Pancreatic adenocarcinoma is a highly malignant tumours of the digestive system with an extremely poor prognosis and an average 5-year survival rate of approximately 10 %(Reference Lin, Yang and Jin1,Reference Chen, Li and Sun2) . As one of the leading causes of cancer death worldwide, the incidence of pancreatic adenocarcinoma has increased approximately twice than in 1996(Reference Rawla, Sunkara and Gaduputi3,Reference Siegel, Miller and Jemal4) . It is predicted that by 2030, pancreatic adenocarcinoma will surpass breast, prostate and colorectal cancer as the second leading cause of cancer-related death in the USA(Reference Rahib, Smith and Aizenberg5). Therefore, it is critical to prevent pancreatic adenocarcinoma.
Tea is one of the most widely consumed beverages in the world. Tea polyphenols (mainly including epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG) and epicallocatechin gallate (EGCG)) are the main components of tea that are effective against cancer(Reference Farhan6). Experimental studies have shown that tea is able to exert anticancer effects through antioxidant activity(Reference Zheng, Ryu and Kwon7,Reference Lambert and Elias8) , cell cycle regulation(Reference Shankar, Ganapathy and Hingorani9–Reference Shimizu, Deguchi and Lim11), the inhibition of receptor tyrosine kinase pathway, immune system modulation and control of epigenetic modification, and others(Reference Shirakami and Shimizu12). Epidemiological studies have found that tea intake reduces the risk of developing a variety of malignant tumours (such as liver cancer, colon cancer, oral cavity cancer, and oesophageal cancer)(Reference Huang, Lu and Min13–Reference Ren, Freedman and Kamangar15). Tea extract may inhibit pancreatic adenocarcinoma through multiple pathways in most laboratory studies(Reference Suhail, Rehan and Tarique16). However, epidemiological studies remain controversial, and most of the meta-analysis results do not support the preventive effect of tea on pancreatic adenocarcinoma(Reference Abe and Inoue17,Reference Wei, Chen and Zhu18) . Blasiak et al. found that green tea extract decreased the levels of AP-1 transcription factor subunits and inhibited the VEGF-A mRNA levels(Reference Blasiak, Chojnacki and Szczepanska19). Yang et al. found that EGCG treatment targeted HIF-1α and thereby suppressed VEGF-A activation(Reference Yang and Li20). Shimizu et al. suggested that EGCG might inhibit the activation of the VEGF/VEGFR axis by inhibiting the expression of HIF-1α and several major growth factors(Reference Shimizu, Shirakami and Sakai21).
Angiogenesis is a crucial process for tumour survival(Reference Shaw, Dwivedi and Bhattacharya22). Vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) are able to promote tumorigenesis by multiple pathways, such as inhibiting the tumour immune microenvironment and promoting tumour angiogenesis(Reference Shimizu, Shirakami and Sakai21–Reference Zhang, Yao and Gao24). In pancreatic cancer, VEGF is able to act as a common pathway for multiple mechanisms to promote pancreatic carcinogenesis(Reference Hao, Ji and Pan25–Reference Huang, Li and Xu27).
Traditional epidemiological studies are often biased due to the presence of multiple confounding factors. Mendelian randomisation (MR) analyses used genetic variation associated with risk factors as instrumental variables (IV) to assess whether there is a causal effect or whether there is a spurious association on disease outcome due to reverse causality in an observational setting(Reference Sekula, Del Greco and Pattaro28). Therefore, this study investigated the causal relationship between tea intake and pancreatic adenocarcinoma risk through a two-sample study and further explored mediating effect by two-step MR analysis.
Materials and methods
Data collection
The tea intake-related dataset (ukb-b-6066) which included 9 851 867 SNP and 447 485 individual samples was downloaded from UK Biobank. The mean and standard deviation values for the dataset were 3·49631 and 2·84 255 cup/d, respectively. Types of tea mainly include black tea and green tea. The data pertaining to tea intake were sourced from questionnaires, which contained inquiry ‘How many cups of tea do you drink each day? (Include black and green tea)’ with a range of 0–99 cups. Participants were instructed to provide their average intake over the past year. The pancreatic adenocarcinoma-related dataset (bbj-a-140) which included 8 885 075 SNP and 196 187 individual samples was downloaded from Biobank Japan. The VEGF-A (prot-b-22) and VEGF-D (prot-b-65) were obtained from the genome-wide association studies data determined by Folkersen L et al. (Reference Folkersen, Fauman and Sabater-Lleal29) These datasets included 5 270 646 SNP and 3394 individual samples of European. The VEGF-A isoform 121 (prot-a-3197), VEGF-C (prot-a-3199), VEGFR-2 (prot-a-1622) and VEGFR-3 (prot-a-1129) were obtained from the genome-wide association studies data determined by Sun BB et al. (Reference Sun, Maranville and Peters30) The flow chart of our study and the methodology with relation to the overall exposure–disease association and the mediating factors is shown in Fig. 1.
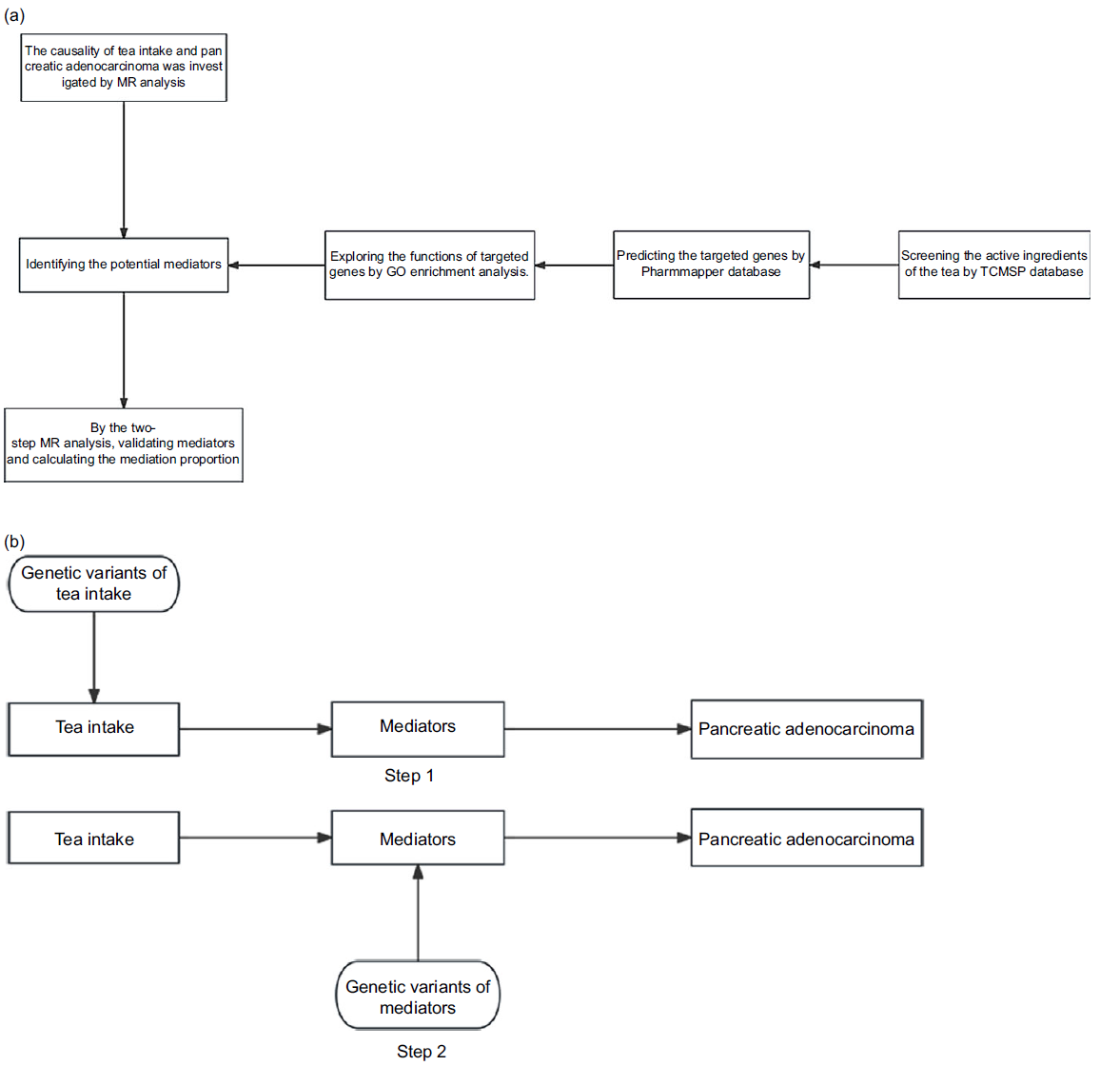
Fig. 1. Flow charts: (a) flow chart of the study procedures; (b) flow chart of the two-step Mendelian randomisation analysis. Step 1: Genetic variant of tea intake is used as an instrument for the exposure of tea intake to estimate the causal impact of the exposure on mediators (identified by pharmacological analysis) of the association between the tea intake and the risk of pancreatic adenocarcinoma; step 2: Genetic variant of mediators is used as an instrument for the mediators to establish the causal impact of the mediators on the risk of pancreatic adenocarcinoma (causal effect of tea intake on the risk of pancreatic adenocarcinoma (total effects) = beta0; causal effect of tea intake on the mediators = beta1; causal effect of the mediators on the risk of pancreatic adenocarcinoma = beta2; mediating effects = beta1 × beta2; direct effects = beta0-beta1 × beta2). MR, Mendelian randomisation; GO, gene ontology.
Selection of instrumental variables
The IV in this study was selected by the following criteria: (1) IV were strongly correlated with the exposure: we used the ‘P < 5e-08’ of the correlation as the criterion for the SNP selection. If the number of SNP IV did not meet the analysis requirements, the standard was relaxed to ‘P < 1e-05’ based on previous research experience(Reference Liang and Fan31,Reference Wootton, Lawn and Millard32) . (2) Exclusion of IV with linkage disequilibrium: the criteria was ‘linkage disequilibrium (LD) r2 < 0·001 within a 10 000 kb distance’. (3) Exclusion of IV associated with confounding factors: we removed SNP associated with confounding factors according to Phenoscanner (http://www.phenoscanner.medschl.cam.ac.uk/). (4) Exclusion of weak IV with F-value < 10 (F-value indicates the association strength magnitude, and the calculation formula: F = (N-2) * (2 * ((beta)^2) * eaf * (1-eaf))/(1-(2 * ((beta)^2) * eaf * (1-eaf)), beta: the effect size; eaf: the effect allele frequency).
Identifying potential mediator
Fig. 1(a) shows that mediators were selected for consideration in analysis. By TCMSP database (https://old.tcmsp-e.com/tcmsp.php), we obtained oral bioavailability (OB) and drug-likeness (DL) for the main ingredients of tea (EC, EGC, ECG and EGCG). ‘OB ≥ 30 %’ and ‘DL ≥ 0·18’ were the criterion for active ingredients of the tea.
We obtained the structure model of the main active ingredients of the tea by Pubchem database (https://pubchem.ncbi.nlm.nih.gov/). According to the structure model of the main active ingredients of the tea, we predicted the targeted genes by Pharmmapper database (http://lilab-ecust.cn/pharmmapper/index.html).
Through the gene ontology (GO) enrichment analysis, we explored the functions of targeted genes. Based on the results of GO enrichment analysis, we predicted the potential mediator.
Analysis methods
In this study, we used five MR analysis methods (MR-Egger, Weighted median, Inverse variance weighted (IVW), Simple mode and Weighted mode) to determine the relationship between exposure and outcome. IVW was considered as the main method of MR in the study and through multiple methods to verify the consistency of the results(Reference Ma, Chen and Liu33). MR-Egger and IVW of Cochran Q assess heterogeneity. The intercept of MR-Egger regression is to appraise for pleiotropy. Leave-one-out method determined that the causal relationship between exposure and outcome was not affected by a single SNP. All the analyses were completed using R 4.1.1 software, and the program package adopted included ‘TwoSampleMR’, ‘gwasglue’ and ‘MRPRESSO’. The ‘P < 0·05’ was considered statistically significant.
Results
The causal effect of tea consumption on pancreatic adenocarcinoma
Using the ukb-b-6066 dataset, we obtained 4022 SNP that are strongly related to tea consumption (P < 5e-08). After excluding SNP with linkage disequilibrium, forty-one SNP were remained. Subsequently, we eliminated nine SNP (rs1453548, rs2472297, rs9302428, rs132904, rs10741694, rs9937354, rs4808940, rs57631352 and rs11587444) associated with confounding factors (diabetes, smoke and anticancer immunisation). Due to the presence of palindromic sequences, five SNP (rs11164870, rs2273447, rs2783129, rs56348300 and rs713598) were also removed. According to Poisson test, nine SNP (rs2351187, rs1481012, rs17245213, rs17576658, rs2117137, rs2478875, rs72797284, rs12591786 and rs34619) which are significant different between ukb-b-6066 and bbj-a-140 were additionally deleted (P < 0·05). Finally, we selected sixteen SNP as IV for subsequent two-sample MR analysis (effect of tea consumption on pancreatic adenocarcinoma).
The results of two-sample MR analysis showed that genetically predicted increased tea consumption was associated with a decreased risk of pancreatic adenocarcinoma (IVW: OR: 0·111 (0·014, 0·853), P = 0·033, Fig. 2(a)). The results of five methods of MR analysis (MR-Egger: OR: 0·493 (0·003, 77·037); Weighted median: OR: 0·133 (0·008, 2·129); IVW: OR: 0·111 (0·014, 0·853); Simple mode: OR: 0·036 (0·001, 2·430); Weighted mode: OR: 0·115 (0·007, 1·846)) are consistent (Fig. 2(a)). The effect size of each SNP of tea consumption on pancreatic adenocarcinoma is shown in Fig. 2(b). Besides, the Cochran’s Q statistics of MR-Egger and IVW (MR-Egger: Q = 15·057, P = 0·374; IVW: Q = 15·490, P = 0·417) did not show a significant heterogeneity. There was no significant intercept (intercept = –0·032; se = 0·050. P = 0·536), indicating the absence of directional pleiotropy in statistics (Fig. 2(c)). We iteratively removed one SNP and performed IVW using the remaining SNP and did not observe significantly outlying SNP (Fig. 2(d)).
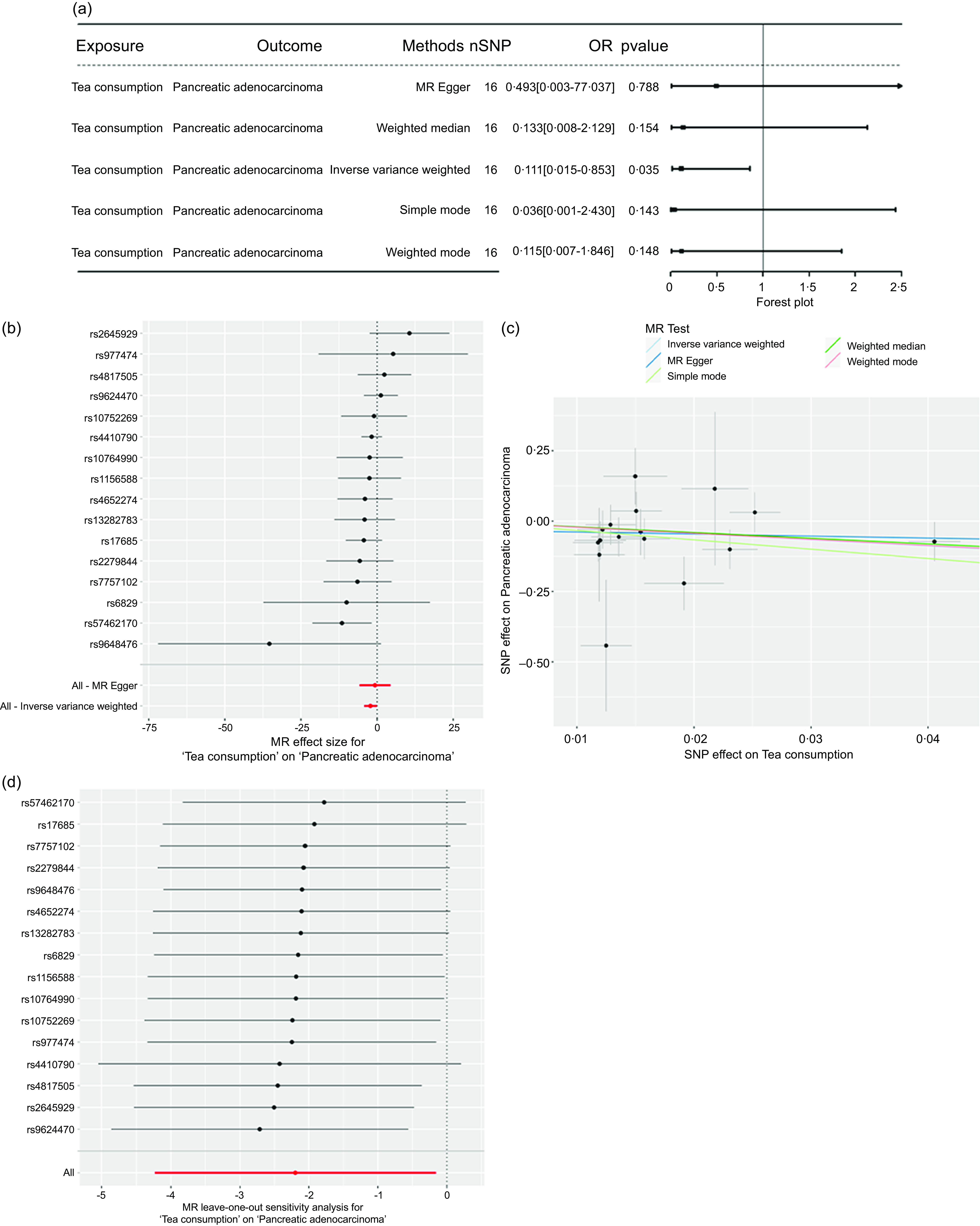
Fig. 2. The causal effect of tea consumption on pancreatic adenocarcinoma: (a) genetically predicted increased tea consumption was associated with a decreased risk of pancreatic adenocarcinoma; (b) the effect size of each SNP for tea consumption on pancreatic adenocarcinoma; (c) there was statistically no directional pleiotropy; and (d) there was no apparent outlying SNP.
Level of vascular endothelial growth factor and vascular endothelial growth factor receptor were potential mediator
To explore the potential mediators, we used pharmacological analysis to predict the targeted gene functions of the main active components in tea. By using the TCMSP database, we obtained the OB and DL values for EC, EGC, and ECG and EGCG (Table 1). The ent-Epicatechin and (-) -Epigallocatechin-3-gallate were used as the main active components of tea for the next analysis (OB > 30 %, DL > 0·18). Through the Pubchem database, we obtained the molecular structure of ent-Epicatechin and (-) -Epigallocatechin-3-gallate. We predicted the targeted genes of ent-Epicatechin and (-) -Epigallocatechin-3-gallate by Pharmmapper database, and then a total of seventy-three targeted genes for ent-Epicatechin and seventy-one targeted genes for (-) -Epigallocatechin-3-gallate were obtained. We identified a total of fifteen representative targeted genes (ACADM, AZGP1, CCNE1, GAN, NAT1, NR3C2, PDK2, PRKD2, RARG, RUVBL1, SPEN, SUOX, USP19, VEGFA and VEGFB) which are the targeted genes for both ent-Epicatechin and (-) -Epigallocatechin-3-gallate (Fig. 3(a)). Using the GEPIA database (the method is one-way ANOVA), we performed a differential analysis of the expression of these genes in pancreatic adenocarcinoma. The results showed that all of these fifteen representative targeted genes were differentially expressed in pancreatic adenocarcinoma (P < 0·05, online Supplementary Fig. S1). Subsequently, we performed a GO enrichment analysis on these fifteen representative targeted genes. The results of GO enrichment analysis showed that these fifteen representative targeted genes were enriched in VEGF pathway (Fig. 3(b)). Therefore, we speculated that the VEGF and VEGFR were potential mediators.
Table 1. The OB and DL of main tea polyphenols
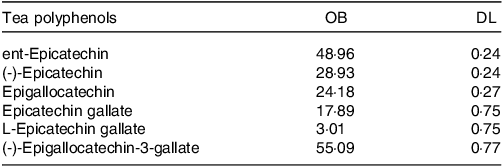
OB, oral bioavailability; DL, drug-likeness.
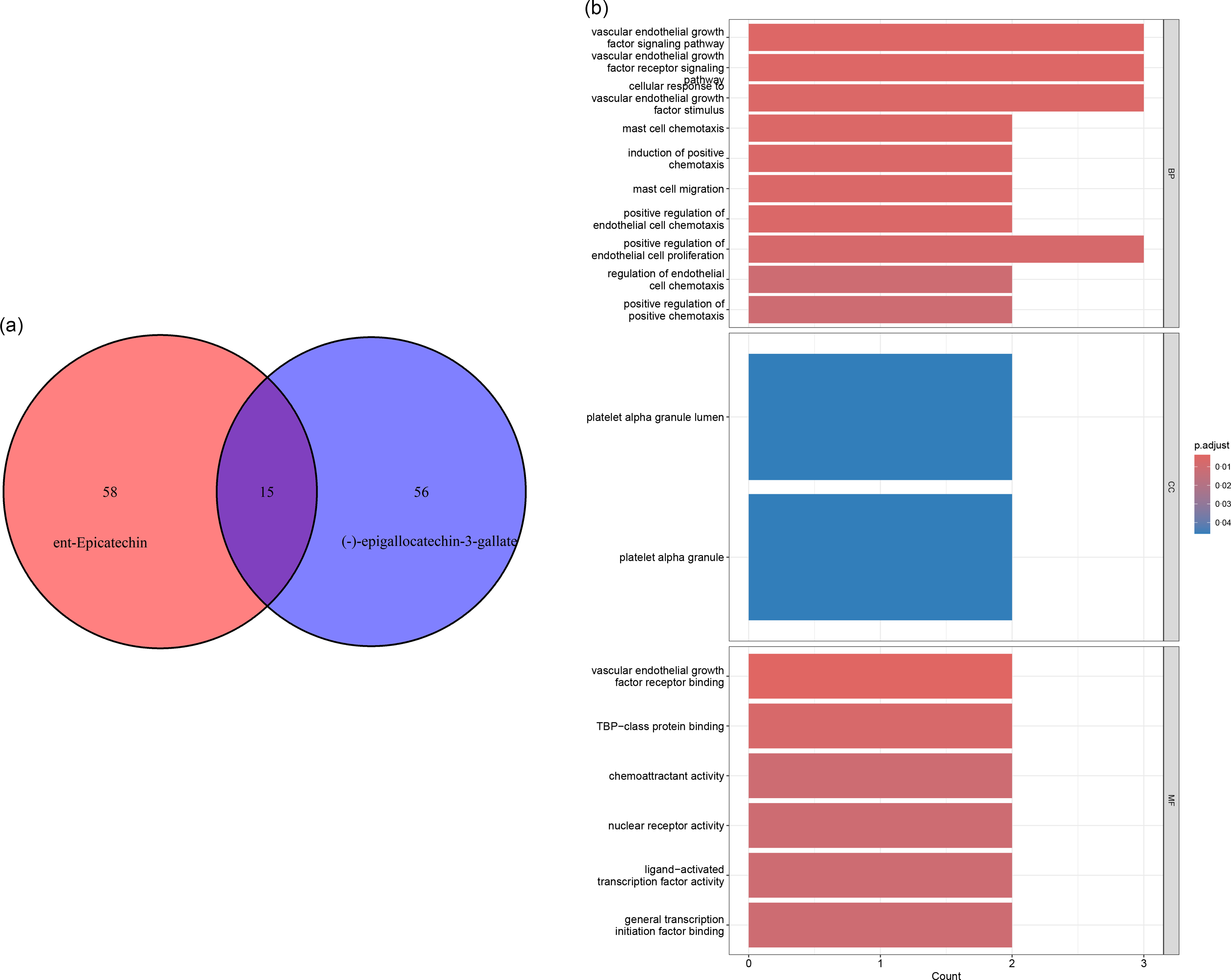
Fig. 3. Pharmacological analysis to predict the function of targeted genes of main active components of tea: (a) fifteen representative targeted genes were determined; (b) the results of gene ontology (GO) enrichment analysis showed that these fifteen representative targeted genes enriched in vascular endothelial growth factor (VEGF) pathway.
The causal effect of tea consumption on vascular endothelial growth factor and vascular endothelial growth factor receptor
Given the results of GO analysis of fifteen representative targeted genes for main active components of tea, we further explored the causal effects of tea consumption on VEGF and VEGFR (Fig. 4). The results of MR analysis showed that genetically predicted increased tea consumption was associated with a decreased level of VEGF-D (IVW: OR: 0·435 (0·203, 0·931), P = 0·032). The results of the five MR analysis methods (MR-Egger: OR: 0·550 (0·091, 3·311); Weighted median: OR: 0·489 (0·171, 1·401); IVW: OR: 0·435 (0·203, 0·931); Simple mode: OR: 0·481 (0·095, 2·439); Weighted mode: OR: 0·491 (0·168, 1·439)) are consistent. However, no causal association was observed between tea consumption and VEGF-A (IVW: OR: 0·847 (0·395, 1·816), P = 0·670), VEGF-A (isoform 121) (IVW: OR: 1·352 (0·743, 2·459), P = 0·323), VEGF-C (IVW: OR: 1·139 (0·627, 2·072), P = 0·669), VEGFR-2 (IVW: OR: 0·701 (0·376, 1·307), P = 0·264) and VEGFR-3 (IVW: OR: 0·562 (0·279, 1·131), P = 0·106). The effect size of each SNP for tea consumption on VEGF and VEGFR is shown in online Supplementary Fig. S2. The Cochran’s Q statistics of MR-Egger and IVW did not show a significant heterogeneity (online Supplementary Table S1). The scatter plot showed that there was statistically no pleiotropy (online Supplementary Fig. S3). We iteratively removed one SNP and used the remaining SNP for inverse variance weighting, and no apparent outlying SNP was observed (online Supplementary Fig. S4).
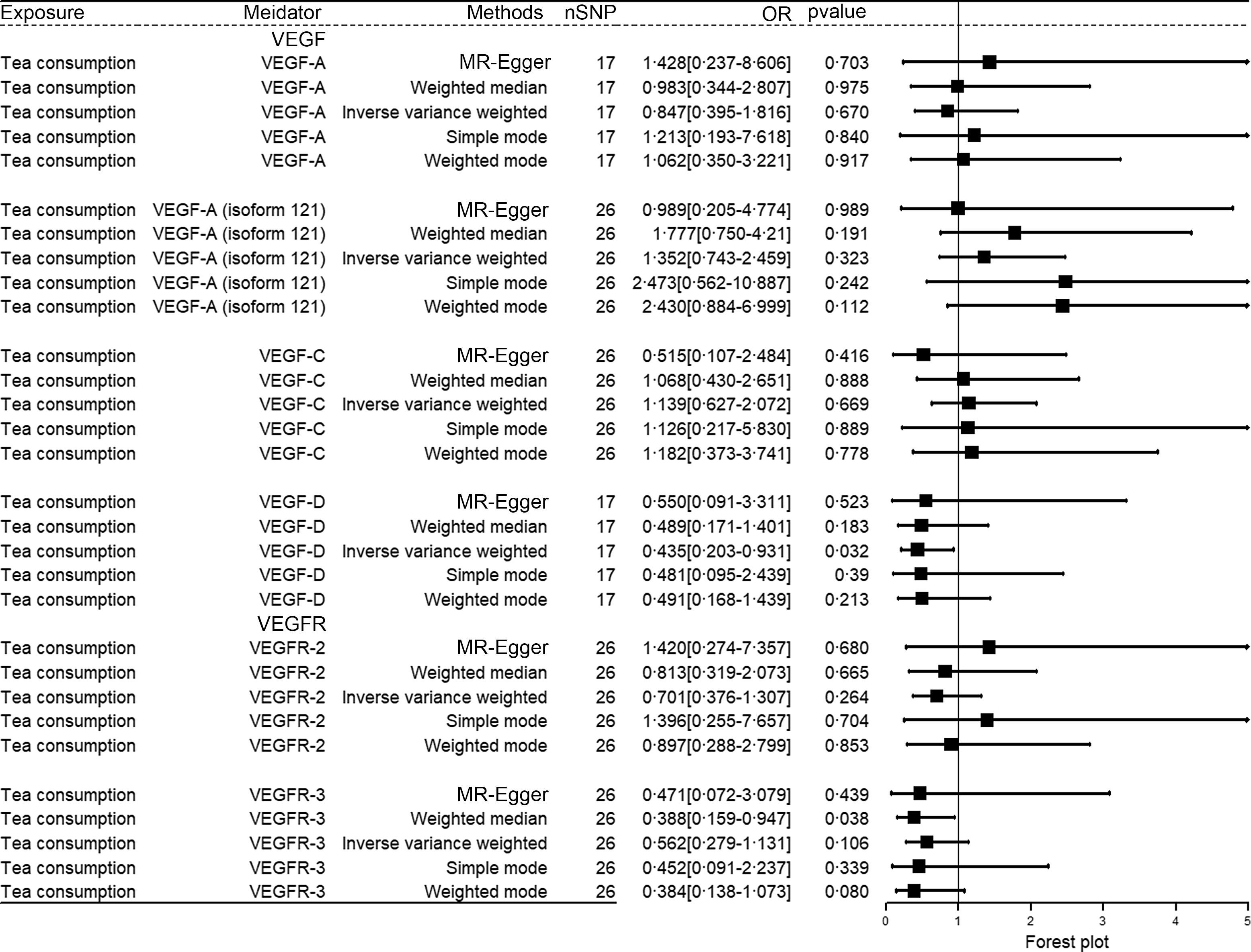
Fig. 4. The causal effect of tea consumption on VEGF and VEGFR. VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
The causal effect of VEGF-D on pancreatic adenocarcinoma
Due to the number of SNP IV was not meet the analysis requirements, we reset the correlative standard (between tea consumption and instrumental SNP) to ‘P < 1e-05’. We used five MR analysis methods to determine the relationship between VEGF-D and pancreatic adenocarcinoma (Fig. 5(a)). The results of MR analysis showed that increased VEGF-D was associated with a reduced risk of pancreatic adenocarcinoma in genetic prediction (IVW: OR: 1·835 (1·109, 3·038), P = 0·018). The results of the five MR analysis methods (MR-Egger: OR: 1·558 (0·387, 6·278); Weighted median: OR: 1·522 (0·797, 2·907); IVW: OR: 1·835 (1·109, 3·038); Simple mode: OR: 1·966 (0·966, 4·000); Weighted mode: OR: 1·658 (0·810, 3·392)) are consistent. The effect size of each SNP for VEGF-D on pancreatic adenocarcinoma is shown in Fig. 5(b). Besides, the Cochran’s Q statistics of MR-Egger and IVW (MR-Egger: Q = 1·805, P = 0·875; IVW: Q = 1·866, P = 0·932) did not show a significant heterogeneity. There was no significant intercept (intercept = 0·029; se = 0·117. P = 0·815), indicating a statistical absence of directional pleiotropy (Fig. 5(c)). We iteratively removed one SNP and performed IVW using the remaining SNP, and no significant outlier SNP were observed (Fig. 5(d)).
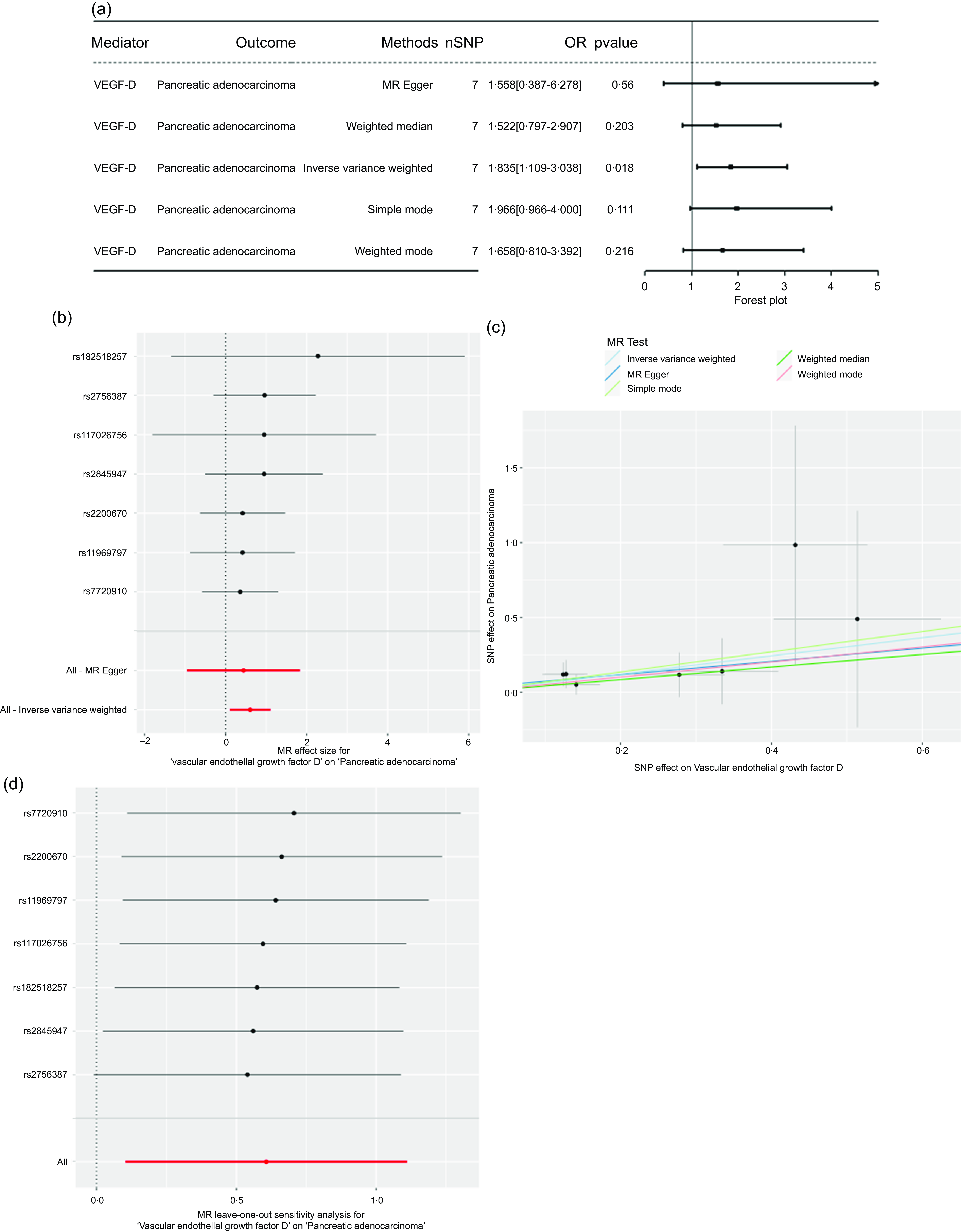
Fig. 5. The causal effect of VEGF-D on pancreatic adenocarcinoma: (a) genetically predicted increased VEGF-D was associated with an increased risk of pancreatic adenocarcinoma; (b) the effect size of each SNP for VEGF-D on pancreatic adenocarcinoma; (c) there was statistically no directional pleiotropy; and (d) there was no apparent outlying SNP. VEGF, vascular endothelial growth factor.
Mediation proportion
Based on the MR analysis results, we used VEGF-D as the mediator and calculated the mediation proportion. The results showed that the mediating proportion was beta0-beta1 × beta2 = 0·230 (0·066–0·394).
Discussion
In this study, we found that in genetic prediction, increased tea consumption was associated with a decreased risk of pancreatic adenocarcinoma by the results of two-sample MR analysis based on genome-wide association studies data. The conclusion was consistent with the findings of case–control studies of Wang et al. and Liu et al. (Reference Wang, Zhang and Sun34,Reference Liu, Chen and Wang35) Our results underscore the significance of tea consumption in the prevention of pancreatic adenocarcinoma and mediating effects of VEGF-D in this process, which provides novel insights for future investigations.
Then, we selected fifteen representative targeted genes (ACADM, AZGP1, CCNE1, GAN, NAT1, NR3C2, PDK2, PRKD2, RARG, RUVBL1, SPEN, SUOX, USP19, VEGFA and VEGFB) by pharmacological analysis. Differential analysis indicated that these fifteen genes were significantly different in pancreatic adenocarcinoma and normal pancreatic tissues. Previous studies have also showed a connection between these targeted genes and pancreatic adenocarcinoma(Reference Yang, Gu and Zhang36–Reference Costache, Ioana and Iordache42).
We preliminarily predicted VEGF and VEGFR as potential mediators by GO enrichment analysis. Similarly, McMillan et al. and Shankar et al. showed that catechin can inhibit pancreatic cancer by inhibiting VEGF and VEGFR(Reference Shankar, Ganapathy and Hingorani9,Reference McMillan, Riggs and Jackson43) . However, previous study only performed with experimental study in tumour cells or animal models. It is important and more realistic to validate the role of VEGF and VEGF in tea and pancreatic adenocarcinoma in epidemiological studies. The results of this study showed that VEGF and VEGFR may be the potential mediator in causal effect of tea consumption on pancreatic adenocarcinoma. Subsequently, we performed the two-step MR analysis to validate it and calculate the mediation proportion. The results of MR analysis showed that genetically predicted increased tea consumption was associated with a decreased level of VEGF-D. Mineva et al. and Rashidi et al. believed that EGCG suppresses VEGF by multiple pathways(Reference Mineva, Paulson and Naber44,Reference Rashidi, Malekzadeh and Goodarzi45) . By using the Weighted median method, we found that the genetically predicted tea consumption was able to be associated with the decrease of VEGFR-3 (P < 0·05). Since heterogeneity and pleiotropy were not statistically considered in the results of this analysis, we believed that the IVW method is the priority choice method(Reference Bowden and Holmes46). The results of IVW did not show a causal relationship between tea consumption and VEGFR-3. Subsequently, we explored the causal effect of VEGF-D on pancreatic adenocarcinoma by MR analysis. The results showed that genetically predicted level of VEGF-D is associated with pancreatic adenocarcinoma. This result is consistent with the findings of previous experimental studies mentioned above(Reference Shankar, Ganapathy and Hingorani9,Reference McMillan, Riggs and Jackson43) . Therefore, our study found that genetically predicted increased tea consumption is associated with a reduced risk of pancreatic adenocarcinoma, with VEGF-D mediating 23 % effect of tea consumption on the risk of pancreatic adenocarcinoma.
The strengths of this study are as follows: (1) genome-wide association studies-based MR analysis was used in this study, which greatly controlled confounding factors; (2) this study combined pharmacological analysis and two-step MR analysis to explore the mediators between tea consumption and risk of pancreatic adenocarcinoma, which also strengthened the interpretability of the MR analysis results; and (3) the results were validated by applying multiple kinds of MR methods with different model assumptions, and the effects of outlier and pleiotropy were comprehensively assessed. Also, the consistency and reliability of study results were validated.
However, this study has some limitations: the limited reference annotated gene set failed to match all predicted target genes and thus may have result in part of targeted genes not included, which may lead us to ignore some other potential mediators.
In conclusion, combining MR analysis and pharmacological analysis, this study found that genetically predicted tea consumption was inversely associated with pancreatic adenocarcinoma risk, 23 % of which was mediated by VEGF-D. However, more experimental studies and epidemiological studies are still needed to validate this conclusion.
Acknowledgements
The author(s) received no financial support for the research, authorship and/or publication of this article.
Y. O. undertook the article conception, the data analysis and the paper writing. B. Z. completed the article revision and the technical guidance. All authors performed the paper review and revision.
The authors declare no conflicts of interest.
The study did not require approval from the ethics review committee.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114524002393









