It is usually considered that a herbivorous fish could utilise carbohydrate much better than a carnivorous fish. The suitable dietary carbohydrate level is about 45 % for rohu (Labeo rohita), a herbivorous fish(Reference Mohapatra, Sahu and Chaudhari1), but is only 17 % for golden pompano (Trachinotus ovatus), a carnivorous fish(Reference Zhou, Ge and Niu2). Considering the high cost of feeds, it is necessary to improve carbohydrate utilisation in fish. However, much more still needs to be done about the complete characteristics and regulation of glucose homoeostasis in fish with different feeding habits.
Fish possess a range of enzymes for digesting and absorbing simple or complex forms of carbohydrates(Reference Ray, Ghosh and Ringø3). Previous research reported that omnivorous fish, such as common carp (Cyprinus carpio) and goldfish (Carassius auratus), exhibit higher levels of amylase activity than carnivorous fish such as rainbow trout (Oncorhynchus mykiss) and gilthead seabream (Sparus aurata)(Reference Hidalgo, Urea and Sanz4). The transmembrane transportation of glucose requires the assistance of Na-dependent GLUT 1 (SGLT1) in the enterocyte membrane and facilitative GLUT type 2 (GLUT2) in the basolateral membrane(Reference Kamalam, Medale and Panserat5). Previous work suggested that levels of SGLT1 mRNA were significantly up-regulated following a glucose load, but oral glucose did not up-regulate GLUT2 mRNA levels in rainbow trout(Reference Polakof and Soengas6). To the contrary, subsequent research showed that high levels of dietary starch significantly increased the transcriptional level of GLUT2 in blunt snout bream (Megalobrama amblycephala)(Reference Liang, Mokrani and Chisomo-Kasiya7). However, few studies have paid attention to the role of carbohydrate digestion and absorption in maintaining glucose homoeostasis of different species of fish, particularly with regard to the role of amylase.
After glucose loading, the clearance rate of blood glucose is thought to be much faster in herbivorous fish than in carnivorous species(Reference Kamalam, Medale and Panserat5,Reference Moon8,Reference Hemre, Mommsen and Krogdahl9) . Previous research has reported that the activity of pyruvate kinase (PK) in grass carp(Reference Yuan, Zhou and Liang10) and gilthead seabream(Reference Fernández, Miquel and Córdoba11) can be elevated by high levels of dietary carbohydrate. However, other studies have reported that dietary carbohydrate has no effect on the activity or expression of PK in Senegalese sole (Solea senegalensis)(Reference Dias, Rueda-Jasso and Panserat12) or rainbow trout(Reference Skiba-Cassy, Panserat and Larquier13). In general, after glucose loading, the relative expression or the activity of key enzymes in gluconeogenesis significantly decreased, for example, the mRNA levels of glucose-6-phosphatase (G6Pase) significantly decreased in gibel carp (Carassius gibelio)(Reference Jin, Yang and Zhu14). However, a failed regulation of G6Pase was reported in European seabass (Dicentrarchus labrax)(Reference Enes, Panserat and Kaushik15) and gilthead seabream(Reference Enes, Panserat and Kaushik15,Reference Caseras, Meton and Vives16) . In fish, excess ingested glucose could be stored as glycogen and this depends on feeding habits and nutritional status(Reference Kamalam, Medale and Panserat5). In largemouth bass (Micropterus salmoides)(Reference Ma, Mou and Pu17) and golden pompano (T. ovatus)(Reference Zhou, Ge and Niu2), levels of hepatic glycogen have been reported to increase with the increase of dietary carbohydrate. In some fish, excessive amounts of ingested glucose can also be transferred to body lipid storage. Sterol regulatory element-binding protein (Srebp1) and carbohydrate-responsive element binding protein (chrebp) are two key regulatory factors of glucose-induced lipogenesis. Experiments involving rainbow trout and blunt snout bream showed significant changes in the expression of these two factors in response to dietary carbohydrate(Reference Craig and Moon18,Reference Prisingkorn, Jakovlic and Yi19) . The expression of fatty acid synthase (fas) has also been shown to be up-regulated in rainbow trout fed with a high carbohydrate diet(Reference Skiba-Cassy, Panserat and Larquier13). Despite fish, particularly carnivorous fish, display a low ability to utilise carbohydrates and maintain glucose homoeostasis, some studies have reported that carnivorous fish, such as rainbow trout(Reference Panserat, Capilla and Gutierrez20,Reference Dai, Panserat and Terrier21) and gilthead seabream(Reference Peres, Gonçalves and Oliva-Teles22), can adapt their metabolic response to glucose loading, although the ability to adjust is limited. Therefore, more attention should be paid on the different metabolic reactions to glucose loading within species in order to have a better understanding glucose metabolism in fish with different feeding habits.
Grass carp (Ctenopharyngodon idella), a herbivorous fish, and Chinese longsnout catfish (Leiocassis longirostris Günther), a carnivorous fish, are two commonly cultured species in China. Previous studies reported that the optimal level of dietary starch for grass carp was 33 % which was much higher than that in Chinese longsnout catfish (10 %)(Reference Pei, Xie and Wu23,Reference Tian, Liu and Yang24) . After a glucose load, grass carp had an efficient clearance of glucose by increasing the activity of hepatic hexokinase(Reference Li, Liu and Dong25). Compared with grass carp, Chinese longsnout catfish has a lower ability of utilising dietary carbohydrates. Furthermore, the difference between grass carp and Chinese longsnout catfish in glucose homoeostasis and metabolism is still unclear. Therefore, in the present study, we characterised glucose homoeostasis in grass carp and Chinese longsnout catfish following the oral administration of starch and compared the pathway related to carbohydrate metabolism such as, digestion, transportation, gluconeogenesis, glycolysis, glycogen synthesis and glucose-induced lipogenesis. The data of the models in Chinese longsnout catfish and grass carp contribute to improve the present understanding of glucose intolerance.
Materials and methods
Experimental animals
Grass carp (64·12 (se 9·50) g) were obtained from a commercial fish farm (Hanshou, Changde, Hunan, China). Chinese longsnout catfish (19·63 (se 3·08) g) were obtained from another fish farm (Shishou, Jingzhou, Hubei, China). Prior to the oral administration of starch, both grass carp and Chinese longsnout catfish were cultured in an indoor recirculating system for 2 weeks to acclimatise. During this period, all fish were fed to apparent satiety twice per d with experimental feeds which had been formulated specially for these two species (Table 1); we also ensured that carbohydrate levels were appropriate for each species. During both the acclimation period and the experimental period, water quality was maintained as follows: temperature 22 (se 2)°C, ammonia–N content 0·15 (se 0·03) mg/l, dissolved O 6·71 (se 0·10) mg/l and pH 6·81 (se 0·12). Photoperiod was 12 h dark–12 h light; the period of daylight was 08.00–20.00 hours.
Table 1. Proximate composition of diets used to feed grass carp and Chinese longsnout catfish

* Maize starch was obtained from Yufeng industrial group.
Oral starch administration
The administration of starch was performed by gavage using a lavage tube (outer diameter 1·00 mm). Prior to the oral administration of starch, all fish underwent fasting for a period of 48 h in order to ensure that their digestive tract was empty and that they had a basal level of plasma glucose. Then, fish were randomly selected, anaesthetised with MS-222 (80 mg/l; Sigma), immediately weighed and administered with 3 g of a mixture of water and maize starch (water–starch at a ratio of 3:2) per 100 g body weight. Maize starch (gelatinisation degree of starch is 4·15 % and performed according to Holm et al. (Reference Holm, Lundquist and Bjorck26); the total digestibility of starch in vitro is 49·79 % and performed according to Tang et al. (Reference Tang, Iji and Choct27)) was obtained from Yufeng industrial group. Another group of fish was administered with saline (0·9 %) as a sham treatment in order to assess the effects of oral administration on plasma glucose levels. Following the administration of starch, six fish were sampled immediately; these samples were designated as time 0 h. And, time 0 h was used as the control group. Further samples were collected at 1, 3, 6, 12, 24 and 48 h. One aquarium (150 litres) was used for each sampling time in order to minimise the stress of sampling. A total of eighty-four grass carp and eighty-four Chinese longsnout catfish were sampled.
At each time point, six fish from each tank were anaesthetised with MS-222 (80 mg/l) and samples of blood, liver and anterior intestine were acquired. Blood was taken from dorsal vessels using a syringe and then centrifuged at 3000 g (4°C) for 10 min to obtain plasma samples. Samples of liver and anterior intestine were collected immediately after blood sampling and were quickly frozen in liquid N2 and stored at −80°C for further analysis. All experiments were conducted in accordance with the Guiding Principles for Care and Use of Laboratory Animals approved by the Institute of Hydrobiology, Chinese Academy of Science (approval ID: IHB20140724).
Biochemical assays
The proximate composition of feeds was analysed following Association of Official Analytical Chemists methods(28). Total protein content (N × 6·25) was determined after acid digestion with the Kjeldahl method, using a 4800 Kjeltec Analyzer Unit (FOSS Tecator). Total lipid content was determined using ether extraction in a Soxtec system (Soxtec System HT6; Tecator), with diethyl ether as the extraction liquid. Gross energy was determined by an Oxygen bomb calorimeter (Parr 6200; Parr Instrument Company). Glucose, TAG, cholesterol levels in plasma and glycogen contents in liver were measured with commercial assay kits (Nanjing Jiancheng Bioengineering Institute). The activity of amylase in intestine and PK was measured by commercial assay kits (Nanjing Jiancheng Bioengineering Institute). The activity of phosphofructokinase (PFK), fructose 1,6-bisphosphatase (FBP) and glucose-6-phosphate dehydrogenase (G6PDH) was measured using commercial assay kits (Beijing Solarbio Science and Technology Co. Ltd). The activity of G6Pase in liver was determined by spectrophotometric assay using commercial assay kits (Suzhou Comin Biotechnology Co. Ltd). One unit of enzyme activity is defined as the amount of enzyme that will generate 1 nmol/ml NADH per min at 25°C. The activity of glucokinase (GK) in liver was determined by the methods described by Panserat et al. (Reference Panserat, Médale and Blin29). The protein concentration of liver homogenate was determined by the Coomassie Brilliant Blue G250, using bovine serum albumin as the standard.
Quantitative PCR analysis
Total RNA was extracted from liver samples using TRIzol Reagent (Ambion, Life Technologies) in accordance with the manufacturer’s instructions. RNA quality was assessed by agarose gel electrophoresis. Total RNA was then reverse-transcribed using an M-MLV First-Strand Synthesis Kit (Invitrogen). The primers for PCR were designed according to published sequences for grass carp and Chinese longsnout catfish in GenBank (Tables 2 and 3). The primers used for glucose-6-phosphatase catalytic subunit (g6pc) in grass carp were based on those reported previously by Li et al. (Reference Li, Yuan and Liang30).
Table 2. Primers used in the PCR analysis for grass carp
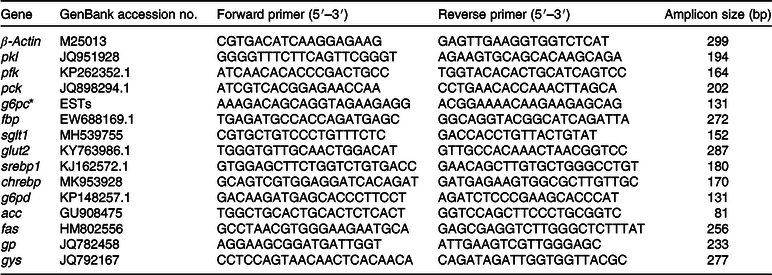
pkl, Pyruvate kinase (liver type); pfk, phosphofructokinase; pck, phosphoenolpyruvate carboxykinase; g6pc, glucose-6-phosphatase catalytic subunit; fbp, fructose 1,6-bisphosphatase; sglt1, Na-dependent GLUT 1; srebp1, sterol regulatory element-binding protein; chrebp, carbohydrate-responsive element binding protein; g6pd, glucose 6-phosphate dehydrogenase; acc, acetyl-CoA carboxylase; fas, fatty acid synthase; gp, glycogen phosphorylase; gys, glycogen synthase.
* According to Li et al. (Reference Li, Yuan and Liang30).
Table 3. Primers used in the PCR analysis for Chinese longsnout catfish
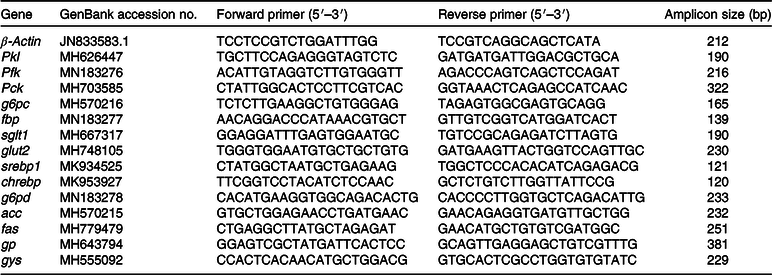
pkl, Pyruvate kinase (liver type); pfk, phosphofructokinase; pck, phosphoenolpyruvate carboxykinase; g6pc, glucose-6-phosphatase catalytic subunit; fbp, fructose 1,6-bisphosphatase; sglt1, Na-dependent GLUT 1; glut2, glucose transporter 2; srebp1, sterol regulatory element-binding protein; chrebp, carbohydrate-responsive element binding protein; g6pd, glucose 6-phosphate dehydrogenase; acc, acetyl-CoA carboxylase; fas, fatty acid synthase; gp, glycogen phosphorylase; gys, glycogen synthase.
Real-time PCR analysis was carried out using LightCycle 480 SYBR Green I Master Mix with a LightCycle® 480 II system (Roche). All reactions were performed in a 6 µl volume including 2 µl cDNA template, 0·24 µl forward and reverse primer, 0·52 µl ddH2O and 3 µl LightCycle 480 SYBR Green 1 Master. Negative controls (in which the template was replaced by ddH2O) were amplified in the same plate. PCR conditions were as follows: pre-incubation 95°C for 5 min, followed by forty cycles of amplification (95°C for 10 s, 60°C for 20 s, 72°C for 10 s). A melting curve was then created (0·5°C increments from 65 to 95°C) to confirm the amplification of a single product. β-Actin was chosen as an internal reference for normalisation. In the pretrial of our experiment, it has been found that the gene expression of β-actin was very stable in the target tissues of grass carp and Chinese longsnout catfish. The amplification efficiency of the genes analysed in the present study varied from 1·95 to 2·05. Final data were calculated according to the method described by Vandesompele et al. (Reference Vandesompele, Preter and Pattyn31).
Statistical analysis
All data were analysed using SPSS 20 for Windows. Duncan’s multiple range test was used to detect the significance of differences between groups followed by one-way ANOVA. Before all analyses, all data were tested for the normality of distribution (one-sample Kolmogorov–Smirnov test) and homogeneity of variances (Levene’s test) among different time points. Statistical significance was set to P < 0·05, and data are presented as mean values with their standard errors (n 6).
Results
Plasma glucose
Plasma glucose levels did not change significantly following the administration of oral saline (P > 0·05). In grass carp, plasma glucose rose from 4·61 (se 0·33) to 12·56 (se 0·81) mmol/l over the first 3 h after starch administration and returned to baseline by 6 h; there was no significant difference in plasma glucose level when compared between 0 and 6 h (Fig. 1(A)). In Chinese longsnout catfish, the plasma glucose levels at 6, 12 and 24 h were significantly higher than those at 0 h (P < 0·05). There was no significant difference in plasma glucose levels when compared between 48 and 0 h (P > 0·05).

Fig. 1. Changes in the plasma glucose levels of (A) grass carp and (B) Chinese longsnout catfish following the oral administration of starch. Each point represents the mean of six replicates. a,b,c Unlike letters indicate significant differences (P < 0·05) between different sampling times.  , Starch;
, Starch;  , saline.
, saline.
Digestion and transportation
Following the oral administration of starch, we detected significantly higher levels of amylase activity at 1 and 3 h compared with 0 h (P < 0·05) in the anterior intestine of grass carp (Fig. 2(A)). However, there were no significant differences in terms of amylase activity in the anterior intestine of Chinese longsnout catfish following the oral administration of starch (Fig. 2(B)).
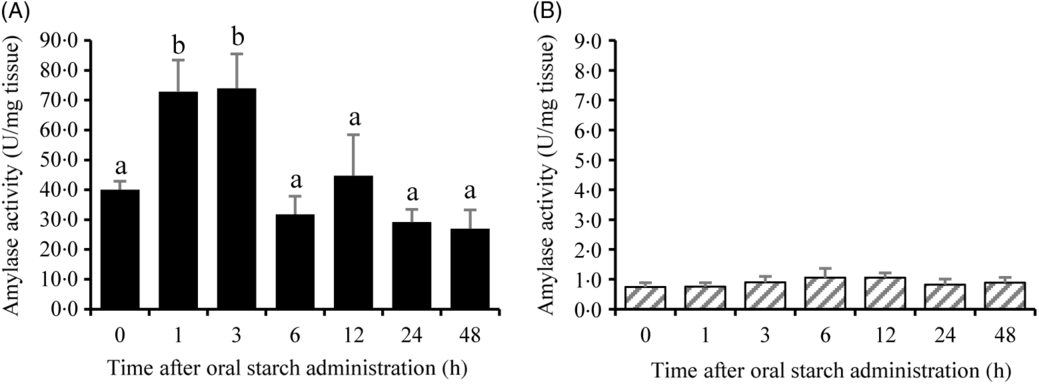
Fig. 2. Amylase activity in the anterior intestine of (A) grass carp and (B) Chinese longsnout catfish following the oral administration of starch. Each bar represents the mean of six replicates. a,b Mean values with unlike letters are significantly different (P < 0·05).
In grass carp, the highest levels of mRNA expression for sglt1 were detected 1 h after the administration of starch; expression levels then decreased steadily from 3 to 12 h (Fig. 3(A)). In Chinese longsnout catfish, the mRNA levels of sglt1 at 3 h were significantly higher than at 12, 24 and 48 h (Fig. 3(B)). There was no significant change in the transcriptional levels of glut2 in the intestine of grass carp (P > 0·05) (Fig. 3(C)), although the mRNA levels of glut2 increased significantly (P < 0·05) at 6 and 12 h in the liver of grass carp, reaching a peak at 12 h (Fig. 3(E)). The expression of glut2 in the livers of Chinese longsnout catfish was significantly higher (P < 0·05) at 12 h (Fig. 3(F)).
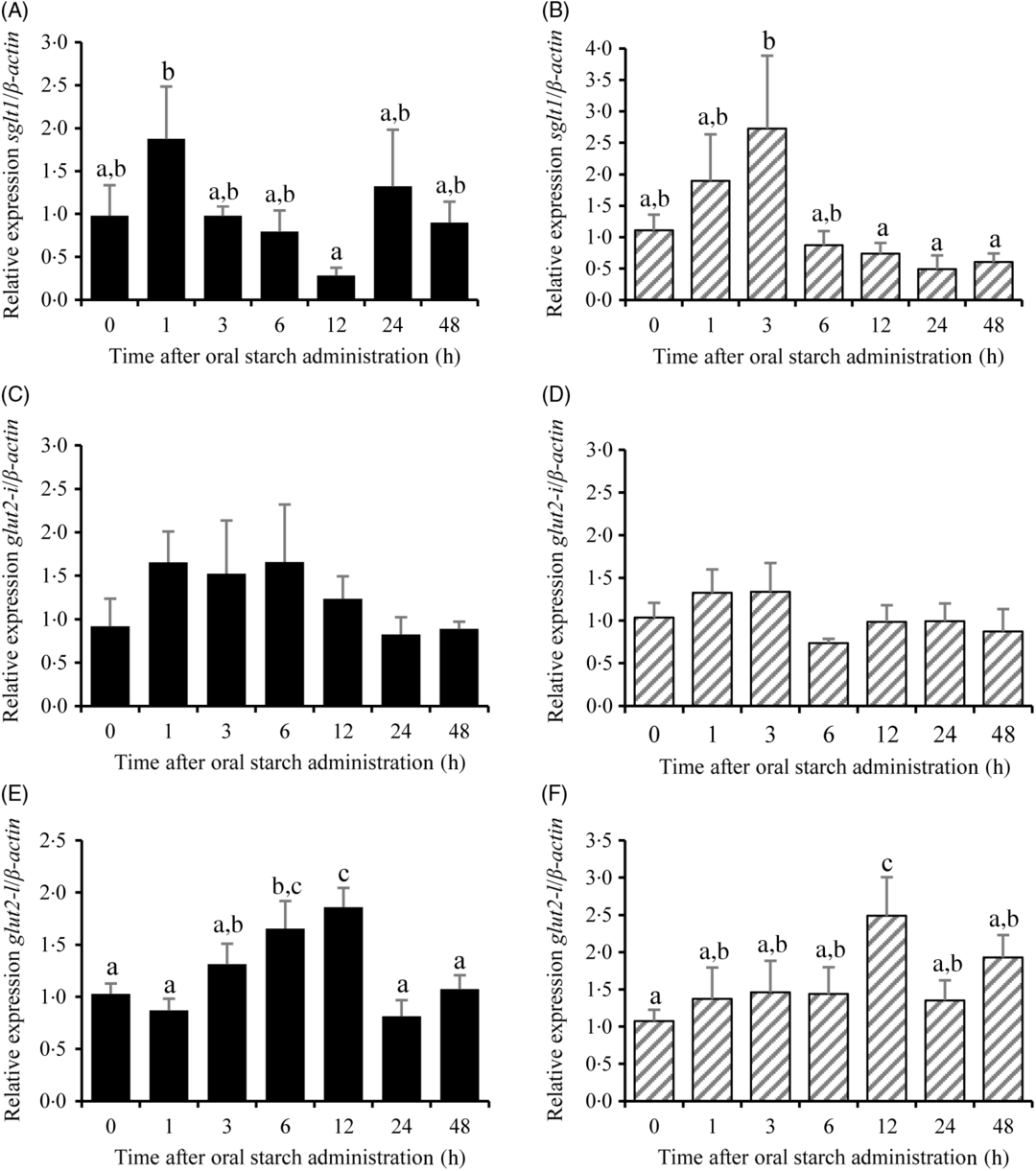
Fig. 3. Relative expression of GLUT in grass carp and Chinese longsnout catfish following the oral administration of starch: (A) Na-dependent GLUT 1 (sglt1) in the intestine of grass carp; (B) sglt1 in the intestine of Chinese longsnout catfish; (C) GLUT type 2 (glut2) in the intestine of grass carp; (D) glut2 in the intestine of Chinese longsnout catfish; (E) glut2 in the liver of grass carp; and (F) glut2 in the liver of Chinese longsnout catfish. Each bar represents the mean of six replicates. a,b,c Mean values with unlike letters are significantly different (P < 0·05).
Hepatic gluconeogenesis
In grass carp, the activity of G6Pase, which plays a key role in catalysing the final step of gluconeogenesis, decreased significantly until 12 h after the oral administration of starch (P < 0·05) but then returned to baseline by 24 h (Fig. 4(A)). Similarly, the expression of g6pc, which encodes G6Pase, decreased significantly (P < 0·05) until 12 h after administration but then increased significantly (P < 0·05) at 48 h (Fig. 4(C)). However, in Chinese longsnout catfish, both liver G6Pase activity and g6pc expression remained unchanged after starch treatment (Fig. 4(B) and (D)). FBP and phosphoenolpyruvate carboxykinase (PEPCK) are both rate-limiting enzymes for catalysing gluconeogenesis. The enzymes activity and mRNA levels of FBP and the mRNA levels of pck, which encode PEPCK, showed no significant changes in either grass carp or Chinese longsnout catfish following the administration of starch (P > 0·05).
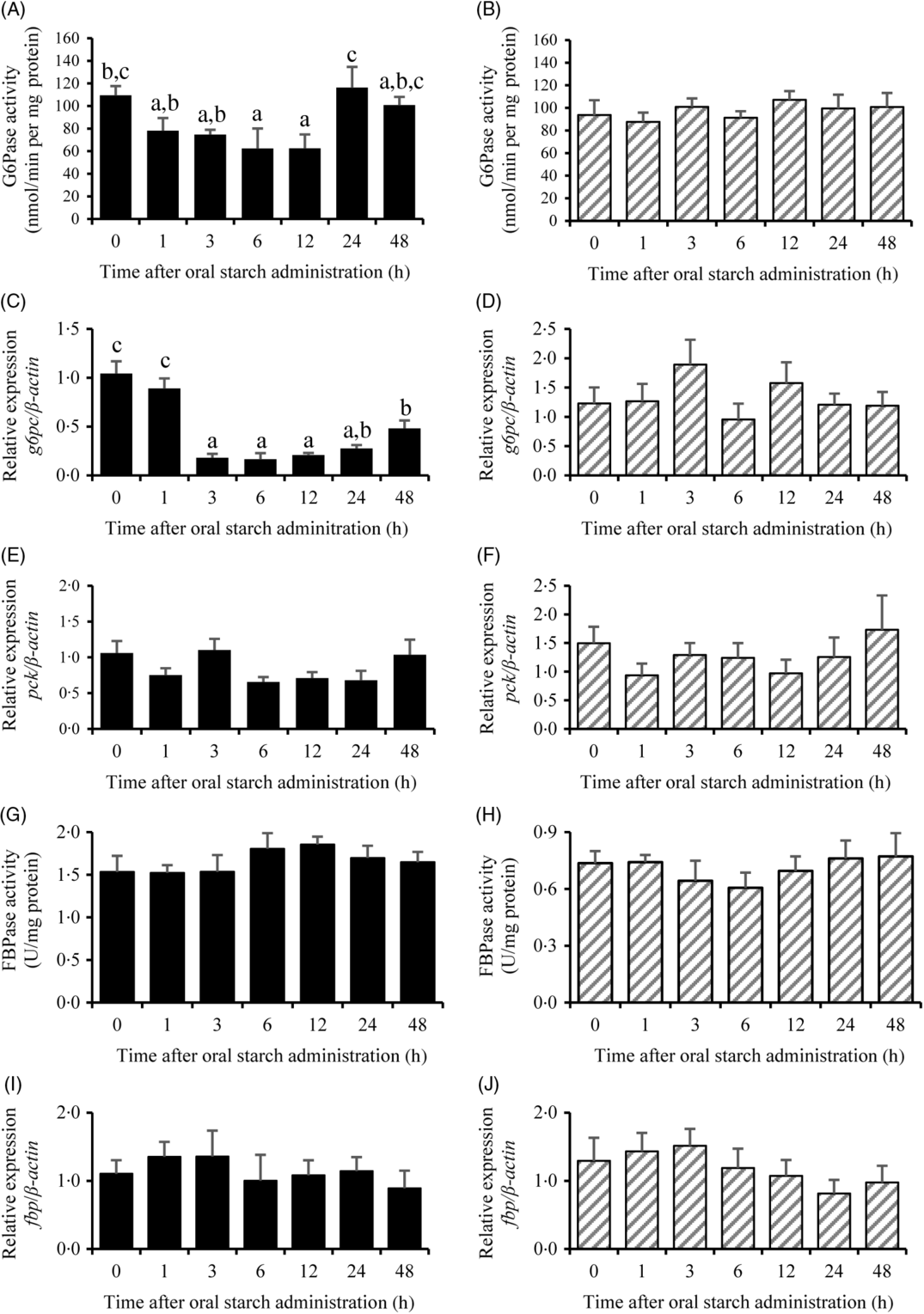
Fig. 4. Enzymic activity and expression levels of hepatic gluconeogenesis in grass carp and Chinese longsnout catfish following the oral administration of starch. Enzymic activity of glucose-6-phosphatase (G6Pase) in (A) grass carp and (B) Chinese longsnout catfish; expression levels of glucose-6-phosphatase catalytic subunit (g6pc) in (C) grass carp and (D) Chinese longsnout catfish; expression levels of phosphoenolpyruvate carboxykinase (pck) in (E) grass carp and (F) Chinese longsnout catfish; enzymic activity of FBPase in (G) grass carp and (H) Chinese longsnout catfish; expression levels of fructose 1,6-bisphosphatase (fbp) in (I) grass carp and (J) Chinese longsnout catfish. Each bar represents the mean of six replicates. a,b,c Mean values with unlike letters are significantly different (P < 0·05).
Hepatic glycolysis
A significant increase in hepatic GK activity was detected in grass carp (P < 0·05) at 3 and 6 h after the oral administration of starch (Fig. 5(A)). We also detected a significant increase in the hepatic activity of GK (P < 0·05) in Chinese longsnout catfish (Fig. 5(B)) at these same time points. PK plays a key role in catalysing the final step of glycolysis. The activity of hepatic PK in grass carp increased slowly following the administration of starch and peaked at 12 h; at this time point, activity was significantly higher (P < 0·05) than that at 0 h (Fig. 5(C)). Furthermore, the mRNA levels of pk were significantly increased (P < 0·05) at 12 h after the administration of starch (Fig. 5(E)). There was no significant change in the activity and transcription of PK at any of the sampling time points in the livers of Chinese longsnout catfish (Fig. 5(D) and (F)). Furthermore, there were no significant changes in the activity of enzymes and expression levels of pfk at any of the sampling time points following the administration of starch; this was the case for both grass carp and Chinese longsnout catfish (P > 0·05) (Fig. 5(G)–(J)).

Fig. 5. Enzymic activity and expression levels of hepatic glycolysis in grass carp and Chinese longsnout catfish following the oral administration of starch. Enzymic activity of (A) glucokinase in grass carp; (B) glucokinase in Chinese longsnout catfish; (C) pyruvate kinase (PK) in grass carp; (D) PK in Chinese longsnout catfish; expression level of (E) pyruvate kinase (liver type) (pkl) in grass carp; (F) pkl in Chinese longsnout catfish; enzymic activity of phosphofructokinase (PFKase) in (G) grass carp and (H) Chinese longsnout catfish; expression level of pfk in (I) grass carp and (J) Chinese longsnout catfish. Each bar represents the mean of six replicates. a,b,c Mean values with unlike letters are significantly different (P < 0·05).
Hepatic glycogen
In grass carp, the expression levels of hepatic glycogen synthase (gys) and glycogen phosphorylase (gp) were significantly higher (P < 0·05) at 6 h than at 0 h. However, there was no significant change in terms of glycogen content (P > 0·05) in the livers of grass carp following the administration of starch (Fig. 6(A), (C) and (E)). In contrast to grass carp, there was a significant increase in glycogen content in the livers of Chinese longsnout catfish from 12 to 48 h (P < 0·05). Furthermore, the expression levels of hepatic gp were significantly reduced (P < 0·05) in Chinese longsnout catfish but there was no significant change in the mRNA expression of gys (Fig. 6(B), (D) and (F)).

Fig. 6. Hepatic glycogen content in (A) grass carp and (B) Chinese longsnout catfish; relative expression of hepatic glycogen synthase (gys) in (C) grass carp and (D) Chinese longsnout catfish; relative expression of hepatic glycogen phosphorylase (gp) in (E) grass carp and (F) Chinese longsnout catfish following the oral administration of starch. Each bar represents the mean of six replicates. a,b,c Mean values with unlike letters are significantly different (P < 0·05).
Glucose-induced lipogenesis
For all sampling time points, plasma TAG and cholesterol levels were consistently higher in grass carp than in Chinese longsnout catfish (Fig. 7). Plasma cholesterol in grass carp increased significantly (P < 0·05) 1–3 h after the administration of starch, compared with 0 h. In Chinese longsnout catfish, levels of plasma cholesterol increased significantly (P < 0·05) 12–24 h after administration (Fig. 7(B)).

Fig. 7. Changes in (A) plasma TAG and (B) plasma cholesterol in grass carp and Chinese longsnout catfish following the oral administration of starch. Each point represents the mean of six replicates. a,b,c Unlike letters indicate significant differences (P < 0·05) between sampling times for grass carp. A,B,C Unlike letters indicate significant differences (P < 0·05) between sampling times for Chinese longsnout catfish.  , Chinese longsnout catfish;
, Chinese longsnout catfish;  , grass carp.
, grass carp.
The expression levels of hepatic chrebp were significantly up-regulated (P < 0·05) in grass carp 6 h after the oral administration of starch (Fig. 8(A)). However, there were no significant changes in the levels of hepatic chrebp in Chinese longsnout catfish (Fig. 8(B)). In grass carp, the relative expression of hepatic srebp1 increased significantly (P < 0·05) at 3 and 6 h (Fig. 8(C)). The activity of glucose 6-phosphate dehydrogenase (g6pd) in grass carp significantly elevated at 3 and 6 h (Fig. 9(A)). Compared with 0 h, the relative expression of hepatic g6pd increased significantly in the Chinese longsnout catfish at 24 and 48 h (P < 0·05) (Fig. 9(D)). The mRNA levels of acetyl-CoA carboxylase (acc) increased at 1–6 h, peaked at 6 h and then returned to baseline at 12 h in the livers of grass carp (Fig. 8(E)). Compared with 0 h, the expression levels of acc were significantly higher (P < 0·05) at 24 and 48 h in the livers of Chinese longsnout catfish (Fig. 8(F)). The expression levels of hepatic fas in grass carp increased significantly at 1–6 h (P < 0·05).

Fig. 8. Relative expression of hepatic carbohydrate-responsive element binding protein (chrebp), sterol regulatory element-binding protein (srebp1), acetyl-CoA carboxylase (acc) and fatty acid synthase (fas) in grass carp and Chinese longsnout catfish following the oral administration of starch. (A) chrebp in grass carp; (B) chrebp in Chinese longsnout catfish; (C) srebp1 in grass carp; (D) srebp1 in Chinese longsnout catfish; (E) acc in grass carp; (F) acc in Chinese longsnout catfish; (G) fas in grass carp; (H) fas in Chinese longsnout catfish. Each bar represents the mean of six replicates. a,b,c Mean values with unlike letters are significantly different (P < 0·05).

Fig. 9. Enzymic activity and expression of hepatic glucose-6-phosphate dehydrogenase (G6PDH) in grass carp and Chinese longsnout catfish following the oral administration of starch. Enzymic activity of G6PDH in (A) grass carp and (B) Chinese longsnout catfish; relative expression of g6pd in (C) grass carp and (D) Chinese longsnout catfish. Each bar represents the mean of six replicates. a,b,c Mean values with unlike letters are significantly different (P < 0·05).
Discussion
Our analysis revealed an interesting difference in the carbohydrate metabolism of fish in that the oral administration of starch led to an increase in plasma glucose levels; glucose peaked at 3 h in grass carp and 6 h in Chinese longsnout catfish. The time taken to reach a glucose peak following the oral administration of starch was previously reported to be 3 h in tilapia (Oreochromis niloticus)(Reference Lin, Liou and Shiau32) and 6 h in white sturgeon (Acipenser transmontanus)(Reference Deng, Refstie and Hung33). However, after intraperitoneal injection of glucose, plasma glucose concentrations peaked at 1 h in both tilapia(Reference Chen, Wang and Pi34) and blunt snout bream(Reference Li, Xu and Zhang35). Differences in the timing required to reach a glucose peak between present study and those published previously are not only related to species differences but also related to differences in the methods used to administer carbohydrates. In addition, the peak levels of plasma glucose in grass carp were almost three times higher than that in Chinese longsnout catfish. This difference could be due to a range of reasons. Firstly, digestion and absorption play a key role in the initial steps of glucose metabolism; amylase plays a critical role during these processes. A previous comparative study, featuring species with different food habits, indicated that the amylase activity of goldfish was almost fifty-eight times higher than that of rainbow trout(Reference Hidalgo, Urea and Sanz4). We observed a similar result in the present study that the amylase activity of grass carp was 54- to 82-fold higher than Chinese longsnout catfish following the administration of starch. The activity of amylase had a close relationship with the utilisation of dietary carbohydrates(Reference Polakof, Panserat and Soengas36). Dietary supplementation of α-amylase enhanced the digestibility of starch and G6PDH activity in the liver of Rohu carp (L. rohita)(Reference Castillo and Gatlin37,Reference Kumar, Sahu and Pal38) . After a meal with high digestibility (97–99 %) of starch, gluconeogenesis was not particularly depressed, while lipogenic pathways were enhanced in gilthead seabream(Reference Enes, Panserat and Kaushik39). Interestingly, after the administration of starch, the amylase activity of grass carp increased significantly at 1 and 3 h and then returned to baseline at 6 h; Chinese longsnout catfish, however, showed no changes in the activity of amylase. Our results relating to amylase were supported by the fact that intestinal amylase activity was previously reported to be induced significantly with increased levels of dietary carbohydrate in common carp (C. carpio)(Reference Keshavanath, Manjappa and Gangadhara40) and golden pompano (T. ovatus)(Reference Zhou, Ge and Niu2), although no changes were reported for southern catfish (Silurus meridionalis Chen)(Reference Gao and Luo41). Differences in the activity of amylase in grass carp and Chinese longsnout catfish could potentially explain the differences in the way that plasma glucose increases in these fish following exposure to an excess of carbohydrate.
The absorption of glucose by the blood from the enteric cavity is facilitated by two key GLUT, Na-dependent GLUT 1 (SGLT1) and the facilitative GLUT2(Reference Kellett, Brot-Laroche and Mace42). Sglt1 expression is directly regulated by dietary carbohydrate(Reference Dyer, Daly and Salmon43,Reference Margolskee, Dyer and Kokrashvili44) , and the mRNA levels of glut2 in zebrafish intestine were previously shown to be regulated by refeeding(Reference Castillo, Crespo and Capilla45). In the present study, the mRNA levels of sglt1 in grass carp and Chinese longsnout catfish increased in a manner similar to plasma glucose and then decreased at a time point when plasma glucose levels peaked. A previous study involving rainbow trout showed that the expression levels of sglt1 increased following the oral administration of glucose(Reference Polakof, Álvarez and Soengas46). These data imply that SGLT1, located in the brush border/apical membrane, could play a role in rising plasma glucose levels in fish. However, in the present study, the expression of glut2 in the intestine of grass carp and Chinese longsnout catfish did not show any significant change following the oral administration of starch, as also shown previously in the rainbow trout(Reference Polakof, Álvarez and Soengas46,Reference Kamalam, Panserat and Aguirre47) .
Gluconeogenesis is a main pathway to produce glucose in order to raise glycaemia in fish. Phosphoenolpyruvate carboxykinase, FBP and G6Pase are the key enzymes in regulation of hepatic gluconeogenesis. Previous studies have reported that the expression of pck was not affected by glucose injection in the liver of tilapia(Reference Chen, Wang and Pi34) and the transcription level of fbp was not influenced in common carp after a high carbohydrate meal(Reference Panserat, Plagnes-Juan and Kaushik48). In line with the previous studies, we found that the expression levels of pck and fbp were not affected by the oral administration of starch in either grass carp or Chinese longsnout catfish. However, reduced expression levels of pck and fbp were previously reported in gibel carp after a glucose load(Reference Jin, Yang and Zhu14). Our data showed that the transcriptional expression and enzymic activity of G6Pase were inhibited in grass carp but not in Chinese longsnout catfish. This was supported by previously published results which stated that the effect of dietary carbohydrate on G6Pase in fish is species-specific(Reference Kamalam, Medale and Panserat5,Reference Caseras, Meton and Vives16,Reference Panserat, Plagnes-Juan and Kaushik48) . A failure to inhibit the gluconeogenic pathway was previously reported to be responsible for postprandial hyperglycaemia in rainbow trout(Reference Panserat, Capilla and Gutierrez20,Reference Panserat, Médale and Brèque49) ; this appears to also be the case for Chinese longsnout catfish. However, similar to our present observations in grass carp, a previous study involving roho labeo (L. rohita) reported that the activity of G6Pase was significantly reduced after a high carbohydrate meal(Reference Alexander, Sahu and Pal50). Another study reported that the expression of G6Pase was inhibited in gibel carp following the injection of glucose(Reference Jin, Yang and Zhu14). Therefore, compared with herbivorous fish, carnivorous fish appear to exhibit lower abilities to inhibit gluconeogenesis for the maintenance of glucose homoeostasis.
Another interesting result was that plasma glucose levels in grass carp returned to baseline at 6 h after the oral administration of starch; in Chinese longsnout catfish, glucose levels did not return to baseline until the 48 h time point. The time returned to baseline after oral starch administration was previously reported to be 8 h in tilapia(Reference Lin, Liou and Shiau32) and 15 h in white sturgeon (A. transmontanus)(Reference Deng, Refstie and Hung33). These results indicated that Chinese longsnout catfish, a carnivorous fish, has a poor capacity for clearing blood glucose. Glycolysis is the primary pathway for utilising glucose and converting glucose into pyruvate. The transcriptional expression and activity of hepatic GK, a rate-limiting enzyme of glycolysis, are strongly induced by dietary carbohydrate(Reference Panserat, Nicole and Sergio51,Reference Song, Marandel and Dupont-Nivet52) . Previous research in gilthead seabream proved that the activity of GK increased significantly 6–8 h after feeding(Reference Caseras, Metón and Fernández53). In line with gilthead seabream, the activity of GK in grass carp and Chinese longsnout catfish was highly induced by the oral administration of starch. Significantly increased activity of GK was also reported in blunt snout bream after a glucose load(Reference Li, Xu and Zhang35). The expression level of pfk is considered to represent an effective indicator for postprandial hepatic glycolysis in tilapia(Reference Chen, Zhang and Chen54); however, our data showed that the activity and transcription levels of hepatic pfk were not stimulated by the oral administration of starch in grass carp and Chinese longsnout catfish. Similar results were previously published for gibel carp(Reference Jin, Yang and Zhu14) and tilapia(Reference Chen, Wang and Pi34). High levels of dietary carbohydrate are also known to significantly affect PK in fish, another rate-limiting enzyme in glycolysis, although this effect is species-dependent(Reference Kamalam, Medale and Panserat5). In the present study, the activity and relative expression of hepatic PK increased and peaked at 12 h in grass carp but then decreased; there were no significant changes in the expression of hepatic PK in Chinese longsnout catfish. The present results concurred with previous studies reporting significant changes of hepatic PK expression in grass carp(Reference Yuan, Zhou and Liang10) but no significant changes in rainbow trout(Reference Skiba-Cassy, Panserat and Larquier13,Reference Panserat, Plagnesjuan and Kaushik55) . Therefore, in Chinese longsnout catfish, it appears that PK, not GK, is responsible for the prolonged postprandial period of hyperglycaemia.
In order to maintain glucose homoeostasis, excessive amounts of glucose can be stored as glycogen. Previous studies reported a significant increase in hepatic glycogen content following glucose loading in hybrid grouper(Reference Li, Sang and Zhang56) and tilapia(Reference Chen, Wang and Pi34). After intraperitoneal injection of glucose, the expression of hepatic gys1 increased significantly and liver glycogen accumulated in tilapia(Reference Chen, Wang and Pi34). However, in the present study, hepatic glycogen synthesis and catabolysis were significantly induced by the administration of oral starch but did not affect the hepatic glycogen content in grass carp. However, in Chinese longsnout catfish, there was no change in hepatic glycogen synthesis and reduced levels of hepatic glycogen catalysis; collectively, this resulted in a significant increase in hepatic glycogen content. This indicated that the increased hepatic glycogen content in Chinese longsnout catfish was not the result of increased levels of glycogen synthesis but rather the reduced catabolism of glycogen. In addition, our present data showed that hepatic glycogen content in grass carp was almost 9·7 times higher than that in Chinese longsnout catfish at 0 h. Therefore, the storage and metabolism of hepatic glycogen after oral starch administration in grass carp and Chinese longsnout catfish appear to be very different.
Lipogenesis plays an important role in glucose metabolism because excess ingested glucose can be stored in the form of lipid(Reference Kamalam, Medale and Panserat5). Increased plasma TAG levels have been reported after a glucose load in both tilapia(Reference Chen, Wang and Pi34) and European seabass(Reference Peres, Gonçalves and Oliva-Teles22). Similar results were evident in the present study; while plasma levels of TAGs and total cholesterol were increased in both species, grass carp possessed markedly higher levels than Chinese longsnout catfish following the oral administration of starch. This indicated that grass carp may possess better ability to convert carbohydrate to lipid than Chinese longsnout catfish. Carbohydrates could provide NADPH for lipogenesis and G6PDH is one of the key enzymes in this process. In line with previous studies(Reference Enes, Panserat and Kaushik39,Reference Kamalam, Medale and Kaushik57,Reference Likimani and Wilson58) , elevated activities and expressions of G6PDH induced by carbohydrates were reported in grass carp. However, the absence of a clear effect of oral starch administration on the activity of G6PDH was found in Chinese longsnout catfish. SREBP and ChREBP directly activate the expression of genes related to the synthesis of TAGs and cholesterol(Reference Horton, Goldstein and Brown59). The present study found that the oral administration of starch induced high expression levels of srebp1 and chrebp in grass carp but not in Chinese longsnout catfish. A previous study showed that the expression of chrebp was induced in blunt snout bream in response to high dietary levels of carbohydrate(Reference Prisingkorn, Jakovlic and Yi19). Another study reported that the increased expression of chrebp enhanced the expression of target genes involved in glycolysis, glycogen storage and lipogenesis(Reference Conde-Sieira, Ceinos and Velasco60). In the present study, the up-regulated expression of srebp1 (3 and 6 h), chrebp (6 h), acc (6 h), fas (1, 3 and 6 h) in the livers of grass carp following the oral administration of starch indicated that the process of lipogenesis had been activated. In a previous study, increased levels of glucose led to the increased expression of srebp1 but not evident in fas (Reference Jin, Yang and Zhu14). In rainbow trout, srebp1 was up-regulated after the injection of insulin but the expression levels of g6pd, acc and fas remained unchanged(Reference Jin, Panserat and Kamalam61). In another study with rainbow trout, the transcription level of srebp1 did not increase with dietary carbohydrates but the enhanced activity of FAS and mRNA level of g6pd were observed(Reference Kamalam, Medale and Kaushik57). In the present study, although the expression levels of srebp1 and chrebp did not change in the livers of Chinese longsnout catfish, we still observed the up-regulation of g6pd (24 h), acc (24 and 48 h) and fas (3 and 48 h) following the oral administration of starch. Increased relative expression levels of acca (acetyl-CoA carboxylase α) and fas have also been reported in tilapia following glucose injection and feeding(Reference Chen, Wang and Pi34,Reference Chen, Zhang and Chen54) . These results indicated that glucose-induced lipogenesis plays an important role in glucose metabolism but still needs more attention in different fish.
Conclusions
The ongoing investigations into the multiple ways of carbohydrate utilisation emphasise the need to understand the difference in glucose homoeostasis between different fish. In the present study, grass carp, but not Chinese longsnout catfish, exhibited a rapid and significant increase in plasma glucose following the oral administration of starch. This was further supported by the induction of amylase activity in this species. The present findings also suggested that grass carp exhibited a faster clearance rate of plasma glucose than Chinese longsnout catfish. This finding was related to the significant inhibition of gluconeogenesis and enhanced levels of glycolysis, hepatic glycogen metabolism and glucose-induced lipogenesis in grass carp. The present study indicated that grass carp and Chinese longsnout catfish, species of fish with significantly different requirements for dietary carbohydrate, exhibited clear differences in glucose homoeostasis and carbohydrate metabolism and with regard to digestion, transportation, gluconeogenesis, glycolysis, glycogen synthesis and lipogenesis. The present study provides a good reference for glucose homoeostasis, especially with the model of Chinese longsnout catfish.
Acknowledgements
The authors are grateful to Guanghan Nie for his technical help. We also thank Hongyan Li, Wenjie Xu and Cui Liu for their help with laboratory samples.
The present study was supported by the National Key R&D Program of China (2018YFD0900400), the China Agriculture Research System (CARS-46), the National Natural Science Foundation of China (31672670 and 31972771), the Fund Project in State Key Laboratory of Freshwater Ecology and Biotechnology (2019FBZ02) and the Key Project of Hubei Provincial Science and Technology Department (2015BBA226).
D. H. designed the experiment. J. S. performed the experiment and prepared the manuscript under the supervision of D. H. Y. G., L. M. and L. X. contributed to data analysis. Y. Y. helped with chemical analysis. S. C., J. J., H. L., X. Z. and S. X. provided suggestions on the experimental design and the manuscript.
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.















