The metabolic syndrome (MetS) comprises pathological conditions that include insulin resistance, arterial hypertension, visceral adiposity and dyslipidaemia, which favour the development of CVD(Reference Reaven1). Existing evidence suggests that the MetS is rising in both developed(Reference Ford, Giles and Mokdad2) and developing countries, such as Brazil(Reference Grundy3).
Currently, many factors are thought to be involved in the beneficial cardiovascular effects of fish oil n-3 fatty acids(Reference Deckelbaum, Leaf and Mozaffarian4), such as alteration of plasma lipid profile by decreasing plasma TAG content; reduced agonist-induced activation of NF-κB and increased PPARγ; decreases in inflammatory markers and in vascular cell adhesion molecules; and decreased synthesis of inflammatory PG and leukotrienes. On the other hand, soya-based products and their components(Reference Sacks, Lichtenstein and Van Horn5) are involved in improving blood lipid profiles, and particularly in decreasing total and LDL-cholesterol; besides, soya products can also reduce blood glucose levels in postmenopausal women with type 2 diabetes mellitus and the MetS. Fish or fish oil consumption has been recommended as part of disease management to prevent death due to CHD, both as a preventive measure and after a coronary event, largely due to its properties that prevent fatal CHD and sudden cardiac death(Reference Deckelbaum, Leaf and Mozaffarian4). Dietary soya has also been recommended for its potential role in CVD, although the recommendation has changed from soya protein or isoflavones to soya products because of their high content of polyunsaturated fats, fibre, vitamins and minerals and low content of saturated fat(Reference Sacks, Lichtenstein and Van Horn5).
Elevated blood pressure (BP) results from environmental factors, genetic factors and interactions among these factors. Among the environmental factors that affect BP, dietary factors have a prominent and probably predominant role(Reference Appel, Brands and Daniels6). Several clinical trials and meta-analysis have identified nutritional strategies using soya-based products(Reference Jenkins, Kendall and Jackson7–Reference He, Gu and Wu9) or fish oil(Reference Gelijnse, Giltay and Grobbee10) as non-invasive approaches to managing and reducing the risk of elevated BP.
Fish oil n-3 fatty acids and soya-based products may exert some of their beneficial actions through changes in pro-inflammatory and anti-inflammatory (adiponectin) adipocytokines, and endothelial function(Reference Gelijnse, Giltay and Grobbee10–Reference Stirban, Nandrean and Götting14). In a recent report, our group showed an increase in NO levels and a decrease in diastolic BP (DBP) in patients with the MetS receiving a soya-based product (kinako)(Reference Simão, Lozovoy and Simão15).
To the best of our knowledge, the present study is the first to report the effects of fish oil and a soya-based product, used in isolation and concomitantly, on inflammatory markers and endothelial function measured by NO in women with the MetS. Our hypothesis is that a decrease in SBP and/or DBP with soya or fish oil ingestion could be partially explained by an increase in adiponectin and NO levels and that the association of the supplements would lead to better results than their isolated use.
Therefore, the aim of the present study was to verify the effects of fish oil and soyabean intake on inflammatory markers, endothelial function and BP.
Subjects and methods
A total of 100 (n 100) women with the MetS from ambulatory patients of the University Hospital of Londrina, Paraná, Brazil were contacted by telephone, and thirty-one were considered ineligible (Fig. 1). The remaining sixty-nine (n 69) women were randomised and began to participate in the study (Fig. 1). No subject was paid to participate in the study. Their motivation was related to the intake of a non-pharmacological therapy that was practically without side effects. The mean age of the patients was 47·9 (sd 9·98) years. The distribution of postmenopausal women (n 40) between the groups was similar. The control and fish oil groups included ten postmenopausal women, the kinako group included eleven, and the combined fish oil and kinako group included nine. The exclusion criteria were thyroid, renal, hepatic, gastrointestinal or oncological diseases and utilisation of lipid-lowering drugs, oestrogen replacement therapy, drugs for hyperglycaemia, and fish oil or soya supplements. Patients who were taking antihypertensive drugs were not excluded and were allowed to continue taking the same dose of the drugs. None of the subjects followed a specific diet before the study began.

Fig. 1 Schematic of subject flow and reasons for exclusion.
The patients were instructed not to change their usual diets, alcohol intake, level of physical activity or other lifestyle factors throughout the intervention period. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Ethical Committee of the University of Londrina, Paraná, Brazil (study protocol CEP 298/05). Written informed consent was obtained from all subjects/patients.
Study design
Before beginning the study, the patients were followed up on a regular basis. Patients were randomly assigned to one of four groups after stratification by age and BMI. The first group (control group, n 15) was only directed to maintain its usual diet; the second group (kinako group, n 15) received 29 g/d of kinako (toasted ground soya bean, with 50 mg of isoflavones and 12·95 g/d of soya protein) at lunch and dinner; the third group (fish oil group, n 19) received 3 g/d of fish oil n-3 fatty acids (ten capsules); and the fourth group (fish oil and kinako group, n 16) received 3 g/d of fish oil n-3 fatty acids and 29 g/d of kinako. Each fish oil capsule contained 180 mg of EPA and 120 mg of DHA. The capsules were given at breakfast, lunch and dinner. The subjects were recommended to refrain from resting after meals to avoid unpleasant effects. All the groups were evaluated at the beginning of the study and after 90 d. Interviews were conducted to ensure that there was no change in lifestyle factors throughout the study. Kinako was provided by Good Soy, Uberaba, Minas Gerais, Brazil, and its quality was certified by the Brazilian Farming Research Company (Embrapa-Soja). The nutrient composition of 29 g of kinako was as follows: 539·53 kJ (128·95 kcal), 12·95 g protein, 6·35 g carbohydrate, 5·75 g lipids, 3·95 g fibre, 83·25 mg Ca, 2·75 mg Fe, 3·5 μg vitamin A, 0·23 mg vitamin B1, 0·075 mg vitamin B2, 0·61 mg niacin and 50 mg isoflavones.
Anthropometric measurements and biochemical parameters were assessed at the beginning of the study and after 90 d.
The MetS was defined following the Adult Treatment Panel III criteria(Reference Jacobs16), when three of the following five characteristics were confirmed: (1) abdominal obesity: waist circumference (WC) ≥ 88 cm; (2) hypertriacylglycerolaemia ≥ 1·695 mmol/l; (3) low levels of HDL-cholesterol ≤ 1·295 mmol/l; (4) high BP: ≥ 130/85 mmHg; (5) high fasting glucose ≥ 6·1 mmol/l.
Steps taken to optimise compliance
Various measures were taken to optimise and assess patient compliance(Reference Santana, Mandarino and Cardoso17). Before each trial began, it was ensured that the patients understood that they could be allocated to any group. Boxes of fish oil capsules were handed out at the initial interview and at the two later visits. The subjects were asked to return the boxes at each visit so that the number of capsules taken could be estimated by questioning the patients and counting the remaining capsules. Kinako compliance was measured by questioning the patients and counting the kinako packages when patients returned for their clinical and nutritional evaluation.
Anthropometric and blood pressure measurements
Body weight was measured to the nearest 0·1 kg by using an electronic scale, with individuals wearing light clothing and no shoes; all patients were weighed in the morning. Height was measured to the nearest 0·1 cm using a stadiometer. BMI was calculated as weight (kg) divided by height (m) squared. WC was measured with a soft tape on standing subjects midway between the lowest rib and the iliac crest. Following these, three BP measurements, taken with a minute's interval between them after the subject had been seated, were recorded. The mean of these measurements was used in the analysis(Reference Pickering, Hall and Appel18).
The subjects had been seated for at least 5 min in a chair with back support and with feet on the floor, and smoking, alcohol and exercise were avoided for at least 30 min before the measurement. BP was measured by only one person who was trained and retrained properly. Recommendations of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure were followed(Reference Chobanian, Bakris and Black19).
Inflammatory markers and cytokine measurements
Serum high-sensitivity C-reactive protein was measured using a nephelometric assay (Dade-Behring). Additionally, serum TNF-α, IL-6 and adiponectin were measured by a sandwich ELISA using a commercial immunoassay (R&D System).
Nitric oxide
Serum NO metabolite levels were assessed by nitrite (NO2−) and nitrate (NO3−) concentration according to the Griess reaction, supplemented by the reduction of nitrate to nitrite with Cd(Reference Guevara, Iwanejko and Dembińska-Kieć20, Reference Navarro-Gonzálvez, Benayas and Arenas21).
Statistical analysis
The distribution of the number of medications was analysed by a χ2 test. The Wilcoxon matched pairs test was performed to verify changes from baseline (intra-group changes). ANOVA was performed to compare differences between ages. The Kruskal–Wallis with post hoc Dunn's test was performed to verify differences between BMI and to compare differences across treatment groups (inter-group changes). Data are presented as the means and standard deviations or medians and 25th–75th percentiles. Significance was set at P < 0·05.
Results
Overall, four patients withdrew from the study: two patients in the kinako group and two patients in the group that received fish oil and kinako. All subjects (n 65) who completed the study reached a level of compliance above 90 % of fish oil and kinako ingestion.
In general, the participants had no major complaints, except for the fishy taste and self-limited diarrhoea reported by three patients in the fish oil groups. According to the self-reports, each subject's lifestyle was unchanged throughout the study. In all groups, subjects did not drink alcohol regularly. There were no differences between groups with respect to age, smoking status, Ca channel blockers, β blockers and diuretics (Table 1). Despite their influence on adiponectin levels, for ethical reasons we could not ask the patients to stop using antihypertensive (angiotensin-converting enzyme inhibitors) medication during the study, but there was no statistically significant difference between groups when they were compared. There were no significant differences in any laboratory parameter studied at baseline.
Table 1 Characteristics of the patients with the metabolic syndrome
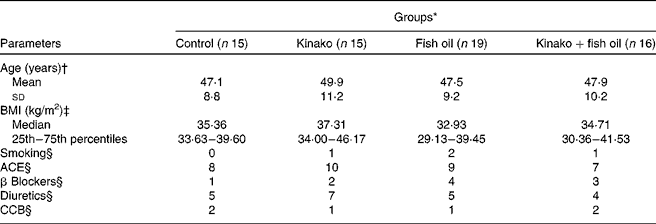
ACE, angiotensin-converting enzyme; CCB, Ca channel blocker.
* See the Study design section for the diets given to each group. None of the groups differed with respect to the parameters measured.
† ANOVA was performed to compare differences between ages.
‡ The Kruskal–Wallis test was performed to verify differences between subjects' BMI.
§ Distribution of number of medications was analysed by a χ2 test.
In relation to baseline values, WC showed a statistically significant decrease in the kinako group (P < 0·05), whereas WHR increased significantly (P < 0·05) after 90 d in the group that received both fish oil and kinako. The group that received fish oil and kinako concomitantly showed a statistically significant decrease in SBP (P < 0·05), whereas there was a significant decrease in DBP in the control group (P < 0·05), kinako group (P < 0·01) and fish oil group (P < 0·01) after 90 d in relation to the baseline values (Table 2).
Table 2 Anthropometric and blood pressure measurements in patients with the metabolic syndrome at baseline, and after 90 d of intervention‡ (Medians and 25th–75th percentiles)

WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Mean value was significantly different from that at baseline: * P < 0·05, ** P < 0·01.
† Percentage change from baseline was significantly different from that for the kinako+fish oil group (P < 0·05).
‡ The Wilcoxon matched pairs test was performed to verify changes from baseline (intra-group changes). The Kruskal–Wallis with post hoc Dunn's test was performed to compare differences across treatment groups (inter-group changes). Significance was set at P < 0·05.
§ See the Study design section for the diets given to each group.
Differences across treatment groups showed a statistically significant decrease (P < 0·05) in DBP in the kinako group after 90 d when compared to the results obtained from the combined fish oil and kinako group (Table 2).
In relation to inflammatory markers, there were significant increases in IL-6 values in the kinako group (P < 0·05) and in adiponectin values after 90 d in the kinako (P < 0·01) and fish oil (P < 0·05) groups in relation to the baseline values. The C-reactive protein and TNF-α values did not differ significantly in any group (Table 3).
Table 3 Inflammatory markers and endothelial function in women with the metabolic syndrome using soya or fish oil at the beginning of the study and after 90 d‡ (Medians and 25th–75th percentiles)

CRP, C-reactive protein.
Mean value was significantly different from that at baseline: * P < 0·05, ** P < 0·01.
† Percentage change from baseline was significantly different from that for the kinako+fish oil group: P < 0·05.
‡ A Wilcoxon matched pairs test was performed to verify changes from baseline (intra-group changes). The Kruskal–Wallis with post hoc Dunn's test was performed to compare differences across treatment groups (inter-group changes). Significance was set at P < 0·05.
§ See the Study design section for the diets given to each group.
Serum NO metabolite levels showed a significant increase (P < 0·05) in the kinako and fish oil groups after 90 d when compared to the baseline values (Table 3).
Differences between treatment groups showed a statistically significant increase (P < 0·05) in IL-6 in the kinako group compared with data from the combined fish oil and kinako group (Table 3).
Discussion
To our knowledge, this is the first study to report a simultaneous increase in adiponectin and NO levels in patients with the MetS consuming a soya-based product or fish oil. Conceivably, these data can explain, at least partially, the BP decrease found in the present study with kinako or fish oil ingestion.
Effects of fish oil and kinako on inflammatory markers and adiponectin
In the present investigation, as in most supplementation studies with n-3 fatty acids or soya(Reference Daamsgard, Frokiaer and Andersen22, Reference Maskarinec, Oum and Chaptman23), we found no effect on C-reactive protein or TNF-α, but unexpectedly there was an increase in IL-6 levels with kinako ingestion. The current understanding of the role of IL-6 in the context of obesity is ambivalent. IL-6 can be viewed as a proinflammatory cytokine which induces C-reactive protein secretion, but may also be regarded as an anti-inflammatory cytokine as it induces the synthesis of IL-1 receptor antagonist and the release of soluble TNF receptor, leading to reduced activity of proinflammatory cytokines(Reference Ait-Oufella, Taleb and Mallat24). There is also growing evidence that IL-6 has a role in inducing lipolysis and decreasing appetite and weight gain, thus controlling obesity-associated pathologies(Reference Berg and Scherer25). In the present study, we found a significant decrease in WC in the kinako group, which favours the latter hypothesis. Soya-based products, due to their high contents of protein and fibre, may have beneficial effects on satiety(Reference Allison, Gadbury and Schwartz26). They may even produce weight loss with a decrease in fat mass but not muscle mass in overweight and obese subjects(Reference Deibert, König and Schmidt-Trucksaess27). On the other hand, the lack of increase in IL-6 levels in the group that ingested soya and fish oil concomitantly could be explained by a decrease in IL-6 levels that may occur with fish oil ingestion(Reference Ciubotaru, Lee and Wander28); however, IL-6 levels did not decrease in the fish oil group in the present study.
Similar to data of the present study, some experimental studies have reported beneficial anti-inflammatory effects of soya protein or isoflavone, shown by increasing adiponectin(Reference Nagasawa, Fukui and Funahashi29, Reference Charles, Yuskavage and Carlson30). Charles et al. showed an increase in adiponectin levels in healthy, normal-weight, postmenopausal women with high doses of isoflavones (20 g of soya protein with 160 mg of isoflavone).
n-3 Fatty acids have been shown to increase adiponectin levels in both experimental(Reference Itoh, Suganami and Satoh31, Reference Shirai and Suzuki32) and human studies(Reference Itoh, Suganami and Satoh31, Reference Lara, Economou and Wallace33). Itoh et al. (Reference Itoh, Suganami and Satoh31) reported that EPA (1·8 g/d) increases adiponectin secretion possibly through the improvement of the inflammatory changes in obese adipose tissue in rodent models of obesity and human obese subjects. Lara et al. (Reference Lara, Economou and Wallace33) also verified a trend towards an increase in plasma adiponectin, independent of weight change with 125 g/d of salmon consumption (2·4 g/d n-3 fatty acids) in non-obese healthy subjects. Experimental studies have revealed that the transcription factor PPARγ is perhaps the main mechanism by which n-3 fatty acids may increase adiponectin levels(Reference Banga, Unal and Tripathi34). Of note, n-3 fatty acids act like the thiazolidinediones, which are PPARγ agonists and are used in the treatment of insulin-resistant and diabetic subjects(Reference Tao, Wang and Gao35). However, different from our results, some studies have not found an adiponectin increase with n-3 fatty acids supplementation in healthy(Reference Daamsgard, Frokiaer and Andersen22), overweight to moderately obese subjects(Reference Kratz, Swarbrick and Callahan36), or in patients with type 2 diabetes(Reference Kabir, Skurnik and Naour37). There are some concerns about those studies related to using olive oil as placebo(Reference Daamsgard, Frokiaer and Andersen22). These concerns include the following: olive oil is not an inert compound and has shown beneficial anti-inflammatory effects(Reference Barbosa, Simão and Dichi38); the anti-inflammatory low dose of 1·8 g/d n-3 fatty acids(Reference Kabir, Skurnik and Naour37), as circulating adiponectin, is associated with plasma n-3 fatty acids concentration(Reference Fernández-Real, Vendrell and Ricart39); and these studies analysed small samples(Reference Kratz, Swarbrick and Callahan36).
Effects of fish oil and kinako on endothelial function
The present study is in line with others that show an improvement in endothelial function mediated by NO, in healthy postmenopausal women using isoflavone(Reference Hall, Formanuik and Harnpanich40) or in postmenopausal women with the MetS using a soya nut diet (30 g/d)(Reference Azadbakht, Kimiagar and Mehrabi13). Experimental studies indicate that isoflavone can stimulate the production of NO via oestrogen receptor-mediated activation of endothelial NO synthase (eNOS)(Reference Squandrito, Altavilla and Squandrito41). Recently, a meta-analysis(Reference Li, Liu and Bai42) showed that oral isoflavone supplementation does not improve endothelial function in women with high baseline flow-mediated dilatation values, but it does lead to significant improvement in postmenopausal women with low baseline flow-mediated dilatation values, an endothelial marker predominantly mediated by NO(Reference Doshi, Naka and Payne43). Our data showed low baseline serum NO metabolite levels, which may have contributed to the favourable results obtained with kinako.
n-3 Fatty acids have also shown an improvement in endothelial function mediated by NO in subjects with hypercholesterolaemia(Reference Goodfellow, Bellamy and Ramsey44) and diabetes mellitus(Reference Stirban, Nandrean and Götting14). Collectively, in vitro studies suggest that endothelial-dependent vessel relaxation is due to the enhancement of NO release(Reference Jung, Torrejon and Tighe45). However, another study in hypertriacylglycerolaemic subjects(Reference Kelley, Siegel and Fedor46) did not show an NO increase with high doses (7·5 g/d) of DHA; however, the authors used olive oil as the placebo. Olive oil modulates NO biosynthesis and increases eNOS expression(Reference Schmitt and Dirsch47), and therefore it is not an ideal placebo.
Of note, whereas fish oil action on NO may result from increased expression of eNOS(Reference Nyby, Matsumoto and Yamamoto48), it can be hypothesised that the relatively higher amount of the NO precursor, arginine, in the amino acid profile of soya protein(Reference Erdman49) could explain, at least in part, its effects on NO concentration. The l-arginine/NO pathway plays a critical role in maintaining normal endothelial function by causing vasodilatation(Reference Bai, Lun and Yang50).
Effects of fish oil and kinako on blood pressure
Although dietary behaviour could have influenced the non-significant decline in SBP and the significant reduction in DBP in the control group, there were no major changes in subjects' habitual intake as well as in other factors, such as physical activity and also in NO and adiponectin levels.
Similar to our findings, soya intake has been shown to have an inverse association with BP both in longitudinal studies(Reference Yang, Shu and Jin8) and in small-scale clinical trials(Reference Jenkins, Kendall and Jackson7, Reference He, Gu and Wu9). However, in contrast to our results, Azadbakht et al. (Reference Azadbakht, Kimiagar and Mehrabi51) used soya protein or soya nuts (30 g/d) in MetS patients but did not show a significant decrease in BP.
Human studies have also shown that fish oil can lower BP. A meta-regression analysis of randomised trials verified that at doses between 3 and 5·6 g/d, fish oil reduced BP in hypertensive individuals by up to 5·5/3·5 mmHg(Reference Gelijnse, Giltay and Grobbee10). Furthermore, n-3 fatty acids intake is related inversely to BP, including in non-hypertensive persons, in seventeen population-based samples in China, Japan, the UK and the USA(Reference Ueshima, Stamler and Elliot52).
Because there was no significant increase in adiponectin and NO levels in the group that received concomitant kinako and fish oil, decreased SBP in this group may be justified by other pathophysiological mechanisms, other than adiponectin and NO.
In the present study, although the decrease in DBP in the kinako group could be explained by the concomitant decrease in WC, there was a decrease in SBP in the group that received kinako and fish oil, reinforcing the assumption that the effects of kinako and fish oil on BP are beyond weight loss.
We had also expected a synergistic effect with both supplements in DBP, which has not occurred. Perhaps, the small number of individuals has not allowed this finding.
Link between the effects of adiponectin, endothelial function and blood pressure
Numerous epidemiological studies based on different ethnic groups have identified adiponectin deficiency as an independent risk factor for endothelial dysfunction, hypertension, CHD, myocardial infarction and other cardiovascular complications. On the other hand, decreased bioavailability and/or impaired production of NO is considered a major cause of endothelial dysfunction, which is now recognised as one of the earliest changes in atherosclerosis(Reference Goldstein and Scalia53, Reference Zhu, Cheng and Vanhoutte54).
In experimental studies, adiponectin has been shown to enhance NO production in cultured aortic endothelial cells(Reference Tan, Xu and Chow55), to significantly increase eNOS (83 %) and reduce inducible NO synthase (70 %) in hyperlipidaemic rats(Reference Li, Wang and Zhang56) and to improve obesity-related hypertension in mice(Reference Ohashi, Kihara and Ouchi57). Adiponectin has anti-inflammatory effects and augments blood flow by enhancing NO production and activating eNOS, and it may act as a modulator of vascular remodelling by suppressing smooth muscle cell migration, which possibly plays a role in the regulation of atherosclerosis(Reference Ziemke and Mantzoros58). Human studies have also shown that hypoadiponectinaemia is closely linked to endothelial dysfunction in healthy(Reference Shimabukuro, Higa and Asahi59), and in hypertensive subjects(Reference Ouchi, Ohishi and Kihara60). Although other mechanisms, aside from NO, can explain the effects of soya and fish oil on BP improvement, NO has been considered the principal mediator of vasodilatation caused by endothelial cells. NO plays a major role in regulating BP, and its deficient bioactivity is an important component of hypertension(Reference Tan, Xu and Chow55, Reference Hermann, Flammer and Lüscher61). Hypertensive subjects have increased generation of reactive oxygen species, which scavenge NO, thereby reducing NO bioavailability(Reference Hermann, Flammer and Lüscher61).
Thus, it is conceivable that the present findings of adiponectin and NO increase may explain, at least in part, the BP decrease with fish oil or kinako ingestion in MetS patients.
Limitations of this study are the small number of subjects and the antihypertensive drugs the patients were taking, such as angiotensin-converting enzyme inhibitors, which may elevate plasma adiponectin levels(Reference Zhu, Cheng and Vanhoutte54) and β blockers, which may have synergistic antihypertensive action with fish oil n-3 fatty acids(Reference Howe62). However, statistical analyses between the groups were not significantly different, and the patients' medication regimens remained unchanged during the intervention period.
In relation to the consistency of our initial hypothesis, the aforementioned limitations, especially the small number of patients, may justify the changes verified with adiponectin and NO levels on DBP when soya or fish oil was ingested separately, but not in combination. On the other hand, these limitations may also explain the synergistic effects verified in SBP, despite a non-significant increase in NO and adiponectin levels.
In conclusion, the findings of increased serum adiponectin and NO metabolite levels after 90 d, both in the fish oil group and the kinako group, reinforce the importance of the influence of adiponectin and NO levels on DBP decrease in patients with the MetS. A synergistic beneficial effect was verified with the concomitant use of fish oil and kinako on SBP but was unrelated to changes in adiponectin and NO levels. Further investigation is warranted to confirm the results obtained in the present study.
Acknowledgements
The present research was supported by the National Council of Brazilian Research – CNPq. The Good Soy Company supplied the kinako. The authors' responsibilities were as follows: A. N. C. S. collected the data, designed the study, interpreted the results and wrote the manuscript; M. A. B. L., L. D. B. and T. N. C. S. collected the data and interpreted the results; H. K. M. performed cytokine analysis; T. M. performed the statistical analysis; and I. D. developed the hypothesis tested in the study, designed the study, interpreted the results and wrote the manuscript. None of the authors had a competing financial interest or any other conflict of interest in relation to the present study.






