Cr and Fe are essential micronutrients for human health and play an important role in human metabolism. Cr is involved in lipid and carbohydrate metabolism, and the most frequent manifestation of Cr deficiency is hyperglycaemia. In addition, hyperglycaemia, neuropathy and weight loss have been observed in a classic case of Cr deficiency in patients on total parenteral nutrition, with these abnormalities being corrected with supplementation of Cr to the parenteral nutrition solution. This nutrient has also been associated with CVD and gene expression(Reference Anderson, Mahan and Escott-Stump1). The US Institute of Medicine(2) estimates a Cr adequate intake of 25 μg/d for women aged 19–30 years. The toxicity of Cr(III), the chemical form present in foods, is low enough to provide a sufficient safety margin between usual consumed and harmful amounts, as humans cannot oxidise Cr(III) to potentially carcinogenic Cr(VI) compounds(Reference Anderson, Mahan and Escott-Stump1–Reference Gatto, Kelsh and Mai3).
Fe is a component of a number of proteins, including enzymes and Hb. Almost two-thirds of Fe in the body is found in Hb present in circulating erythrocytes, and it is important for the transport of oxygen to tissues throughout the body for metabolism. Most of the remaining 15 % is in the myoglobin of muscle tissue and a variety of enzymes necessary for oxidative metabolism and many other functions in all cells. The estimated average requirement for women aged 19–30 years is 8·1 mg/d(2). Fe deficiency is considered the most common single nutrient deficiency disease in the world, affecting most seriously women, children and adolescents(Reference Anderson, Mahan and Escott-Stump1). This deficiency is produced by an unbalance between requirements and the quantity of the mineral that is ingested, absorbed and utilised. Considerable amounts of Fe must be provided by the diet in an available form, as the bioavailability of dietary Fe appears to be an important determinant of Fe status.
In general, estimates of the total element content in food and diets are insufficient, and its bioavailability also needs to be considered. Mineral bioavailability has gained increasing interest in the field of nutrition. In vivo studies are both expensive and laborious, and the possibility of measuring certain parameters during the experiments is often limited(Reference Perales, Barberá and Lagarda4). In vitro methods of simulated digestion are an alternative for calculating the percentage of the mineral that is transformed into absorbable forms in the digestive tract (bioaccessibility). These procedures are rapid, usually inexpensive, and they allow individual experimental variables to be easily controlled. The results are usually expressed as the dialysable fraction under given experimental conditions such as pH, enzyme addition and temperature(Reference Perales, Barberá and Lagarda4, Reference Cabrera-Vique and Bouzas5).
The aim of the present study was to determine Cr and Fe content in duplicate meals, which represent the habitual diet served in a female university residence in Granada (Spain), in order to test their adequacy to dietary reference intakes. Moreover, an in vitro method that simulates gastrointestinal conditions by employing a dialysis membrane was used to determine the Cr and Fe dialysable fractions. Electrothermal atomic absorption spectrometry was used as an analytical technique.
Materials and methods
Reagents
Standard solutions of Cr and Fe (1·00 (sd 0·002) g) (Tritisol; Merck, Darmstadt, Germany) were used, diluted as necessary to obtain working standards. High-quality concentrated HNO3 (65 %), perchloric acid (70 %) and vanadium pentaoxide (Merck) were used for sample mineralisation. Magnesium nitrate (Merck) was used as the chemical modifier for Cr determination. Ammonium molybdate (Merck) was used to precondition the furnace tubes. All reagents used were of analytical grade. Pepsin (Sigma P7000 porcine pancreas; Sigma-Aldrich Chemie GmbH, Steinheim, Germany), pancreatin (Sigma P1500 porcine pancreas) and bile salts (Sigma B8756) were used to simulate gastric and intestinal digestion. A pepsin solution was prepared by dissolving 16 g of pepsin in 100 ml of 0·1 m-HCl; a pancreatin–bile extract mixture was prepared by dissolving 4 g of pancreatin and 25 g of bile extract in 1 litre of 0·1 m-NaHCO3. NaOH (0·5 m) and HCl (6 m) were used to adjust pH. Dialysis tubing Spectra with a molecular mass cut-off of 10–12 kDa (‘Visking size 3–20/32’; Medicell International, Liverpool, London, UK) was used. The dialysis tubing was freed of trace metal impurities by boiling in 2 % (w/v) NaHCO3, 0·1 % (w/v) SDS and 0·01 m-EDTA disodium salt for 30 min, followed by thorough washing with bidistilled deionised water. It was then preserved in 20 % ethanol solution.
Apparatus
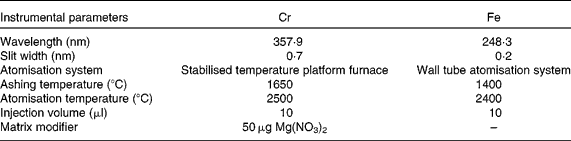
A Perkin-Elmer 1100B double-beam atomic absorption spectrometer equipped with deuterium arc background correction (Perkin-Elmer, Norwalk, CT, USA) and hollow Cr and Fe cathode lamps made by the same manufacturer were used. In addition, a Perkin-Elmer HGA-700 graphite furnace atomiser was used. Pyrolytically coated graphite tubes (ref. B013-5653) and pyrolytic graphite platforms (ref. B012-1092) were obtained from Perkin-Elmer. Ar of 99·999 % purity (Sociedad Española de Oxígeno, Barcelona, Spain) at 300 ml/min was used as the internal gas during all stages except atomisation, when the flow was stopped. Background-corrected integrated absorbance was used as the analytical signal. Furnace conditions were optimised on the basis of time–temperature assays (Table 1); these conditions obviated most matrix interferences and other sources of unspecific absorption. A Moulinex blender, model 327 (Moulinex, Bangnolet, France), of which different parts were Teflon-coated following Van Cauwenbergh et al. (Reference Van Cauwenberg, Hendrix and Robberecht6), was used for sample homogenisation. To determine the total Cr content in the diets, the samples were dried using a microwave oven (Moulinex) and mineralised in an acid digestion block (Selecta, Barcelona, Spain). A thermostatic Selecta water-bath (Selecta) and a Radiometer model 26 pH meter (Radiometer, Copenhagen N.V., Denmark) were used for the in vitro assays.
Table 1 Instrumental parameters for chromium and iron determination in duplicate meals by electrothermal atomic absorption spectrometry
Material
In order to decrease the risk of contamination, the use of glassware was reduced to a minimum, and plastic (polypropylene) vessels and pipette tips were used. All materials were washed with HNO3 and rinsed several times with bidistilled deionised water.
Sampling strategies
To determine the dietary intake of minerals and trace elements, study designs that include the collection and preparation of ready-for-consumption food are believed to produce the most realistic and reliable results(Reference García, Cabrera and Lorenzo7–Reference Roussel, Andriollo-Sanchez and Ferry9). Duplicate portion samples of sixty-three different meals, corresponding to twenty-one breakfast, twenty-one lunch and twenty-one dinner, were taken over twenty-one consecutive days at a female university residence in Granada (Spain) that provides full board to 165 students aged 18–24 years. These meals were the only food served in the residence, and no drinks other than milk, coffee or water were included. Due to the impossibility of quantifying water consumption, this was not included in the present study. Food samples were subjected to a simulated eating procedure using normal knives and forks. The food items were sliced, and the inedible parts discarded. The remaining parts were weighed and then homogenised in the blender.
Combined weight and estimated dietary records were completed in parallel with the duplicate diet collection. All the foods as well as the size of the portions were recorded. The food content of the diet was transformed into energy and nutrient values using the Spanish Food Composition Tables(Reference Mataix, Mañas and Llopis10) and AYS44 Diet Analysis software, supplied by ASDE, S.A. (Valencia, Spain).
Sample preparation
The dried and homogenised samples were mineralised in an acid digestion block. A detailed description of duplicate meals treatment before determining total Cr and Fe by electrothermal atomic absorption spectrometry is reported elsewhere(Reference Cabrera-Vique and Bouzas5).
In vitro method for estimating chromium and iron dialysability
The simulated gastrointestinal digestion procedure and the in vitro absorption estimation were carried out in accordance with previously reported methods(Reference Cabrera-Vique and Bouzas5, Reference García, Cabrera and Lorenzo11). The technique measures the Fe and Cr fraction dialysed from a sample under simulated gastrointestinal conditions and, therefore, available for absorption. Fe and Cr dialysability is expressed as the percentage of dialysed element in relation to the total content in the duplicate meal. Dialysis mineral percentages were calculated as follows: dialysis (%) = 100 × D/C, where D is the dialysed mineral content (μg/g sample) and C is the total mineral content (μg/g sample).
Statistical analysis
Interpretation of the data was performed using the statistical software package SPSS 13.0 for Windows (SPSS, Chicago, IL, USA). Results are expressed as means and standard deviations. The normal distribution of variables and the homogeneity of variances were checked by the Kolmogorov–Smirnov and the Bartlett test, respectively. Comparisons were made using Student's t test when the variable fulfilled parametric conditions and the Kruskal–Wallis test when the conditions were non-parametric. Additionally, correlations by Pearson's or Spearman's test (for parametric and non-parametric conditions, respectively) and regression models were employed.
Results and discussion
Method validation
For method validation, the detection limit, sensitivity, precision and accuracy were tested for each element. The slopes of the aqueous and standard addition calibration graphs were compared. To check the similarity of slopes, Student's t test was applied; the results showed that the values were similar, with an absence of matrix effects (slope ratios approaching 1). Thus, calibration was performed using the aqueous standard, which greatly simplified the analysis. The reliability of the method was further corroborated by using a certified reference material obtained from the International Atomic Energy Analytical Quality Services of Vienna (IAEA-H-9 mixed human diet). The paired t test showed good agreement at a significance level of 0·05 %. The results are summarised in Table 2.
Table 2 Analytical characteristics for chromium and iron determination in convenience and fast foods
(Mean values and standard deviations or ranges)
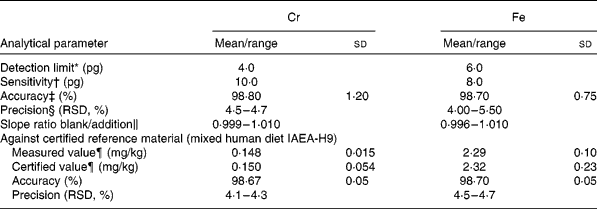
* Calculated according to International Union of Pure and Applied Chemistry rules and corresponding to three times the standard deviation of the blank (n 10).
† Expressed as characteristic mass in pg/0·0044 absorbance units.
‡ Results from recovery assays of five randomly chosen samples.
§ Relative standard deviation (RSD) for ten replicate determinations in each of five samples.
‖ Application of the standard addition method of five randomly chosen samples.
¶ Means and standard deviations at 95 % CI about the mean (n 10), referred to dry weight.
Chromium and iron levels in duplicate meals
Cr content in the analysed duplicate meals corresponding to each day ranged from 98·50 to 120·80 μg, with a mean of 110 μg (Table 3), which surpassed the adequate intake recommendations(2). Mean dietary Cr intake was similar to that reported by García et al. (Reference García, Cabrera and Lorenzo7) and Schuhmacher et al. (Reference Schuhmacher, Domingo and Llobet12) in duplicate diet studies of Spanish families, but these authors included water consumption. Cr intakes less than 110 μg/d have been observed in Finland(Reference Räsänen, Laitinen and Stirkkinen13), Belgium(Reference Van Cauwenberg, Hendrix and Robberecht6) and France(Reference Roussel, Andriollo-Sanchez and Ferry9). Anderson et al. (Reference Anderson, Bryden and Polansky14) reported data on Cr dietary intake from a duplicate plate technique of 23·1 (sd 2·9) and 38·8 (sd 6·5) μg/d for American women and men, respectively. On the other hand, higher intakes have been reported by Ysart et al. (Reference Ysart, Miller and Crews15), who even showed a Cr intake of 340 μg/d for the general population from the UK and neither quantified drinking-water consumption. Additional data on Cr daily intake in different countries according to other authors are summarised in Table 4.
Table 3 Total content and dialysable fraction of chromium and iron in daily duplicate meals from a female university residence
(Mean values and ranges, n 21)

* Data refer to the fresh weight of the edible portion.
Table 4 Chromium daily dietary intake in different countries according to other authors

In the present study, the highest Cr levels were observed in meals that include meat, a high content of spices and aromatic herbs and chocolate. In previous studies, we analysed several foods and beverages that are widely consumed in Spain, in order to estimate their possible contribution to the total dietary Cr intake. A high Cr presence in dairy products, meat, stimulant drinks and infusion (especially tea and coffee), whole cereals, brown sugar, spices and aromatic herbs was observed(Reference García, Cabrera and Lorenzo7, Reference Lendínez, Lorenzo and Cabrera16). In accordance with Storelli(Reference Storelli17), we observed that seafood consumption (except some cephalopods) does not represent an important contribution to daily dietary Cr intake.
Fe content in the analysed daily duplicate meals ranged from 9·50 to 40·00 mg, with a mean of 18·50 mg (Table 3). These values are similar to those reported by Velasco-Reynold et al. (Reference Velasco-Reynold, Navarro-Alarcón and López-García de la Serrana18) for duplicate meals provided daily in hospitals in Granada, Spain (17·7 mg; range 9·51–42·00 mg). Our mean value of 18·50 mg/d is higher than the estimated average requirement of 8·1 mg/d proposed by the US Institute of Medicine(2) and is similar to the Fe intake estimated in other Spanish epidemiological studies, as reported by Fernández-Morales et al. (Reference Fernández-Morales, Aguilar Vilas and Mateos Veja19) in female adolescents (16·63–17·21 mg/d), although higher than that reported by the enKid study in women aged 18–24 years (12·9 mg/d)(Reference Serra-Majem, Ribas-Barba and Pérez-Rodrigo8). Women from countries such as Germany(Reference Heseker, Adolf and Eberhardt20), The Netherlands(Reference Van Dokkum, Schneijder and Van Erp-Baart21) and Norway(Reference Frost-Anderson, Nes and Sandstad22) present Fe intake levels of about 10–11 mg/d, while the lowest values are found in Denmark, where women aged 19–24 years have an Fe intake of 8·5 mg/d(Reference Elmadfa and Weichselbaum23). Additional data are summarised in Table 5.
Table 5 Iron daily dietary intake in young women from different countries according to other authors

* Vegans.
† Omnivores.
The highest levels of Fe in the analysed duplicate meals appeared in meals that include liver terrine, blood sausage, meat, dry fruits or chocolate; in general, food of animal origin, such as meat, fish and their products, would be primary sources of Fe. In any case, the analysed meals do not supply Fe levels close to the recommended upper limit (45 mg/d)(2).
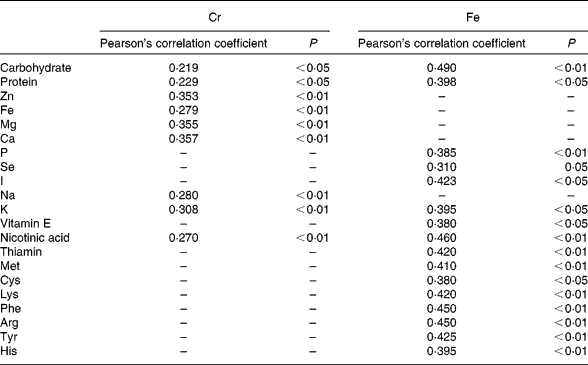
The intake of a given element may be related to that of other nutrients, particularly minerals and vitamins(Reference Anderson, Mahan and Escott-Stump1, 2). In addition, Van Cauwenbergh et al. (Reference Van Cauwenberg, Hendrix and Robberecht6) suggested that Cr intake may increase in parallel to energy intake. We observed a significantly positive and linear correlation among total Cr levels and energy intake, and carbohydrate, protein, Zn, Fe, Mg, K, Na, Ca and nicotinic acid content (Table 6), similar to the relationships reported by García et al. (Reference García, Cabrera and Lorenzo7). Positive correlations were observed between total Fe levels and carbohydrate, protein, different amino acids, P, Se, I, K, vitamin E and nicotinic acid content. In agreement with Velasco-Reynold et al. (Reference Velasco-Reynold, Navarro-Alarcón and López-García de la Serrana18), the present results show that the total Fe supplied in meals is directly related to its macronutrient content.
Table 6 Statistically significant correlations between total chromium and iron and other nutrients in the analysed duplicate meals
We consider that the implementation of a total diet study offers the advantage of providing more realistic exposure data since foods are analysed ‘as-consumed’. In addition, it provides a good tool for identifying the population or age groups that are most exposed (children, the elderly, etc.) and identifies the main food or food groups contributing to the exposure when food sampling is based on an individual approach. Thus, food consumption is monitored, and useful trends are identified for orienting food safety programmes.
In vitro chromium and iron dialysable fraction
In vitro simulation of the digestive process and the subsequent quantification of the concentrations of the soluble and/or dialysable compounds in the gastrointestinal medium (bioaccessibility determination) represent one of the most effective methods of identifying an efficient source of a nutrient in a food product. In vitro methods are routinely used to estimate the bioaccessible amounts of essential elements in diets. Table 3 shows the results of the Cr and Fe dialysable fraction after in vitro simulation of the digestion process in the duplicate meal samples. These results express the element fraction, as a percentage, that would be available for absorption by intestinal cells (bioaccessible Cr and Fe).
The Cr dialysable fraction in the analysed daily meals ranged from 0·50 to 1·50 %. García et al. (Reference García, Cabrera and Lorenzo11) obtained similar results (0·4–1·6 %) using an in vitro method for a total dietary intake ranging from 16 to 117 μg/d. These authors reported that Cr bioaccessibility is higher for low levels of daily dietary intake ( < 40 μg/d) than for levels of 40–80 μg/d; for high levels (>80 μg/d), there was an increase in the dialysable fraction. This influence of the total Cr content in the analysed meals on the Cr dialysable fractions has also been observed. Paustenbach et al. (Reference Paustenbach, Hays and Brien24) reported values of 2 % in drinking-water, and Cabrera-Vique & Bouzas(Reference Cabrera-Vique and Bouzas5) found 0·38–1·05 % in convenience and fast foods.
In the present study, wide variability in the Fe dialysable fraction was observed, with levels ranging from 7·75 to 11·80 % (Table 3). A similar variability was also reported by Velasco-Reynold et al. (Reference Velasco-Reynold, Navarro-Alarcón and López-García de la Serrana18) in hospital duplicate diets (4·81 (sd 3·25) %) and by Cámara et al. (Reference Cámara, Amaro and Barberá25) in Spanish school meals (0·23–19·0 %). We observed that a high animal protein and ascorbic acid content in the meals significantly enhanced the Fe dialysable fraction. This fact has been reported previously, as has the finding that animal proteins can counteract the phytate and tanin inhibitor effect(Reference Velasco-Reynold, Navarro-Alarcón and López-García de la Serrana18). The mean Fe dialysable fraction estimated in the present study (9·80 %) was similar to the Fe in vitro availability in diets using Caco-2 cells, as reported by Mesías et al. (Reference Mesías García, Seiquer and Delgado-Andrade26). In Western countries, the Fe absorbed fraction is about 10 %(Reference Velasco-Reynold, Navarro-Alarcón and López-García de la Serrana18, Reference Mesías García, Seiquer and Delgado-Andrade26).
Adherence of the analysed diets to the Mediterranean diet
The beneficial health effects of the Mediterranean diet are commonly attributed to the association of regular physical activity with a complex combination of dietary characteristics. This has been corroborated by numerous epidemiological and experimental nutrition studies(Reference Mesías García, Seiquer and Delgado-Andrade26, Reference Mesías, Seiquer and Muñoz-Hoyos27). In order to determine the adherence of a diet to the Mediterranean diet patterns, Serra-Majem et al. (Reference Serra-Majem, Ribas and Ngo28) developed the Mediterranean Diet Quality Index, based on principles sustaining Mediterranean dietary patterns as well as those that undermine it. The index ranges from 0 to 12 and is based on a sixteen-question test. On applying this test to the diet provided at the university residence, we obtained an index of 9, which represents optimal adherence to the Mediterranean diet. This is as expected in view of the high dietary content of legumes, cereals, fish, fresh fruits and vegetables, which is characteristic of the Mediterranean patterns. Taking into account this index and the analytical data obtained, we consider that a diet based on the Mediterranean-style plan supplies adequate amounts of Fe and Cr for young women. In the same way, Mesías et al. (Reference Mesías, Seiquer and Muñoz-Hoyos27) reported the beneficial effect of Mediterranean dietary patterns on dietary Fe utilisation in male adolescents aged 11–14 years.
In summary, the present study shows that a balanced and varied diet based on the Mediterranean-style diet plan supplies adequate amounts and bioaccessibility of Fe and Cr for young women. The data obtained should also be useful for international comparison and enhance our knowledge of the nutritional value of these nutrients.
Acknowledgements
We thank Glenn Harding for revising the English text of the manuscript. The present study was supported by AGR141 Research Group, financed by Junta de Andalucía (Government of Spain). C. C.-V. designed the present study and collected the data, and M. M. contributed to the data analysis. Both the authors participated in the writing of the manuscript. Neither of the authors declares a conflict of interest.








