of cold 5 % MPA and 1 mm-EDTA followed by centrifugation at 21 000 g for 10 min at 4°C. A volume of 10 μl of that supernatant was injected into a Waters 2695 separation module coupled with a Waters 2465 ECD (Nihon Waters K.K., Tokyo, Japan) and separated by using an Atlantis dC18 5 μm column (4·6 × 150 mm; Nihon Waters K.K.) combined with an Atlantis dC18 5 μm guard column (4·6 × 20 mm; Nihon Waters K.K.). The mobile phases consisted of 50 mm-phosphate buffer (pH 2·8), 0·54 mm-EDTA, and 2 % methanol. The flow rate was 1·3 ml/min, and temperatures for column and ECD were set at 30°C. Electrical signals were detected by using an ECD with a glassy carbon electrode at +0·6 V. All electrochemical signal data from the ECD were recorded by the Waters Empowere2 software (Nihon Waters K.K.).
Measurement of urinary creatinine
The concentration of creatinine in urine was determined enzymatically by using a Creatinine Test Wako Kit (Wako Pure Chemical Industries, Limited, Osaka, Japan) according to the manufacturer's instructions(Reference Fabiny and Ertingshausen26).
Statistical analysis
The values are expressed as mean values with their standard errors. The 8 h area under the curve (AUC) of increased plasma VC concentration was calculated using the trapezoidal rule as a summary measure of VC bioavailability. Statistical analyses were performed with KareidaGraph software (Synergy Software, Reading, PA, USA). The significance of differences among the treatment groups and at different time points was determined by repeated-measures ANOVA. Differences between means were further evaluated by the Tukey's honestly significant difference test. Differences were considered significant at P < 0·05.
Results
When we compared the two preparations of potatoes, the VC content was 2·24-fold higher in potato chips (57·2 (sem 0·4) mg) than in mashed potatoes (17·7 (sem 0·1) mg)/100 g weight. The water content of potato chips (1·9 (sem 0·1) g/100 g) was marginally 2·5 % of the value of mashed potatoes (76·7 (sem 0·02) g/100 g).
To clarify whether VC from mashed potatoes and potato chips is absorbed in the intestine and transferred to the blood after oral consumption, we serially measured the VC concentrations of the subjects' plasma after consumption of 282 g mashed potatoes and 87 g potato chips, both of which contained 50 mg of VC. Subsequently, each subject also received 50 mg VC in water and water as controls. The four groups of food were administered at 4-week intervals. At the initial measurement of baseline plasma VC concentrations among the recipients of each food group, no significant differences (P = 0·3) were found for mashed potatoes (34·0 (sem 4·2) μmol/l), potato chips (40·2 (sem 3·1) μmol/l), VC in water (41·5 (sem 2·0) μmol/l) and water (37·2 (sem 2·1) μmol/l). To compare the later changes after oral consumption of mashed potatoes, potato chips, VC in water and water, we calculated the time course of increased plasma VC concentrations by subtracting the initial baseline values from the total at each subsequent blood/urine sampling (Fig. 2). In plasma, VC concentrations from mashed potatoes and potato chips almost linearly increased at first in a time-dependent manner after oral consumption and then reached maximal levels at 3 h. At the 3 h mark, the increase in plasma VC concentrations from mashed potatoes was 8·3 (sem 1·3) μmol/l and from potato chips was 9·7 (sem 1·1) μmol/l. These increases were higher than those of water (0·3 (sem 0·5) μmol/l; P = 0·002 and P = 0·0003, respectively), but not significantly different from each other (P = 0·8) or from that of VC in water (12·3 (sem 1·7) μmol/l). In fact, the only significant change in plasma VC concentration was a decrease for potato chips at 5 h after ingestion. These increases of VC in plasma for mashed potatoes were higher than those for water at 1 h (P = 0·046), 1·5 h (P = 0·02), 2 h (P = 0·002), 2·5 h (P < 0·0001), 3 h (P = 0·002), 3·5 h (P = 0·0005), 4 h (P = 0·02), 5 h (P = 0·012) and 6 h (P = 0·04); for potato chips, they were higher than those for water at 1·5 h (P = 0·03), 2 h (P = 0·02), 2·5 h (P < 0·0001), 3 h (P = 0·0003), 3·5 h (P = 0·0003), 4 h (P = 0·006) and 6 h (P = 0·02). On the other hand, these increases of VC in plasma for mashed potatoes were lower than those of VC in water at 1 h (P = 0·0001), 1·5 h (P = 0·048) and 2·5 h (P = 0·02); for potato chips, they were lower than those of VC in water at 1 h (P < 0·0001), 1·5 h (P = 0·02) and 2 h (P = 0·006). Overall, at different time points, the values between mashed potatoes and potato chips were not significantly different (P>0·05). Additionally, VC concentrations in plasma of all four food groups did not return to baseline until 8 h after consumption.

Fig. 2 Increased plasma vitamin C (VC) concentration after consumption of mashed potatoes (![]() ), potato chips (
), potato chips (![]() ), VC in water (
), VC in water (![]() ) and water (●). Values are means, with their standard errors represented by vertical bars (n 5). Values reached statistical significance for group (P < 0·001), time (P < 0·0001) and food group × time interaction (P < 0·0001) by repeated-measures ANOVA. Mean values were significantly different from water: *P < 0·05, **P < 0·01 and ***P < 0·001 (ANOVA and Tukey's honestly significance test). Mean values were significantly different from VC in water: †P < 0·05, ††P < 0·01, †††P < 0·001 (ANOVA and Tukey's honestly significance test). For mashed potatoes, values at 2, 2·5, 3, 3·5, 4, 5, 6, 7 and 8 h were significantly higher than values at 0, 0·5 and 1 h (P < 0·05); values at 1·5 h were significantly higher than values at 0 and 0·5 h (P < 0·05); values at 1 h were significantly higher than values at 0 h (P < 0·05) (repeated-measures ANOVA and Tukey's honestly significance test). For potato chips, values at 3 h were significantly higher than values at 0, 0·5, 1, 1·5, 2 and 5 h (P < 0·05); values at 4 and 8 h were significantly higher than values at 0, 0·5, 1 and 1·5 h (P < 0·05); values at 2, 2·5, 3·5, 5, 6 and 7 h were significantly higher than values at 0, 0·5 and 1 h (P < 0·05); values at 1·5 h were significantly higher than values at 0 and 0·5 h (P < 0·05) (repeated-measures ANOVA and Tukey's honestly significance test). For VC in water, values at 1, 1·5, 2, 2·5, 3, 3·5, 4, 5, 6, 7 and 8 h were significantly higher than values at 0 and 0·5 h (P < 0·01) (repeated-measures ANOVA and Tukey's honestly significance test). For water, values at 5 h were significantly higher than values at 3·5 h (P < 0·05); values at 7 h were significantly higher than values at 0, 0·5, 1, 1·5, 2, 2·5, 3, 3·5 and 4 h (P < 0·05); values at 8 h were significantly higher than values at 0, 0·5, 1·5, 2, 2·5, 3, 3·5 and 4 h (P < 0·05) (repeated-measures ANOVA and Tukey's honestly significance test).
) and water (●). Values are means, with their standard errors represented by vertical bars (n 5). Values reached statistical significance for group (P < 0·001), time (P < 0·0001) and food group × time interaction (P < 0·0001) by repeated-measures ANOVA. Mean values were significantly different from water: *P < 0·05, **P < 0·01 and ***P < 0·001 (ANOVA and Tukey's honestly significance test). Mean values were significantly different from VC in water: †P < 0·05, ††P < 0·01, †††P < 0·001 (ANOVA and Tukey's honestly significance test). For mashed potatoes, values at 2, 2·5, 3, 3·5, 4, 5, 6, 7 and 8 h were significantly higher than values at 0, 0·5 and 1 h (P < 0·05); values at 1·5 h were significantly higher than values at 0 and 0·5 h (P < 0·05); values at 1 h were significantly higher than values at 0 h (P < 0·05) (repeated-measures ANOVA and Tukey's honestly significance test). For potato chips, values at 3 h were significantly higher than values at 0, 0·5, 1, 1·5, 2 and 5 h (P < 0·05); values at 4 and 8 h were significantly higher than values at 0, 0·5, 1 and 1·5 h (P < 0·05); values at 2, 2·5, 3·5, 5, 6 and 7 h were significantly higher than values at 0, 0·5 and 1 h (P < 0·05); values at 1·5 h were significantly higher than values at 0 and 0·5 h (P < 0·05) (repeated-measures ANOVA and Tukey's honestly significance test). For VC in water, values at 1, 1·5, 2, 2·5, 3, 3·5, 4, 5, 6, 7 and 8 h were significantly higher than values at 0 and 0·5 h (P < 0·01) (repeated-measures ANOVA and Tukey's honestly significance test). For water, values at 5 h were significantly higher than values at 3·5 h (P < 0·05); values at 7 h were significantly higher than values at 0, 0·5, 1, 1·5, 2, 2·5, 3, 3·5 and 4 h (P < 0·05); values at 8 h were significantly higher than values at 0, 0·5, 1·5, 2, 2·5, 3, 3·5 and 4 h (P < 0·05) (repeated-measures ANOVA and Tukey's honestly significance test).
Next, we calculated the AUC for increased plasma VC concentration at 8 h after administration to estimate the bioavailability of VC in mashed potatoes and potato chips (Fig. 3). The AUC for mashed potatoes (55·1 (sem 5·7) μmol × h/l) and potato chips (53·6 (sem 8·1) μmol × h/l) were higher than that of water (14·6 (sem 3·3) μmol × h/l; P = 0·004 and P = 0·0048, respectively). On the other hand, these values tended to be 32 % less and were 34 % less than that of VC in water (81·5 (sem 8·9) μmol × h/l; P = 0·06 and P = 0·048, respectively). There was no significant difference between the AUC for mashed potatoes and potato chips (P = 0·99).

Fig. 3 Area under the curve (AUC) for increased plasma vitamin C (VC) concentration until 8 h after consumption of mashed potatoes, potato chips, VC in water and water. Values are means, with their standard errors represented by vertical bars (n 5). Mean values were significantly different from those of water: *P < 0·005 and **P < 0·0001. † Mean value was significantly different from that of VC in water: P < 0·05.
Urinary excretion of VC after consumption of mashed potatoes, potato chips, VC in water and water was similarly measured to reveal their differing effect, if any. Total spontaneous urine volumes over 8 h after intake of mashed potatoes, potato chips, VC in water and water were 321 (sem 15), 331 (sem 47), 326 (sem 73) and 286 (sem 15) ml, respectively, and not significantly different (P = 0·9). Similarly, the total urine creatinine over that 8 h time span did not significantly differ among mashed potatoes, potato chips, VC in water and water (5·30 (sem 0·23), 5·48 (sem 0·24), 5·42 (sem 0·29) and 4·81 (sem 0·34) mmol, respectively, P = 0·3). However, VC in water was excreted at a significantly elevated rate at 3 and 4 h after intake (Fig. 4(a)). Urinary excretion of VC from potato chips was slightly elevated at 4, 5 and 8 h, but not to a significantly different extent from that of VC in mashed potatoes and water until 8 h. Compared with VC in water, urinary excretion of VC at 3 h was 78 and 64 % less than that from mashed potatoes (1·9 (sem 0·9) μmol/mmol creatinine) and from potato chips (3·0 (sem 0·9) μmol/mmol creatinine) but was not a significant factor (P = 0·056 and P = 0·14), respectively. Total amounts of urinary VC excreted at 8 h were 64 and 46 % less than that from mashed potatoes (17·0 (sem 7·5) μmol/mmol creatinine, 1·7 (sem 0·7) mg VC) or potato chips (25·9 (sem 8·8) μmol/mmol creatinine, 2·9 (sem 1·1) mg VC) compared with VC in water (47·9 (sem 17·9) μmol/mmol creatinine, 5·4 (sem 2·2) mg VC), but again, the difference was not statistically significant (P = 0·2 and P = 0·5; Fig. 4(b)).

Fig. 4 (a) Urinary excretion of creatinine-corrected vitamin C (VC) after consumption of mashed potatoes (![]() ), potato chips (
), potato chips (![]() ), VC in water (
), VC in water (![]() ) and water (●). Values are means, with their standard errors represented by vertical bars (n 5). There was no significant main effect of food group (P = 0·14); however, the main effect of time reached significance (P < 0·0001) as did the food group × time interaction (P = 0·0012) by repeated-measures ANOVA. * Mean values were significantly different from water (P < 0·05; ANOVA and Tukey's honestly significance test). For mashed potatoes and water, values at different time points did not show a significant difference (P = 0·7 and P = 0·14, respectively, repeated-measures ANOVA). For potato chips, values at 4 and 8 h were significantly higher than values at 0, 1 and 2 h (P < 0·05); values at 5 h were significantly higher than values at 0 h (P < 0·05) (repeated-measures ANOVA and Tukey's honestly significance test). For VC in water, values at 3 and 4 h were significantly higher than values at 0 and 1 h (P < 0·05) (repeated-measures ANOVA and Tukey's honestly significance test). For water, values at different time points did not show a significant difference (P = 0·14, repeated-measures ANOVA). (b) Urinary excretion of creatinine-corrected VC until 8 h after consumption of mashed potatoes, potato chips, VC in water and water. Mean values were not significantly different among all groups (P = 0·13; ANOVA).
) and water (●). Values are means, with their standard errors represented by vertical bars (n 5). There was no significant main effect of food group (P = 0·14); however, the main effect of time reached significance (P < 0·0001) as did the food group × time interaction (P = 0·0012) by repeated-measures ANOVA. * Mean values were significantly different from water (P < 0·05; ANOVA and Tukey's honestly significance test). For mashed potatoes and water, values at different time points did not show a significant difference (P = 0·7 and P = 0·14, respectively, repeated-measures ANOVA). For potato chips, values at 4 and 8 h were significantly higher than values at 0, 1 and 2 h (P < 0·05); values at 5 h were significantly higher than values at 0 h (P < 0·05) (repeated-measures ANOVA and Tukey's honestly significance test). For VC in water, values at 3 and 4 h were significantly higher than values at 0 and 1 h (P < 0·05) (repeated-measures ANOVA and Tukey's honestly significance test). For water, values at different time points did not show a significant difference (P = 0·14, repeated-measures ANOVA). (b) Urinary excretion of creatinine-corrected VC until 8 h after consumption of mashed potatoes, potato chips, VC in water and water. Mean values were not significantly different among all groups (P = 0·13; ANOVA).
Discussion
In the present study, we show for the first time that the dietary intake of mashed potatoes and potato chips in amounts that contained 50 mg of VC was equally effective at increasing plasma VC concentrations, i.e. both yielded an increase of 24 %. This outcome indicates that (1) VC from ingested potatoes is well absorbed in the intestine and transferred to the blood and (2) processing such as steaming/mashing or frying does not affect the bioavailability of VC in potatoes. Furthermore, after the consumption of mashed potatoes or potato chips, the increased VC concentration in plasma up to the maximal level and for 8 h remained greater, and the urinary excretion of creatinine-corrected VC was relatively lower than both values after the intake of VC in water. Overall, the bioavailability of VC from potatoes, either mashed or fried, exceeded that from VC in water.
Human subjects do not usually eat raw potatoes but, instead, consume them in home-processed and commercial preparations. Han et al. (Reference Han, Kozukue and Young14) reported that potatoes home-processed by boiling, pressure-cooking, frying, sautéing, braising, baking or microwaving lose VC to varying degrees. In that study, the VC content of mashed potatoes (17·7 (sem 0·1) mg/100 g) was at a lower level presumably because the combined processes of steaming, mashing and additional freeze–thawing they applied decreased the VC content. Burg & Fraile(Reference Burg and Fraile15) showed that steaming at 200°C by using a superheated steam oven reduced VC in potatoes by 73 %. Elsewhere, exposing mashed potatoes served to hospitalised patients to a cool-chill-plated catering system resulted in a 76 % loss of VC(Reference McErlain, Marson and Ainsworth27). In contrast, commercial potato chips prepared from the same lot of potatoes contained a much larger amount of VC (57·2 (sem 0·4) mg/100 g). In good accord with the previous report, frying in oil increased VC content per fresh weight of potatoes by decreasing their water content from about 80 to 2 % despite partial loss of VC in the process(Reference Miyoshi, Ishihara and Nakamura16). Thus, commercial potato chips, because of their substantial content of VC and ready availability, are a more efficient source of VC than home-processed potatoes.
In the present study, subjects consumed orally 282 g mashed potatoes and 87 g potato chips, each portion containing 50 mg VC. This amount is suitable for one daily serving in a meal and snack, and almost all of this VC is absorbed in the intestine(Reference Levine, Conry-Cantilena and Wang20, Reference Levine, Wang and Padayatty21). In human subjects, the metabolism and utilisation of orally administered, oxidised VC are equivalent to those of reduced VC(Reference Higasa, Tsujimura and Hiraoka22, Reference Tsujimura, Higasa and Nakayama28–Reference Tsujimura, Higasa and Aono31). Therefore, to make an appropriate comparison, we evaluated the bioavailability of VC in potatoes by measuring the VC content in plasma and its excretion in urine. Previously, a high dose of oral VC drastically increased VC concentration in the blood(Reference Levine, Conry-Cantilena and Wang20, Reference Levine, Wang and Padayatty21). Consistently, though, a quantity of VC intake beyond renal tubules' reabsorption capability is readily excreted into urine(Reference Levine, Conry-Cantilena and Wang20, Reference Levine, Wang and Padayatty21). Levine et al. (Reference Levine, Conry-Cantilena and Wang20, Reference Levine, Wang and Padayatty21) reported that little VC was excreted in urine during the 24 h following a single oral dose of < 50 mg VC, whereas that after >100 mg VC dramatically increased in healthy volunteers. Therefore, such a large dose of oral VC might cause difficulty in examining the bioavailability of VC in food. To overcome this difficulty, we set the amount of orally administered VC in potatoes at 50 mg, which is half the RDA (100 mg) for VC in Japan(32). However, our highly sensitive method for the measurement of VC(Reference Sato, Uchiki and Iwama25) enabled us to detect accurately even slight changes of VC in plasma.
As far as we know, few reports describe the bioavailability of VC from food, raw or processed. In one such report, Mangels et al. (Reference Mangels, Block and Frey33) measured VC depletion–repletion during an 8-week period and found that the bioavailability of VC from oranges, orange juice and cooked broccoli is similar to that of synthetic VC; the exception was raw broccoli, which was 20 % lower. Van het Hof et al. (Reference van het Hof, Tijburg and Pietrzik34) noted that plasma VC concentrations increased after a 4 d diet of vegetables such as broccoli, green peas, whole-leaf spinach, and chopped spinach in accordance with their VC contents. That group also showed that chopping whole-leaf spinach did not improve the bioavailability of VC. Sánchez-Moreno et al. (Reference Sanchez-Moreno, Cano and de Ancos35, Reference Sanchez-Moreno, Cano and de Ancos36) stated that drinking orange juice and Mediterranean vegetable soup (gazpacho) increased plasma VC concentrations in a dose–response manner and that daily consumption for 14 d provided a continuously higher plasma VC concentration than that of the baseline. Also, the consumption of pulsed electric fields-processed orange juice with a long shelf-life increased plasma VC concentration to a similar extent as freshly squeezed orange juice, both in one dose and in daily doses for 14 d(Reference Sanchez-Moreno, Cano and de Ancos37).
Potatoes are consumed worldwide and are the major dietary staple in many countries of Europe and South America. However, this choice is less common in Japan. Interestingly, potatoes and potato chips are an important source of VC (17 mg/d in men and about 7 mg/d in women) for humans, especially those with a low plasma VC status ( < 11·4 μmol/l). Despite this acknowledgement published after a third Glasgow MONICA population survey(Reference Wrieden, Hannah and Bolton-Smith38), it is surprising that there was no evidence about the bioavailability of VC in potatoes. Consequently, the present result in which plasma VC concentration and urinary excretion of VC increased during the 8 h period after the oral intake of mashed potatoes and potato chips is unique and suggests that VC from potatoes is readily absorbed in the intestine and transferred to the blood. Also shown was that the bioavailability of VC from potato chips is closely similar to that from mashed potatoes. Clearly, despite the processing methods of steaming/mashing and frying, VC from potatoes retains adequate bioavailability. Moreover, humans gain more VC from potato chips than from mashed potatoes when comparable proportions are eaten. However, the energy from mashed potatoes and potato chips are 351 kJ and 2318 kJ/100 g, respectively(2). In the present study, the human subjects took 282 g mashed potatoes and 87 g potato chips, which have 990 kJ and 2017 kJ of total energy respectively. Thus, people, especially obese individuals, should hesitate to consume a large amount of potato foods.
In the present study, increased plasma VC concentrations were less at 1, 1·5, 2, 2·5 h after intake of mashed potatoes and potato chips than that of VC in water. However, after 3 h, which was the time of maximal VC level, the concentrations did not differ significantly among the three food groups. However, less VC was lost in urine after potato ingestion than from VC in water alone, corresponding to a slower increase in VC concentration in plasma.
Water consumption, that is, no intake of VC in subjects did not largely affect the increased VC concentration in plasma, the AUC for increased VC in plasma, and the urinary excretion of VC in subjects except for the slight increase of VC from 5 to 8 h after consumption. In fact, to our knowledge, a study examining VC concentration in plasma after water consumption has not been reported previously. Although it is uncertain as to the reason why VC concentration in plasma after water consumption was slightly increased, we considered that long-time fasting over 17 h might increase the VC concentration in plasma via the leakage of VC from cells in tissues such as liver.
Some limitations here are that mashed potatoes and potato chips are solid foods containing much starch; therefore, digestion might take longer and VC absorption in intestinal lumen could be slower compared with VC in water, which has the fastest absorption time. Our time course was set at 8 h after the administration of potatoes, because of ethical concerns for volunteers who underwent overnight fasting. However, to obtain fully convincing conclusions, the study should be extended until 24 h have elapsed, which was not done here because feeding meals to subjects might affect VC metabolism in their bodies. Another possibility is that the transport of VC into tissues from the blood was enhanced by some nutrient(s) of potatoes, which resulted in an apparently slower increase of VC in plasma. That is, extracellular VC is transported into the cytoplasm by the Na dependent VC transporters 1 and 2 in a reduced form and by the GLUT1, GLUT3 and GLUT4 in an oxidised form(Reference Wilson17, Reference May, Qu and Qiao18, Reference Corti, Casini and Pompella39). As almost all VC in the blood exists in a reduced form(Reference Levine, Conry-Cantilena and Wang20), SVCT1 and SVCT2 are mainly responsible for taking up VC from the blood and into the cytoplasm of multiple tissues. SVCT1, which is expressed in such tissues as the intestine, liver, lung, kidney and skin, is involved in whole-body homeostasis(Reference Wilson17). SVCT2, which is expressed in the brain, eye, liver, kidney, intestine, adrenal gland, bone and skeletal muscle, is a high-affinity VC transporter to maintain high VC concentrations in tissues(Reference Wilson17). As recently reported, SVCT2-mediated VC uptake is quickly enhanced by the translocation of cytoplasmic SVCT2 to plasma membranes from various types of stimulation(Reference Wu, Zeng and Taniguchi40). Since the regulation of SVCT2-mediated VC uptake is not fully understood, the present results might imply the existence of novel SVCT2-activating nutrient(s) in potatoes.
We have performed this experiment employing only male subjects. As shown in previous papers(Reference Levine, Conry-Cantilena and Wang20, Reference Levine, Wang and Padayatty21), VC metabolism in human subjects is not largely different between males and females. Thus, it is considered that the present data about the bioavailability of VC in mashed potatoes and potato chips could be applicable to females.
In conclusion, the bioavailability of VC in mashed potatoes and potato chips was documented in the present oral, single-dose study. The consumption of mashed potatoes and potato chips increased the VC concentration in plasma, and less VC tended to be excreted in urine than was consumed. These results explicitly confirm the nutritional value of mashed potatoes and potato chips as a dietary VC source as demonstrated here in a cohort of Japanese males.
Acknowledgements
We thank Ms P. Minick for the excellent English editorial assistance. There was no funding for the present study. There are no conflicts of interest. The authors' contributions are as follows: Y. K., C. H., K. I., S. H., H. M., N. M., H. K. and A. I. designed the research; Y. K., C. H. and M. I. conducted the research; Y. K. and C. H. analysed the data; Y. K. wrote the paper and had primary responsibility for the final content. All authors read and approved the final manuscript.







