Foods made from soyabeans have been consumed in Asia for centuries beginning first with China and then spreading to Japan and other nearby countries. In Asian cuisine, the soyabean is prized in particular for its versatility. Over the past two decades, the popularity of soyafoods has increased markedly in the USA and other Western countries. Soyafoods provide ample amounts of high-quality protein(Reference Rand, Pellett and Young1, Reference Hughes, Ryan and Mukherjea2) and have a healthy fatty acid profile(Reference Wu, Rodgers and Marshall3). However, the increased popularity has occurred largely because of research suggesting that independent of nutrient content soyafoods confer health benefits, especially related to the prevention of chronic disease(Reference Wu, Yu and Tseng4–Reference Zhang, Shu and Li8). There is evidence that soya intake reduces the risk of osteoporosis(Reference Koh, Wu and Wang7, Reference Zhang, Shu and Li8), certain forms of cancer(Reference Yan and Spitznagel5, Reference Messina and Hilakivi-Clarke9, Reference Messina and Wu10) and CHD(Reference Kokubo, Iso and Ishihara11, Reference Zhang, Chen and Huang12). In regard to the latter, the US Food and Drug Administration (FDA) approved a health claim for soyafoods and CHD in 1999 based on the cholesterol-lowering effects of soya protein(13). There are also data indicating that soyafoods reduce CHD risk independent of their effects on lipid levels(Reference Dong, Tong and Wu14, Reference Li, Liu and Bai15).
However, over the past decade, evidence in support of some of the proposed benefits of soyafoods has been less consistent than anticipated(Reference Balk, Chung and Chew16). Even the heart-health claim has recently been challenged(Reference Sacks, Lichtenstein and Van Horn17). Furthermore, there are concerns that soyafoods may adversely affect certain individuals. Many of the proposed benefits and most of the concerns(Reference Sirtori18, Reference Sirtori, Arnoldi and Johnson19) about soyafoods are attributed to their uniquely rich isoflavone content(Reference Murphy, Song and Buseman20). These diphenolic molecules are classified as phyto-oestrogens and in traditional soyafoods each gram of protein is associated with approximately 3·5 mg isoflavones (expressed in aglycone equivalents); consequently, one serving of a traditional soyafood contains about 25 mg isoflavones.
Most notable among the concerns is the fear that because of the oestrogen-like effects of isoflavones(Reference Tham, Gardner and Haskell21, Reference Setchell and McLachlan22), soyafoods may be harmful to women with a history of breast cancer and women at high risk of developing this disease(Reference Helferich, Andrade and Hoagland23). Consequently, many health professionals, uncertain about the benefits and concerned about the detrimental effects, are unsure how best to advise their female patients about the role of soyafoods in heart-healthy diets. Therefore, the purpose of this review is to critically evaluate the proposed coronary effects of soyafoods and to address concerns that soyafoods adversely affect breast tissue.
Effects of soya on circulating LDL-cholesterol levels
Direct effects
The cholesterol-lowering effect of soya protein was first demonstrated clinically in 1967(Reference Hodges, Krehl and Stone24), but not until 1995 did the hypocholesterolaemic effects of soya protein receive widespread recognition. In that year, a meta-analysis of the clinical data by Anderson et al. (Reference Anderson, Johnstone and Cook-Newell25), which included thirty-four trials and thirty-eight different comparisons, found that soya protein lowered LDL-cholesterol (LDL-C) by 12·9 %. The reduction in LDL-C was attributed to the protein or components (isoflavones) associated with soya protein and not to the low saturated fat content of the soyabean, since in nineteen trials the fatty acid content of the soya and control diets was similar. And 4 years later, on the basis of their own analysis, the US FDA approved a health claim for soyafoods and CHD(13). In the subsequent year, the American Heart Association (AHA) recognised the ability of soya protein to lower cholesterol and endorsed the use of soyafoods for people with elevated cholesterol levels(Reference Erdman26).
However, only 2 years following approval of the health claim, the US Adult Treatment Panel III of the National Cholesterol Education Program chose not to endorse the hypocholesterolaemic effects of soya protein, citing inconsistent data and the lack of dose–response information(27). Of greater significance, in their 2006 position paper, the AHA, while continuing to endorse the use of soyafoods for heart-healthy diets because of their ‘high content of polyunsaturated fats, fibre, vitamins and minerals, and low content of saturated fat … ’ concluded that earlier research indicating that ‘soya protein has clinically important favourable effects as compared to other proteins has not been confirmed’(Reference Sacks, Lichtenstein and Van Horn17). Furthermore, the FDA announced in December 2007 their intention to re-evaluate the evidence in support of the health claim, although there is no indication that this re-evaluation has been undertaken because the data are no longer supportive of the claim.
Given the gravitas of the AHA, and their pronounced change in sentiment about the efficacy of soya protein, it is instructive to consider the evidence upon which their reversal is based. Briefly, in an analysis of twenty-two studies, the AHA estimated that soya protein lowered LDL-C only by approximately 3 %(Reference Sacks, Lichtenstein and Van Horn17). In their view, this degree of reduction was insufficient to warrant a health claim. However, the AHA did not actually conduct a formal statistical meta-analysis of the data. When this was recently done, Jenkins et al. (Reference Jenkins, Mirrahimi and Srichaikul6) found that the AHA had underestimated the hypocholesterolaemic effects of soya protein. Rather than lowering LDL-C by approximately 3 %, Jenkins et al. (Reference Jenkins, Mirrahimi and Srichaikul6) estimated that soya protein lowered LDL-C by 4·3 %. Furthermore, when the analysis was limited to eleven of the twenty-two studies which provided evidence that the control and soya-containing diets were nutritionally matched, soya protein was found to lower LDL-C by 5·2 %. This estimate is in line with the results of other recently published meta-analyses(Reference Zhan and Ho28–Reference Anderson and Bush32) (Table 1) and is similar to the cholesterol-lowering effects of soluble fibre, which also has an FDA health claim(Reference Brown, Rosner and Willett33). Although the consensus estimate (4–6 %) of the magnitude of the hypocholesterolaemic effect of soya protein is much more modest than that initially reported by Anderson et al. (Reference Anderson, Johnstone and Cook-Newell25), the decrease is still relevant at both population and patient levels.
Table 1 Change (%) in circulating lipid levels in response to soya protein: results from recently published meta-analyses

↓ , Decrease; LDL-C, LDL-cholesterol; ↑ , increase; HDL-C, HDL-cholesterol; NR, not reported.
* Refers to subject numbers for LDL-C measurements.
† Refers to parallel studies only.
‡ 7·70 mg/l increase.
§ 62·60 mg/l decrease.
The FDA established 25 g/d soya protein as the threshold intake required to lower LDL-C because most trials used at least this much protein, not because lower amounts were shown not to be efficacious. There is, in fact, evidence suggesting that fewer than 25 g/d soya protein is needed to lower cholesterol(Reference Harland and Haffner29, Reference Messina34). Whether exceeding 25 g/d produces larger decreases in LDL-C is unclear. In the overall statistical model in the initial meta-analysis by Anderson et al. (Reference Anderson, Johnstone and Cook-Newell25), the amount of soya protein did not make an impact on cholesterol reduction although when adjusted for baseline cholesterol, it was estimated that 25 and 50 g/d soya protein decreased serum cholesterol concentrations by 89 and 174 mg/l, respectively. Zhan & Ho reported that LDL-C decreased to a larger extent in response to soya protein providing ≥ 80 mg/d isoflavones(Reference Zhan and Ho28). In agreement, Reynolds et al. (Reference Reynolds, Chin and Lees30) found significant correlations between the reduction in LDL-C and soya protein (r − 0·57, P < 0·001) and isoflavone (r − 0·48, P < 0·01) intake. However, Weggemans & Trautwein(Reference Weggemans and Trautwein31) found that there was no dose–response relationship between soya-associated isoflavones and changes in LDL-C. Furthermore, in the meta-regression analysis by Harland & Haffner(Reference Harland and Haffner29), no dose–response relationship between soya protein intake in the range of 15–40 g/d and LDL-C reduction was observed. This finding is particularly noteworthy because this range of soya protein intake more closely reflects the amounts likely to be consumed by free-living populations than the ranges in the other meta-analyses (Table 1). In support of Weggemans & Trautwein(Reference Weggemans and Trautwein31) and Harland & Haffner(Reference Harland and Haffner29), the results of the recent meta-analysis by Anderson & Bush(Reference Anderson and Bush32) also support the notion that neither dose of soya protein (range, 15–50 g/d) nor isoflavones (range, 22–185 mg/d) affect the LDL-C-lowering response.
Participants in the studies in the meta-analyses listed in Table 1 were roughly equally divided between men and women; however, there is some evidence indicating that there are sex differences in the response to soya protein. For example, Zhan & Ho(Reference Zhan and Ho28) found that LDL-C reductions in response to soya protein for men and women were − 0·30 and − 0·14 mmol/l, respectively, although it is not clear whether their analysis controlled for potentially confounding variables such as baseline cholesterol levels. Nevertheless, in agreement, Harland & Haffner(Reference Harland and Haffner29) found greater LDL-C reductions in men than in women.
Indirect effects
In addition to the direct hypocholesterolaemic effect of soya protein, as a result of differences in fatty acid content, Jenkins et al. (Reference Jenkins, Mirrahimi and Srichaikul6) using US National Health and Nutrition Examination Survey III population survey data, estimated that when soyafoods replace commonly consumed sources of animal protein in the diet, LDL-C is reduced by 3–6 %. There was a 4 % reduction in LDL-C when 24 g soya protein – an amount similar to the 25 g/d established by FDA as the threshold intake for cholesterol reduction – replaced a comparable amount of animal protein. Obviously, the displacement effect can vary greatly, as it depends entirely on the types of soyafoods consumed and the types of foods that are replaced.
As an oil seed, soyabeans derive approximately 40 % of their energy from fat, which is much higher than that for other legumes(Reference Messina35) with the exception of peanuts, another oil seed legume. The predominant fatty acid in soya oil, as in many vegetable oils, is the essential n-6 PUFA linoleic acid, which accounts for about 55 % of the total fat content(Reference Wu, Rodgers and Marshall3, Reference Slavin, Kenworthy and Yu36). The soyabean has a moderate amount of oleic acid (approximately 29 %) and a low amount of saturated fat (approximately 12 %). Importantly, unlike many other vegetable oils, soya oil is comprised of approximately 6 % α-linolenic acid, the essential n-3 fatty acid(Reference Wu, Rodgers and Marshall3, Reference Slavin, Kenworthy and Yu36).
Although conventional thinking has been that replacing cholesterol-raising saturated fats with n-6 PUFA will lower cholesterol and CHD risk(Reference Calder37), a recent comprehensive analysis of the clinical data found that to lower CHD, saturated fat needs to be replaced with a mix of n-6 and n-3 PUFA(Reference Ramsden, Hibbeln and Majchrzak38). In fact, according to Ramsden et al. (Reference Ramsden, Hibbeln and Majchrzak38), replacement of saturated fat with n-6 PUFA increases risk(Reference Ramsden, Hibbeln and Majchrzak38). Replacing saturated fat with refined carbohydrate also increases risk, whereas the effect of replacement with monounsaturated fat is unclear(Reference Ramsden, Hibbeln and Majchrzak38–Reference Astrup, Dyerberg and Elwood41). Therefore, the fatty acid profile of soyabeans would appear to be ideally suited for lowering blood cholesterol. On the other hand, the high n-6:n-3 fatty acid ratio (approximately 9:1) has led to concern that soyafoods might elicit a pro-inflammatory response. However, this fear does not appear to be justified(Reference Harris, Mozaffarian and Rimm42).
Linoleic acid is converted in vivo to arachidonic acid, the fatty acid from which pro-inflammatory eicosanoids are produced; however, plasma and tissue levels of arachidonic acid are very tightly regulated(Reference Rett and Whelan43). Further, it is now recognised that some eicosanoids produced from arachidonic acid are anti-inflammatory and possess other desirable attributes(Reference Calder44). To this point, in a cross-sectional study of 915 men aged 40–49 years, both serum linoleic acid and arachidonic acid were found to be inversely related to plasma levels of plasminogen activator inhibitor-1. Because plasminogen activator inhibitor-1 is a primary inhibitor of plasminogen activators, it has an anti-fibrinolytic function.
Finally, given the desirable fatty acid profile of and high-quality protein(Reference Rand, Pellett and Young1, Reference Hughes, Ryan and Mukherjea2) provided by soyafoods, it is not surprising that in research diets shown to dramatically lower blood LDL-C levels, soyafoods have played an important role(Reference Jenkins, Kendall and Faulkner45, Reference Jenkins, Kendall and Marchie46). If one assumes that there is a 4 % reduction in LDL-C resulting from a direct effect of consuming 25 g/d soya protein and a 4 % reduction due to displacement effects, in theory, CHD risk will be reduced by 8–16 % when soyafoods replace conventional sources of protein in Western diets(Reference Law, Wald and Thompson47, Reference Law, Wald and Wu48). Obviously, only long-term clinical trials can definitively show that incorporating soyafoods into the diet reduces coronary events.
Beyond effects on LDL-cholesterol levels
In addition to the effects on LDL-C, meta-analyses have found that soya protein modestly increases (1–3 %) fasting HDL-cholesterol and decreases (5–11 %) TAG levels (Table 1). Each 1 % or 1 mg increase in HDL-cholesterol lowers CHD risk by 2–3 %(Reference Boden49–Reference Grover, Kaouache and Joseph51). Although there is debate about whether an elevated TAG level is an independent predictor of CHD risk(Reference Cullen52), evidence suggests that the role of fasting TAG levels in the aetiology of CHD may be underestimated(Reference Bansal, Buring and Rifai53, Reference Nordestgaard, Benn and Schnohr54). Furthermore, new research suggests that soya protein decreases postprandial TAG levels, elevated levels of which are increasingly viewed as an important CHD risk factor(Reference Santo, Santo and Browne55).
There is also clinical evidence that soyafoods exert coronary benefits independent of their effect on lipid levels. For example, four meta-analyses have found that soya lowers blood pressure(Reference Dong, Tong and Wu14, Reference Liu, Li and Chen56–Reference Taku, Lin and Cai58). The most recently published and comprehensive of these, which included twenty-seven studies, reported that soya lowered systolic and diastolic blood pressure by 2·2 and 1·3 mmHg, respectively(Reference Dong, Tong and Wu14). Decreases in blood pressure of this magnitude can be estimated to reduce risk of stroke, CHD and overall mortality by approximately 10, 5 and 4 %, respectively(Reference McInnes59). There are suggestive data that the hypotensive effects are more pronounced in women than in men(Reference Liu, Li and Chen56) and that traditional soyafoods have a greater impact than isolated soya protein (ISP, which by definition is ≥ 90 % protein)(Reference Hooper, Kroon and Rimm57). However, until the blood pressure-lowering effects of soya are demonstrated in more trials whose primary health outcome is a change in blood pressure, the hypotensive effects of soya should be considered speculative. Work identifying the component(s) responsible for the proposed hypotensive effects is also needed; evidence suggests that both the protein (via peptides formed upon digestion)(Reference Wu and Muir60, Reference Yang, Yang and Chen61) and isoflavone content(Reference Harada and Okajima62, Reference Bitto, Altavilla and Bonaiuto63) of soya may be contributing factors.
Other biological processes and measures related to heart disease that may be favourably affected by various soya components include endothelial function, systematic arterial compliance(Reference Nestel, Yamashita and Sasahara64), arterial stiffness(Reference Hoshida, Miki and Nakagawa65), LDL-C oxidation(Reference Gardner-Thorpe, O'Hagen and Young66) and LDL particle size(Reference Allen, Becker and Kwiterovich67, Reference Desroches, Mauger and Ausman68). However, because of the inconsistent and/or limited data, no conclusions can be made about the effects of soya on these CHD markers except for endothelial function. Endothelial cells in the intima layer of blood vessels are thought to play a central role in inhibiting the development of arteriosclerosis and its thrombotic consequences. For this reason, endothelial function has been referred to as a global indicator of CHD risk(Reference Bonetti, Lerman and Lerman69). The health of the endothelium and factors affecting it can be non-invasively assessed by measuring flow-mediated dilatation (FMD), using ultrasound imaging of the brachial artery.
A recent meta-analysis by Li et al. (Reference Li, Liu and Bai15) that included nine studies and 525 postmenopausal women found that soyabean isoflavones favourably improved endothelial function. However, when the data were sub-analysed, the improvement was seen only in those women with impaired endothelial function at baseline. More specifically, in women with an age-adjusted baseline FMD value < 5·2 %, isoflavone administration resulted in a significant increase (weighted mean difference, 2·22; P = 0·0001) in FMD, whereas in women with a baseline FMD ≥ 5·2 %, there was no benefit (weighted mean difference, 0·24, P = 0·87). A second review of the clinical data also found that isoflavones improve endothelial function(Reference Beavers, Beavers and Miller70). Experimental studies indicate that isoflavones can stimulate the production of NO via oestrogen receptor (ER)–mediated activation of endothelial NO synthase(Reference Squadrito, Altavilla and Squadrito71). However, it may also be that in the long term, isoflavones improve endothelial function by increasing the circulating levels of endothelial progenitor cells, which replace damaged endothelial cells(Reference Chan, Lam and Lau72).
Lack of correction for baseline FMD provides an explanation for the lack of consistency in the literature in this area. Also, although the meta-analysis by Li et al. (Reference Li, Liu and Bai15) showing that isoflavones improve FMD included only studies involving postmenopausal women, there is evidence, albeit limited, suggesting that isoflavones benefit premenopausal women. For example, recently, Hoshida et al. (Reference Hoshida, Miki and Nakagawa73) found that isoflavones (50 mg/d) increased FMD in both pre- (P = 0·004) and postmenopausal (P = 0·019) non-smokers. Furthermore, isoflavones reduced arterial wall stiffness in both premenopausal smokers (P = 0·027) and non-smokers (P = 0·013), while having no effect on postmenopausal women.
Finally, Hodis et al. (Reference Hodis, Mack and Kono74) recently examined the effects of isoflavone-rich ISP on the progression of carotid intima-media thickness (CIMT) in postmenopausal women over a 3-year period. Participants consumed either 25 g/d ISP containing 99 mg isoflavones (n 163) or 25 g milk protein (n 162). At the end of the study, although CIMT progression was 16 % lower in the soya group, the difference was not statistically significant (P = 0·35). Nevertheless, if progression reflects future risk of coronary events, this potential reduction should not be dismissed, especially because the results showed that differences between groups increased with time, suggesting that longer exposure to soya would result in an increased benefit. Furthermore, when the data were sub-analysed, it was found that in comparison to the control, progression was reduced among women in the soya group who were < 5, 5–10, and >10 years post-menopause by 68 % (P = 0·05), 17 % (P = 0·51) and 9 % (P = 0·77), respectively. That the greatest reduction or benefit was seen in early postmenopausal women is consistent with the oestrogen window or timing hypothesis, which maintains that exposure to oestrogen-like compounds early but not late in menopause produces coronary and cognitive benefits(Reference Hodis and Mack75, Reference Sherwin76). Given that the women in the soya group were exposed to high amounts of isoflavones, the oestrogen-timing hypothesis provides a biological basis for such pronounced benefits being observed in early postmenopausal women.
The breast cancer controversy
Introduction
Concerns that soyafoods are contraindicated for women with a history of breast cancer and women at high risk of developing this disease (see Table 2 for a summary of this controversy) are based on the oestrogen-like effects (see next section) of isoflavones and a series of studies conducted primarily by the laboratory of William Helferich at the University of Illinois, which show that isoflavones and isoflavone-containing soya products stimulate the growth of existing mammary tumours in athymic ovariectomised mice implanted with MCF7 cells(Reference Helferich, Andrade and Hoagland23). These cells are derived from a human oestrogen-sensitive breast cancer cell line(Reference Helferich, Andrade and Hoagland23). Data from this model also show that it is the isoflavone genistein that is responsible for tumour stimulation(Reference Ju, Fultz and Allred77) and that this isoflavone inhibits the efficacy of tamoxifen(Reference Ju, Doerge and Allred78) and the aromatase inhibitor letrozole(Reference Ju, Doerge and Woodling79). However, a recently published study by Onoda et al. (Reference Onoda, Ueno and Uchiyama80) failed to confirm the tumour-stimulatory effect of genistein despite the use of an almost identical model. Onoda et al. (Reference Onoda, Ueno and Uchiyama80) noted that in their study before implantation, the MCF-7 cells were cultured in an oestrogen-free environment, whereas in the studies in which tumour stimulation occurred, the cells were exposed to a high concentration of oestrogen (1 nm) before implantation. According to Onoda et al. (Reference Onoda, Ueno and Uchiyama80), this high oestrogen concentration is unphysiological and makes the cells hypersensitive to oestrogenic stimuli. In any event, recent work by Setchell et al. (Reference Setchell, Brown and Zhao81) suggests that because of differences in isoflavone metabolism between nude mice and humans, the former may not be an appropriate model for predicting effects in the latter.
Table 2 Summary of key findings relevant to the soya and breast cancer controversy*
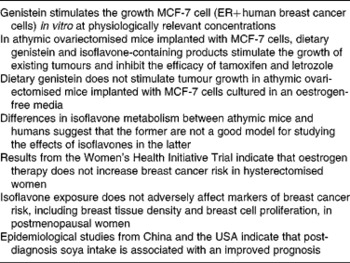
ER, oestrogen receptor.
* See text for references and study details.
Brief overview of isoflavones
The three soyabean isoflavones, genistein, daidzein and glycitein, and their respective glycosides account for approximately 50, 40 and 10 %, respectively, of total soyabean isoflavone content(Reference Murphy, Barua and Hauck82). In Japan, mean isoflavone intake ranges from about 30 to 50 mg/d (expressed as aglycone equivalents)(Reference Messina, Nagata and Wu83). In considering their possible impact on breast tissue, it is important to recognise that although isoflavones are classified as phyto-oestrogens, they are more accurately classified as selective ER modulators (SERM), a group which includes the breast cancer drug tamoxifen and the osteoporosis drug raloxifene(Reference Oseni, Patel and Pyle84). SERM have tissue-selective effects; depending upon the tissue in question, SERM can have oestrogenic effects, anti-oestrogenic effects or no effects at all on tissues affected by oestrogen. This will depend on the types and ratios of ER, the shape of the ligand–receptor complex and the types of co-activators and co-repressors in cells in a particular tissue(Reference Pike85). The tissue selectivity of isoflavones probably derives from their preferential binding to and transactivation of ERβ in comparison to the ERα(Reference Oseni, Patel and Pyle84, Reference Reiter, Beck and Medjakovic86). These two receptors have different tissue distributions in the body and different functions. This is particularly evident in the breast where the activation of ERβ is thought to inhibit the stimulatory and proliferative effects of ERα activation(Reference Speirs, Carder and Lane87).
Although limited, there is considerable evidence supporting the classification of isoflavones as SERM. For example, Carmignani et al. (Reference Carmignani, Pedro and Costa-Paiva88) found that the vasomotor symptom score of postmenopausal women was decreased over a 16-week period by approximately 33, 54 and 55 % in response to a placebo, soya protein containing 53 mg isoflavones, and hormone therapy (HT, oestrogen plus progestin), respectively. However, only HT increased the vaginal maturation index ( < 0·01), a measure of the extent to which the vaginal tissue has been exposed to oestrogenic molecules. Thus, the oestrogen-like effects of isoflavones were apparently sufficient to alleviate menopausal symptoms in a manner similar to HT but unlike HT, isoflavones did not exert an oestrogenic effect on the vagina.
Isoflavones, oestrogen and breast cancer: clinical evidence
It has been postulated that in the high-oestrogen environment of premenopausal women, isoflavones function as oestrogen antagonists and potentially decrease breast cancer risk, whereas in the low-oestrogen environment of postmenopausal women, they function as oestrogen agonists, and increase risk(Reference Bondesson and Gustafsson89, Reference Penttinen-Damdimopoulou, Power and Hurmerinta90). However, although circulating oestradiol levels are 4-fold higher in pre- compared to postmenopausal women, breast tissue oestrogen concentrations are similar(Reference Pasqualini, Chetrite and Blacker91, Reference Geisler92) because of the uptake of oestrogen by the breast from the circulation(Reference Haynes, Straume and Geisler93). Thus, the notion that the effect of isoflavones on breast tissue differs according to the oestrogenic environment may not be physiologically relevant. Furthermore, in the Women's Health Initiative trial, although oestrogen-plus-progestin use increased breast cancer incidence(94) and mortality(Reference Chlebowski, Anderson and Gass95), in hysterectomised women, oestrogen-only use actually decreased risk(Reference Anderson, Limacher and Assaf96, Reference Stefanick, Anderson and Margolis97) and the protective effects were maintained for several years after cessation of therapy(Reference LaCroix, Chlebowski and Manson98). One caveat, however, is that the most recent analysis from the Women's Health Initiative trial suggests that the protective effects of oestrogen may not apply to women at increased risk of breast cancer(Reference Anderson, Chlebowski and Aragaki99).
These findings from the Women's Health Initiative trial are generally supported by the existing epidemiological(Reference Warren100–Reference Chen, Manson and Hankinson104) and clinical data(Reference Greendale, Reboussin and Slone105–Reference Conner, Soderqvist and Skoog108). Granted, the extent to which the findings in women without a uterus can be applied to intact women is unclear; however, there is no reason why women without a uterus should be more or less sensitive to oestrogens. It is true that hysterectomy, even if the ovaries are not removed, may be associated with an earlier endocrine menopause. Such women are therefore more likely to resemble the postmenopausal state and yet are protected by oestrogen therapy.
Clinical studies in which the effects of isoflavone exposure on markers of breast cancer risk have been evaluated are supportive of safety regardless of whether isoflavones are derived from soyafoods or supplements(Reference Messina and Wood109). Higher circulating oestrogen levels, greater breast tissue density and greater in vivo breast cell proliferation, are seen as reflecting an increased cancer risk. To this point, tamoxifen decreases cell proliferation and breast tissue density and breast cancer risk, whereas HT increases these markers and breast cancer risk(Reference Conner, Skoog and Soderqvist107, Reference Conner, Soderqvist and Skoog108, Reference Boyd, Martin and Li110–Reference Conner112). In contrast to the effects of HT, isoflavones generally have no effect on any of these previously referred to markers(Reference Hooper, Ryder and Kurzer113, Reference Huber, Imhof and Schmidt114). One exception is a recently published 6-month study that found in response to very high-dose isoflavone supplementation (154 mg/d genistein), breast cell proliferation increased slightly in premenopausal women, although there were no effects on cell cytology and no effects at all in postmenopausal women, the latter group according to theory, being most vulnerable to isoflavones(Reference Khan, Chatterton and Michel115). Further, the approximate 27 % increase in proliferation observed in premenopausal women is more than an order of magnitude less than the approximately 4- to 10-fold increased breast cell proliferation that occurs in response to HT(Reference Conner, Soderqvist and Skoog108, Reference Murkes, Conner and Leifland116).
Finally, it is worth noting that the clinical findings discussed previously, although supportive of safety, also argue against adult soya intake reducing breast cancer risk as was first proposed more than 20 years ago(Reference Messina and Barnes117–Reference Setchell, Borriello and Hulme119), even though epidemiological data show that among Asian women soya intake is associated with protection against breast cancer(Reference Wu, Yu and Tseng4). Compelling, but still speculative, evidence indicates that to derive protection against breast cancer, soya intake must occur during childhood and/or adolescence(Reference Messina and Hilakivi-Clarke9, Reference Messina and Wu10).
Soya intake and the prognosis of breast cancer patients: epidemiology
Despite the lack of effect of isoflavone exposure on breast tissue, recent epidemiological evidence indicates that soya intake benefits breast cancer patients. Over the past 6 years, six prospective epidemiological studies, three from China(Reference Boyapati, Shu and Ruan120–Reference Kang, Ansbacher and Hammoud122), two from the USA(Reference Guha, Kwan and Quesenberry123, Reference Caan, Natarajan and Parker124) and one from Korea(Reference Woo, Park and Ro125), have evaluated the impact of soya consumption on the prognosis of breast cancer patients. One of these showed there to be no relationship between soya intake and disease-free survival(Reference Boyapati, Shu and Ruan120), four showed that soya intake improved prognosis(Reference Shu, Zheng and Cai121, Reference Guha, Kwan and Quesenberry123, Reference Caan, Natarajan and Parker124, Reference Kang, Zhang and Wang126), whereas the Korean study found that the impact of soya depended upon the type of breast cancer in question(Reference Woo, Park and Ro125). That study(Reference Woo, Park and Ro125) and the largest and longest Chinese(Reference Shu, Zheng and Cai121) and US(Reference Caan, Natarajan and Parker124) studies are briefly described next. When considering the Asian studies, it is worth noting that an argument has been made on the basis of animal studies, that breast tumours that develop in women who consumed soya when young may be different from and respond differently to tamoxifen, than breast tumours in women who did not consume soya early in life(Reference Hilakivi-Clarke, Andrade and Helferich127).
The Shanghai Breast Cancer Survival Study is a population-based cohort study that includes more than 5000 breast cancer survivors(Reference Shu, Zheng and Cai121). During the median follow-up period of approximately 3·9 years, the hazard ratio (HR) plus 95 % CI associated with the highest soya protein intake quartile was 0·71 (95 % CI 0·54, 0·92) for total mortality and 0·68 (95 % CI 0·54, 0·87) for recurrence compared with the lowest intake quartile. Similar protective effects were associated with isoflavone intake. Soya intake was assessed at 6, 18, 36 and 60 months post enrolment and the fourth quartile intake cut-offs for soya protein and isoflavones were >15·31 g/d and >62·68 mg/d, respectively. Soya intake was protective in both pre- and postmenopausal women.
The US study by Caan et al. (Reference Caan, Natarajan and Parker124) involved 2736 breast cancer survivors diagnosed between 1991 and 2000 with early-stage breast cancer, who were participants in the Women's Healthy Eating and Living study. During the median 7·3 years follow-up period, there were 448 new breast cancer events and 271 deaths. The results showed that as isoflavone intake increased, risk of death decreased. Women in the highest isoflavone intake category (cut-off, >16·3 mg/d; median 26·7 mg/d) had a 54 % reduction in risk of death (HR 0·46; 95 % CI 0·2, 1·05; P for trend = 0·02). The benefits of isoflavone intake were most obvious in tamoxifen users (HR 0·26; 95 % CI 0·0, 1·08; P for trend = 0·05). This finding is consistent with the observation that in the prospective Chinese epidemiological study by Kang et al. (Reference Kang, Zhang and Wang126), soya intake improved the efficacy of the aromatase inhibitor anastrozole. The tamoxifen and anastrozole findings are especially noteworthy not only because of the possible implications for breast cancer patients on treatment, but because in ovariectomised athymic mice, genistein was shown to inhibit the efficacy of these drugs(Reference Ju, Doerge and Allred78, Reference Ju, Doerge and Woodling79). Furthermore, both Chinese and US prospective studies show benefits to breast cancer patients of post-diagnosis soya intake, including those treated with tamoxifen. These findings help to address the concern that women who consume soya early in life develop tumours that differ from those who do not.
Finally, in a Korean prospective study by Woo et al. (Reference Woo, Park and Ro125), among the 339 women aged 25–77 years, during the median follow-up period of 32·6 months (range 1·3–52·4 months), there were twenty-five recurrences. The adjusted HR for women in the third isoflavone intake tertile (cut-off, ≥ 14·6 mg/d) was 0·23 (P for trend, 0·01) for women with epidermal growth factor receptor-2 negative (HER2 − ) breast cancer, whereas for women with HER2+ breast cancer the adjusted HR (cut-off, ≥ 15·2 mg/d) was 3·85 (P for trend, 0·17). Only approximately 25 % of breast cancers are HER2+, but this type of breast cancer is more aggressive and is associated with a worse prognosis(Reference Hicks and Kulkarni128). Although these findings raise a cautionary note, no conclusions can be made on the basis of this study because of its limited size, as there were only eight recurrences among the eighty-three HER2+ breast cancer patients. The impact of post-diagnosis soya intake in HER2+ breast cancer patients in a much larger cohort is currently being evaluated.
Summary and conclusions
Soyafoods hold the potential to reduce risk of CHD through multiple mechanisms. Soya protein modestly lowers blood LDL-C(Reference Jenkins, Mirrahimi and Srichaikul6, Reference Zhan and Ho28–Reference Anderson and Bush32) and the isoflavones associated with soya protein improve endothelial function in postmenopausal women(Reference Li, Liu and Bai15). In addition, soyafoods provide high-quality protein(Reference Rand, Pellett and Young1, Reference Hughes, Ryan and Mukherjea2) and have a very favourable fatty acid profile(Reference Wu, Rodgers and Marshall3); consequently, they can be used to displace conventional sources of protein in Western diets which tend to be high-cholesterol and high-saturated-fat animal products(Reference Jenkins, Mirrahimi and Srichaikul6). As a result, blood lipid levels will be favourably affected. There is also intriguing evidence that soyafoods modestly lower blood pressure(Reference Dong, Tong and Wu129) and favourably affect other CHD risk factors. Recent 3-year data suggest that in postmenopausal women, and especially women early in menopause, soya, probably because it contains isoflavones, may slow down the progression of CIMT(Reference Hodis, Mack and Kono130). Nevertheless, soyafoods are controversial because of concerns that the oestrogen-like effects of isoflavones may harm women with a history of breast cancer and women at high risk of developing this disease. However, in contrast to some animal data, clinical evidence indicates that isoflavone exposure does not adversely affect the markers of breast cancer risk, including breast tissue density and breast cell proliferation(Reference Hooper, Ryder and Kurzer113, Reference Huber, Imhof and Schmidt114), and both Chinese and US prospective epidemiological studies indicate that post-diagnosis soyafood consumption improves the prognosis of breast cancer patients(Reference Boyapati, Shu and Ruan120, Reference Shu, Zheng and Cai121, Reference Guha, Kwan and Quesenberry123, Reference Kang, Zhang and Wang126, Reference Caan, Natarajan and Parker131). In the light of these findings, there is no reason to advise health professionals against recommending soyafoods to their patients as part of heart-healthy diets with or without a history of breast cancer. Women with a history of breast cancer will live for many years without succumbing to their disease; and so it is imperative that they recognise the need to adhere to dietary patterns that are protective against a variety of chronic diseases. The consumption of two-to-four servings of soyafoods daily provides the amounts of soya protein and isoflavones associated with the proposed benefits discussed in this review.
Acknowledgements
Each of the authors participated in the writing of this paper and the review of literature discussed. No funding was involved in the writing of this manuscript. M. M. is the executive director of the Soy Nutrition Institute and regularly consults for companies that manufacture and/or sell soyafoods and/or soya supplements. V. M. has no conflicts of interest. D. J. A. J. is on the scientific advisory board of a soyafood company.




