Patients with chronic liver diseases (CLD) of any aetiology present high mortality rates related to different factors, with malnutrition(Reference Anastácio, Ferreira and Ribeiro1–Reference Ferreira, Ferreira Martins and Cunha4) playing a key role. Malnutrition is highly prevalent in this population due to impaired nutrient metabolism, early satiety and poor dietary intake, among others(Reference Anastácio and Davisson Correia5). In particular, decreased protein intake affects the synthesis of all body proteins, including those related to the immune response(Reference Albillos, Lario and Álvarez-Mon6,Reference Robinson, Harmon and O’Farrelly7) , and muscle mass, leading to poorer outcomes(Reference Montano-Loza8), as deleterious effects on energy metabolism regulation, immune function and systemic inflammation can be expected(Reference Biolo, Cederholm and Muscaritoli9).
The liver is constantly exposed to a wide range of products, including those with inflammatory potential(Reference Heymann, Peusquens and Ludwig-Portugall10,Reference Seki and Schwabe11) , requiring physiological processes to control and/or resolve inflammatory responses(Reference Kubes and Mehal12,Reference Racanelli and Rehermann13) . However, multiple overlapping pathways in various liver diseases mediate the dysregulation of the liver inflammatory response, leading to chronic inflammation, which is a hallmark of chronic infection and autoimmune and malignant diseases(Reference Heymann and Tacke14). Therefore, therapeutic strategies targeting the liver should restore its homoeostasis, which means resolving any inflammatory and/or immune dysregulation(Reference Seki and Schwabe11). To favour the synthesis of indispensable proteins responsible for muscle mass maintenance/recovery and the immune response, the choice of high nutritional quality proteins, that is, those that present an adequate amino acid profile, digestibility and bioavailability, might be an alternative therapy for patients with liver disease.
Cows’ milk protein, of which casein and whey proteins make up 80 and 20 %, respectively, has received increasing attention as a potential health-promoting nutrient source(Reference Pereira15). Bovine casein consists of α s1-, α s2-, β- and κ-caseins, which represent 37, 10, 35 and 12 % of whole casein, respectively. Bovine whey protein contains approximately 50 % β-lactoglobulin, 20 % α-lactalbumin and 10 % albumin and lactoferrin, with lactoperoxidase making up the remaining source(Reference de Marchi, Bonfatti and Cecchinato16).
Although there is a growing body of intervention studies evaluating the effects of these compounds on health(Reference Ha and Zemel17–Reference Badr, Ebaid and Mohany19), some authors have suggested that whey proteins could be more effective than casein, as the former present higher digestibility and are rich in essential amino acids, especially the branched-chain amino acids(Reference Devries and Phillips20,Reference Tsien, Davuluri and Singh21) . Furthermore, bioactive peptides derived from whey proteins seem to exert effects on nutritional status recovery and immune modulation(Reference Tang, Moore and Kujbida22–Reference Pennings, Boirie and Senden24). While the positive effects on nutritional status are well recognised(Reference Res, Groen and Pennings25,Reference Hector, Marcotte and Churchward-Venne26) , the immunomodulatory effects of whey proteins, especially under disease conditions, have been less frequently reported in clinical trials. Moreover, most of the studies assessing their immune modulatory effects on cell stimulation, proliferation and cytokine secretion have been conducted with whey protein-specific components or peptides(Reference Madureira, Tavares and Gomes27–Reference Sousa, Lira and Rosa29) rather than the whole protein as a supplement.
Therefore, the aim of this study was to evaluate the effect of whey protein consumption on the nutritional status of end-stage CLD patients and the role of whey protein in the immune modulatory response. We hypothesised that patients supplemented with whey protein would present improvement of their nutritional status, as well as a more effective immune response, further improving their clinical outcomes.
Methods
Subjects
This randomised, double-blind, parallel-arm, clinical trial was carried out with patients with CLD on the waiting list for liver transplantation who were recruited at the Hospital das Clínicas, Universidade Federal de Minas Gerais, Brazil, from July 2014 to August 2016. The study was conducted according to the guidelines laid down in Declaration of Helsinki guidelines, and all procedures involving human patients were approved by the local Institutional Review Board (Comitê de Ética em Pesquisa - COEP/UFMG), under the number CAAE- 27430714.8.0000.5149. Written informed consent was obtained from all patients. This study was also registered at ClinicalTrials.gov under the number NCT02901119 (https://clinicaltrials.gov/ct2/show/NCT02901119?term=NCT02901119&draw=2&rank=1).
The inclusion criteria were age equal to or older than 18 years, patients under regular medical care at the study centre and patients on the waiting list for liver transplantation. Patients who were younger than 18 years old, illiterate, pregnant or breast-feeding; patients with chronic kidney failure or allergies to cows’ milk proteins; and those who had previously undergone transplantation were excluded.
Patients and investigators were blinded to the randomisation. Patients were randomised into the whey protein isolate (WP) group (intervention) and the casein (CA) group (control) through block randomisation with a 1:1 allocation ratio. A random number sequence was generated by Microsoft Office Excel 12.0 (Office 2007) to designate the group. The randomisation and allocation were conducted by the blinded researcher. Patients were provided with sachets containing 20 g of WP or CA supplements, packed in bags with a code for each supplement and were asked to consume one in the morning and one in the evening for 15 d. They were also instructed to maintain their regular diet. Phone calls, at least twice a week, were used to monitor the participants and check if they were taking the supplements. Both patients and researchers were unaware of the treatment assignment, which was revealed only after all the established analyses had been carried out. Body composition, RMR, muscle functionality, food consumption and blood parameters were evaluated before and after the protein supplementation period.
Nutritional status, body composition and RMR assessments
Nutritional status was determined at the beginning of the study through the Subjective Global Assessment, according to Detsky et al. (Reference Detsky, McLaughlin and Baker30). Participants were grouped into categories, suspected malnutrition/moderately malnourished and severely malnourished, for comparison purposes. Anthropometric evaluation included the measurement of the mid-arm circumference (cm), obtained with an inelastic tape, and the triceps skinfold thickness (mm) using a Lange skinfold calliper (Cambridge Scientific Industries), and the mid-arm muscle circumference (cm) was calculated from mid-arm circumference and triceps skinfold thickness using the standard formula: mid-arm muscle circumference = mid-arm circumference – (3·1415 × triceps skinfold thickness); both measurements were conducted according to the established procedures(Reference Frisancho31,Reference Heymsfield, Heymsfield and McManus32) . The values obtained before and after protein supplementation were used to evaluate the intervention effect. Body composition was evaluated by bioelectrical impedance using a single-frequency (electrical current used in the measurement was 800 A and 50 kHz) bioelectrical impedance analyser (Quantum X, RJL Systems) as determined by the current protocols(33). Fat-free mass (kg), fat mass (kg) and phase angle were determined. The RMR was measured by indirect calorimetry in an open-circuit calorimeter (Cosmed) after an overnight fast. Finally, muscle functionality was evaluated by handgrip strength (kg) using a digital dynamometer (Jamar Handgrip Dynamometer, Preston) considering the best of three consistent readings, allowing about 1 min of recovery between each attempt, as per the protocol(Reference Heymsfield, Heymsfield and McManus32,Reference Roberts, Denison and Martin34) . The 6-min walking test (m) was also performed according to the American Thoracic Society protocol(35).
Food consumption
Quantitative food intake data were obtained using a 24-h recall. The 24-h recall was completed according to the multiple pass method, whereby the interviewee is guided by five steps(Reference Moshfegh, Rhodes and Baer36). This method helps the individual remember the food and drink consumed on the day before the interview and to report them in detail, reducing errors in dietary measurement. Diet Pro4R® software (Agromidia Software) was used to calculate daily energy intake, proteins, carbohydrates and lipids.
Clinical assessments
The severity of liver disease was graded using the model for end-stage liver disease (MELD) score in which values equal to or higher than fifteen indicate a more severe disease(Reference Merion37). To better characterise the severity of the disease and the potential effect on the protein supplementation responses, patients were categorised according to the MELD cut-off values. This information and information related to the presence of ascites, oedema and encephalopathy were obtained from the medical records and information provided by the physician taking care of the patient.
Assessment of peripheral biomarkers
Blood was collected in tubes containing heparin or EDTA after an overnight fast before and after protein supplementation. The tubes were centrifuged at 3000 rpm for 10 min at room temperature. The plasma samples were aliquoted in microtubes and stored at –80°C until the analyses. IL-1β, IL-5, IL-6, IL-10, interferon-γ, interferon-γ-induced protein-10 (IP-10), TNF-α, eotaxin, monocyte chemoattractant protein-1 (MCP-1), brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), leptin and insulin were measured using the Human Premixed Multi-Analyte kit – Magnetic Luminex® Screening Assay (R&D Systems). Briefly, microparticles precoated with specific antibodies were added to each well with standards or plasma samples, and they were incubated for 60 min at room temperature in the dark under agitation (900 rpm). After washing the plate (three times with wash buffer), the detection antibody solution was added and the plate was incubated for 30 min at room temperature in the dark. The washing procedure was repeated. A mix with streptavidin-PE solution was added to the plate for 30 min. The washing procedure was repeated again. The samples were resuspended using wash buffer and acquired using the MagPix instrument (Luminex) and xPONENT software (version 3.1; Luminex). The results were converted into pg/ml using the mean fluorescence intensity from the curve standards. The final analysis was carried out with Analyst software 5.1 (Merck Millipore). The concentration of each molecule was calculated using five-parameter regression models.
The plasma adiponectin and the soluble receptors I and II for TNF (sTNFR1 and sTNFR2) were measured by ELISA sandwich according to the procedures supplied by the manufacturer (R&D Systems).
Statistical methods
A preliminary sample estimate (n 29 patients) was determined based on a reported handgrip strength mean improvement (before and after the intervention, respectively) in outpatients with decompensated alcoholic liver disease who received 34 g protein supplementation as a casein-based enteral nutrition product in a controlled trial study(Reference Hirsch, Bunout and De La Maza38). Accordingly, a power of 80 % and a 5 % significance level were adopted. Data related to the sampling process were analysed by the Gpower 3.1.
Descriptive analysis included frequency distribution for categorical variables and measurements of mean and standard deviation for quantitative variables since there was a normal distribution according to the Shapiro–Wilk test. Pearson’s χ 2 test was performed to compare the withdrawal causes according to the treatment group.
The intervention effect was obtained by comparing the means between the WP and CA groups after supplementation using the generalised estimating equation model. The aim of this model was to compare the mean values of the plasma biomarkers and anthropometric variables and the prevalence of oedema, ascites and encephalopathy after the intervention.
The categorical outcome variables were treated as having a binomial distribution with log link function, while the quantitative outcome variables were treated as having a gamma distribution with log link function.
OR, non-standardised coefficients (β) and their respective 95 % CI were calculated for the categorical- and quantitative-dependent variable models. All models were adjusted for baseline, including sex and age.
The working correlation matrix used was unstructured and exchangeable for categorical and quantitative variables, respectively, and its robust estimator covariance matrix was also used.
P values <0·05 were considered statistically significant. All analyses were carried out by the Statistical Package for the Social Sciences (version 20.0, SPSS).
Results
A total of 105 patients with CLD were invited to participate in the study. One subject refused to participate, and four did not meet the inclusion criteria. Of the 100 patients who initiated protein supplementation, five were excluded due to technical problems with the labelling of the sachet supplements. Thus, forty-six patients were allocated to the WP group, and forty-nine were allocated to the CA group, of whom thirty-five and forty patients, respectively, completed the treatment. Study dropout was due to different reasons, such as lack of adherence, intolerance, clinical complications of the disease, liver transplantation and death, without differences between groups (P = 0·948) (Fig. 1).
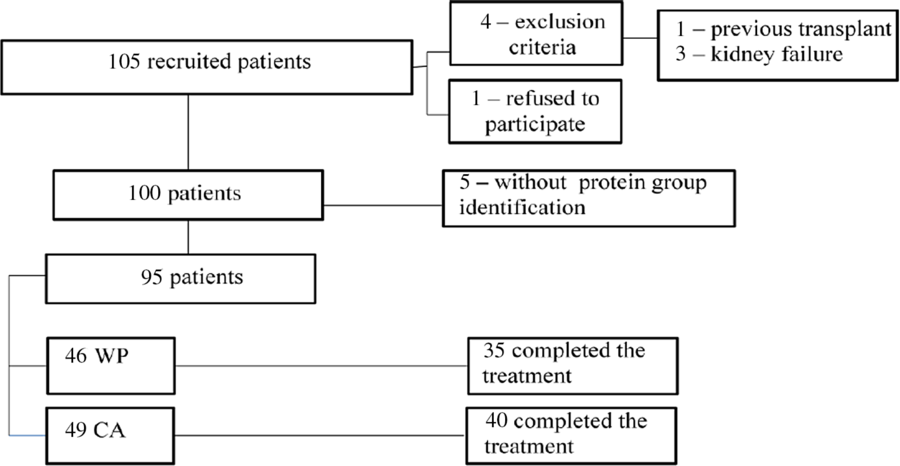
Fig. 1. Flow chart of patient recruitment and distribution in the study. WP, whey protein isolate; CA, casein.
In both groups, the male/female distribution was similar (61·7 and 38·3 %, respectively, in the WP group and 69·4 and 30·6 %, respectively, in the CA group; P = 0·428) as was age (52 ± 9 years in the WP group and 53 ± 11 years in the CA group; P = 0·631).
Alcohol consumption and viral infections were the main causes leading to liver failure in both groups, with similar proportions (66 and 61 % in the WP and CA groups, respectively; P = 0·663), followed by schistosomiasis (Schistosoma mansoni), cancer, and autoimmune and cryptogenic aetiologies.
The MELD scores were similar at baseline (WP group mean MELD = 15, 95 % CI 14, 17; CA group mean MELD = 16, 95 % CI 14, 17; P = 0·796) and at the end of supplementation (β = 0·002; 95 % CI –0·03, 0·04, P = 0·930), even when the patients were stratified by disease severity (Table 1). The proportion of patients with more severe disease was similar in the WP and CA groups (72·3 and 67·3 %, respectively, P = 0·594).
Table 1. Model for end-stage liver disease (MELD) score and clinical parameters of patients with chronic liver disease before and after protein supplementation
(β-Coefficients and 95 % confidence intervals; odds ratios)

CA, casein; WP, whey protein isolate.
* Comparison between CA and WP groups after intervention for quantitative variables.
† Comparison between CA and WP groups after intervention. Significance level P < 0·05. Generalised estimating equations adjusted by sex and age.
‡ Significant difference.
Clinical parameters, that is, ascites, oedema and encephalopathy, were statistically similar in both groups before and after supplementation. Although there was a significant difference in MELD ≥ 15 groups regarding encephalopathy after the intervention, this was related to a lower percentage of patients with this adverse event in the casein group (Table 1).
According to the Subjective Global Assessment, malnutrition was observed in 57·4 and 54·2 % of patients in the WP and CA groups, respectively (P = 0·649). There was no difference in the nutritional status of patients in terms of the MELD scores: malnutrition was seen in 38·5 and 33·3 % (P = 0·778) of patients in the WP and control groups, respectively, with MELD scores <15 and in 64·5 and 62·5 % of those in the WP and control groups, respectively, with the higher MELD scores (P = 0·868) (Table 2).
Table 2. Nutritional status, diet intake and RMR of patients with chronic liver disease before and after protein supplementation
(β-Coefficients and 95 % confidence intervals; odds ratios)
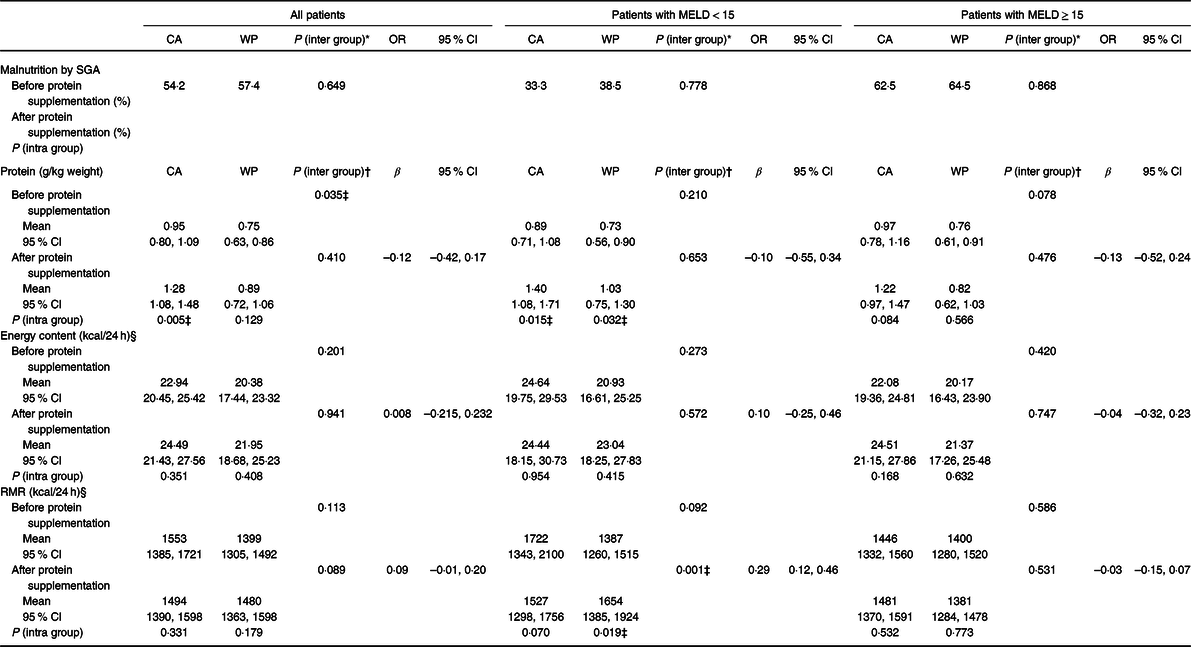
MELD, model for end-stage liver disease; CA, casein; WP, whey protein isolate; SGA, Subjective Global Assessment.
* Comparison between CA and WP groups after intervention.
† Comparison between CA and WP groups after intervention for quantitative variables. Significance level P < 0·05. Generalised estimating equations adjusted by sex and age.
‡ Statistical difference.
§ To convert kcal to kJ, multiply by 4·184.
The protein intake (g/kg of weight) of patients in the WP group was lower at baseline (mean 0·75 g/kg; 95 % CI 0·63, 0·86 g/kg) than that of those in the CA group (mean 0·95 g/kg; 95 % CI 0·80, 1·09 g/kg; P = 0·035), but there was no difference after supplementation (β = –0·12; 95 % CI –0·42, 0·17, P = 0·410). When patients were stratified by MELD score, similar protein intake was seen in both disease severity groups; however, a significant increase after intervention among those with less severe disease (WP and CA MELD < 15 groups) was observed in the intragroup analysis (Table 2). No differences were observed in energetic intake, even when patients were stratified by MELD score (Table 2).
No differences were observed in gastrointestinal symptoms as loss of appetite, nausea and diarrhoea between before (at baseline) and after protein supplementation, in either group or according to the MELD stratification (online Supplementary Table S1).
The RMR increased after WP supplementation among patients with less severe disease (MELD < 15) compared with the RMR in the CA group (β = 0·29; 95 % CI 0·12, 0·46, P = 0·001), with no differences in this parameter between the WP and CA groups in patients in the MELD ≥ 15 category (Table 2).
There were no differences in the body composition parameters (mid-arm muscle circumference, fat-free mass, fat mass and phase angle) or muscle functionality (handgrip strength and 6-min walking test) between before (at baseline) and after protein supplementation in either group or in the MELD-stratified groups (online Supplementary Table S2).
Most peripheral cytokines (TNF, IL-6, IL-1β and interferon-γ) (online Supplementary Table S3), immunomodulatory proteins (sTNFR1, sTNFR2, BDNF and GDNF) and immunomodulatory hormones (adiponectin, insulin and leptin) (online Supplementary Table S4) were similar in both groups at the two assessed time points. Although IL-10 presented a higher level at baseline in the WP group (mean 14·53 pg/ml; 95 % CI 11·54, 17·52 v. CA group, mean 10·81 pg/ml; 95 % CI 8·79, 12·82, P = 0·044), this difference was not maintained after protein supplementation (β = 0·15; 95 % CI –0·10, 0·41, P = 0·241) (Table 3).
Table 3. Plasma immunomodulatory and chemokine proteins of patients with chronic liver disease before and after protein supplementation
(β-Coefficients and 95 % confidence intervals)
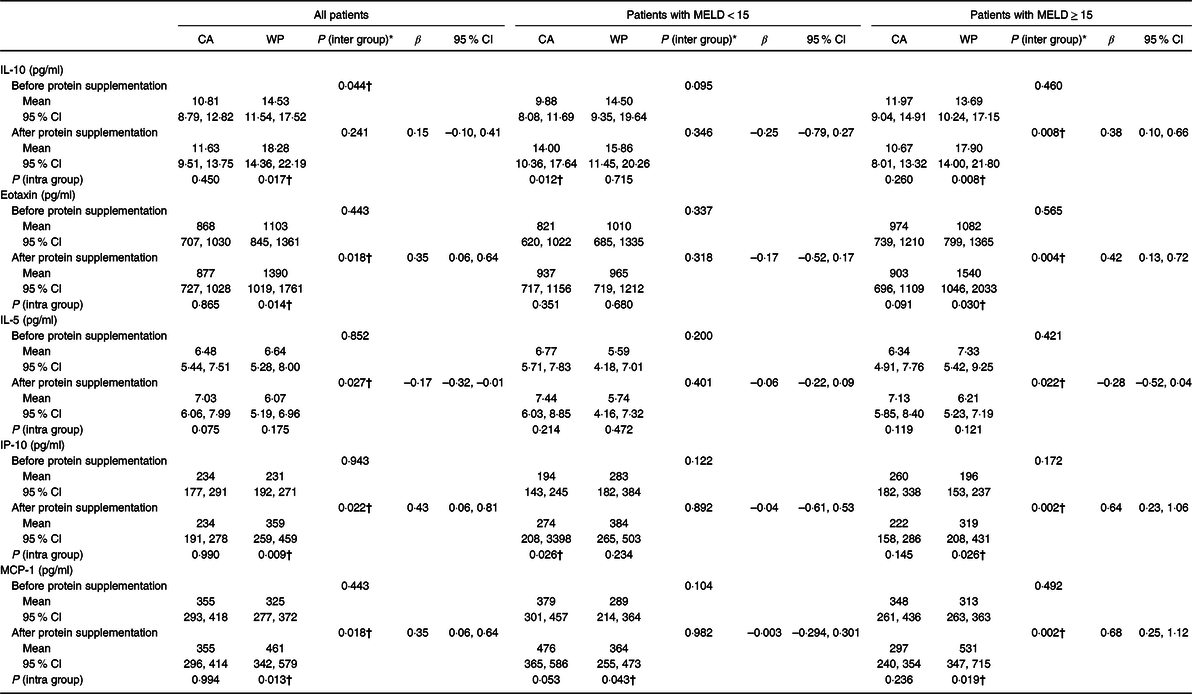
MELD, model for end-stage liver disease; CA, casein; WP, whey protein isolate; IP-10, interferon-γ-induced protein-10; MCP-1, monocyte chemoattractant protein-1.
* Comparison between WP and CA groups after intervention. Significance level P < 0·05. Generalised estimating equations adjusted by sex and age.
† Significant difference.
Differences were observed in the WP group for all measured chemokines. Increased levels of IP-10/CXCL10 (β = 0·43; 95 % CI 0·06, 0·81, P = 0·022), eotaxin-1/CCL11 (β = 0·22; 95 % CI 0·02, 0·42, P = 0·031) and MCP-1/CCL2 (β = 0·35; 95 % CI 0·06, 0·64, P = 0·018) and a decreased level of IL-5 (β = –0·17; 95 % CI –0·31, –0·01, P = 0·027) were observed in the WP group at the end of treatment (Table 3). The same pattern of effects was observed in patients with more severe disease supplemented with WP, for whom increased levels of IP-10/CXCL10 (β = 0·64; 95 % CI 0·23, 1·06, P = 0·002), eotaxin-1/CCL11 (β = 0·42; 95 % CI 0·13, 0·72, P = 0·004) and MCP-1/CCL2 (β = 0·68; 95 % CI 0·25, 1·12, P = 0·002) and lower levels of IL-5 (β = –0·28; 95 % CI –0·52, –0·04, P = 0·022) were also found. Among WP patients with MELD ≥ 15, IL-10 was increased at the end of supplementation period (β = 0·38; 95 % CI 0·10, 0·66, P = 0·008) (Table 3).
Baseline values are demonstrated in supplemental tables (online Supplementary Tables S5 and S6).
Discussion
Patients with end-stage liver disease waiting for liver transplantation who were supplemented with either casein or whey protein for 2 weeks presented no differences in nutritional status or several immune markers after the supplementation period. However, patients who received WP had a different response pattern in regard to IL-10 and chemokines.
Patients waiting for liver transplantation include those with more severe disease, justifying the elevated mean MELD score in the present study, in agreement with previous reports(Reference Ferreira, Ferreira Martins and Cunha4,Reference Pleli, Martin and Kronenberger39) . The more severe the disease, the greater the prevalence of malnutrition, which is frequently associated with clinical complications and immune dysfunction, increasing the mortality rate(Reference Pérez-Reyes, Rivera-Sánchez and Servín-Caamaño40,Reference Schaible and Kaufmann41) . Our results revealed a prevalence of malnutrition of more than 50 % among patients and more than 60 % among those with more severe disease, similar to data reported by other authors(Reference Pérez-Reyes, Rivera-Sánchez and Servín-Caamaño40,Reference Huisman, Trip and Siersema42) , although higher percentages have also been described(Reference Bunchorntavakul, Supanun and Atsawarungruangkit43,Reference Parkash, Jafri and Munir44) . The discrepancy in malnutrition frequency might be related to differences regarding the assessment methods of nutritional status(Reference Cederholm, Jensen and Correia45).
Malnutrition encompasses decreased nutrient intake, loss of muscle mass and functionality, and energy metabolism alterations(Reference Dasarathy46). Poor protein and energetic intake are frequently observed in cirrhotic patients due to restrictive diets and clinical complications such as ascites, anorexia and early satiety(Reference Anand47,Reference Garcia Ferreira, Rezende Anastácio and Soares Lima48) . We found the same pattern of insufficient protein (<1·0 g/kg weight) and energetic intake (approximately 20 kcal (84 kJ)/kg weight) in both groups of patients before the supplementation trial. After supplementation, the protein intake was similar for both the WP and CA groups, including the MELD subgroups, but patients supplemented with WP were unable to reach the minimum protein recommendations for CLD patients (1·2 g/kg weight)(Reference Plauth, Bernal and Dasarathy49). Although we expected to observe effects on muscle mass after WP supplementation due to its higher concentration of branched-chain amino acids, especially leucine, and higher digestibility than CA(Reference Tang and Phillips50,Reference Boirie, Dangin and Gachon51) , these effects were not observed. WP supplementation might have further impacted early satiety due to its faster absorption(Reference Anderson, Luhovyy and Akhavan52–Reference Jakubowicz and Froy54), preventing the patients from reaching adequate protein and energy intake. Both the WP and CA groups had insufficient energy intake before and after treatment, which could negatively affect protein synthesis(Reference Hayashi, Matsumoto and Momoki55). Furthermore, the trial period (15 d of supplementation) may have been too short to assess such differences in addition to the methodological limitations regarding the body compartment assessment, such as anthropometry and bioelectrical impedance, which may not be sensitive enough to detect short-term muscle mass changes.
The effects of physical exercise and insulin on whole-body protein synthesis should be considered(Reference Plauth, Bernal and Dasarathy49). Our patients were physically inactive, and we did not find any effect of the supplementation on insulin levels, although some authors have demonstrated the ability of WP to increase its secretion(Reference Anderson, Luhovyy and Akhavan52,Reference Jakubowicz and Froy54) . The maintenance of muscle and fat masses after protein supplementation may, in part, explain the lack of supplementation effects on insulin, adiponectin and leptin levels(Reference Arnberg, Mølgaard and Michaelsen56–Reference Niu, Kobayashi and Guan59).
Similar to the absence of the effects of supplementation on the amount of muscle mass, no effect was observed on muscle functionality. Our result was similar to that found at baseline by Aamann et al. (Reference Aamann, Dam and Borre60), who, in a prospective study, investigated whether resistance training would increase muscle strength and mass in patients with cirrhosis. Although they reported a positive effect of resistance training on both parameters, they stated that the results can only be generalised to patients with sufficient protein intake, established as 1·2 g/kg of body weight. Our patients did not reach this threshold; therefore, we speculate that as a result, no differences were seen after the intervention on the 6-min walking test. Most likely, for the same reason, no difference was observed in handgrip strength, although baseline values were in accordance with previous authors’ data(Reference Daphnee, John and Vaidya61,Reference Nunes, Bassani and Fernandes62) .
Protein intake usually increases energy expenditure as a result of the energy required for the initial steps of metabolism, storage and oxidation, including urea synthesis(Reference Westerterp-Plantenga, Nieuwenhuizen and Tomé63). However, both WP and CA supplementation did not impact the RMR of most patients, since only MELD < 15 patients supplemented with WP had increased values afterwards. This might be a consequence of the high digestibility of WP compared with that of casein, although this was not seen in the patients with more severe disease, and it could be speculated that these individuals presented with deranged absorption. An elevated amino acid concentration associated with high and fast digestibility implies a metabolic process, which readily increases thermogenesis and, consequently, the RMR(Reference Kassis, Godin and Moille64–Reference Westerterp-Plantenga66).
Immune dysfunction is well established in patients with cirrhosis, regardless of its aetiology. Stimulation of the immune system is a potential target for treatment to improve clinical conditions and nutritional status(Reference Chandra67). WP supplementation impacted immune markers, including those in patients with more severe disease. Our results showed an immunomodulatory effect after WP supplementation reflected by increased levels of eotaxin-1/CCL11, IP-10/CXCL10 and MCP-1/CCL2, including in patients with more severe disease. Proteins whose secretion was stimulated in the WP group are classically involved in the innate response and are mostly produced by macrophages, fibroblast cells, Kupffer cells, endothelial cells, dendritic cells and monocytes.
A pro-inflammatory profile was observed in the WP group. It is important to address that CLD patients have a spectral range from inflammatory to immunodeficiency responses(Reference Albillos, Lario and Álvarez-Mon6). Some authors have demonstrated that increased chemokine production can be harmful to CLD patients, as the exacerbation of inflammation contributes to fibrosis development and cirrhosis evolution(Reference Dirchwolf and Ruf68,Reference Martínez-Esparza, Tristán-Manzano and Ruiz-Alcaraz69) . On the other hand, chemokines, including IP-10/CXCL10, MCP-1/CCL2 and eotaxin-1/CCL11, are responsible for cell migration and the control of several infections, including liver infection(Reference Bone-Larson, Hogaboam and Evanhoff70–Reference Schwabe, Wiley and Marra72). In liver disease patients, both deleterious and beneficial effects exerted by IP-10/CXCL10(Reference Chen, Lv and Wen73–Reference Koniaris, Zimmers-Koniaris and Hsiao76), MCP-1/CCL2(Reference Li, You and Fan77,Reference Baeck, Wei and Bartneck78) and eotaxin(Reference Pham, Bernuau and Durand79–Reference Goh, Henderson and Heredia82) have been described. Therefore, determining whether the increased production seen after WP intake will be harmful or protective for these patients requires further assessments. Inflammatory chemokines contribute substantially to the recruitment of leucocyte populations and will regulate any non-leucocyte tissue cells (epithelial, mesenchymal and endothelial) important in defence mechanisms(Reference Oo, Shetty and Adams83,Reference Hughes and Nibbs84) . In contrast, IL-5 is frequently reported to be involved in liver lesion progression(Reference Reiman, Thompson and Feng85,Reference Tuxun, Apaer and Ma86) . Thus, its decreased production in CLD patients after WP supplementation contributes to our hypothesis that WP plays an immunomodulatory role associated with the increase in IL-10. The latter cytokine has a general suppressive effect, preventing increased exacerbations of innate and adaptive immune responses, controlling detrimental pathological injury(Reference Sziksz, Pap and Lippai87). The reported effects of IL-10 have been mostly beneficial for CLD patients(Reference Horst, Neumann and Diehl88–Reference Zhang90).
The reason WP supplementation exerted these effects on CLD patients, including those with more severe disease, is not well understood since we could not find explanations for these specific findings in the literature. One potential explanation could be related to the effects exerted by whey protein on the liver by increasing apoptotic signals and inflammatory markers(Reference DeKlotz, Roby and Friedlander91–Reference Whitt, Ward and Deniz94). Additionally, potentially increased intestinal permeability due to the weak barrier observed in CLD patients(Reference Liboredo, Vilela and Ferrari95) may increase the risk of food allergies(Reference Wang and Sampson96) and overdosing nutrients, as observed under supplementation conditions(Reference Bischoff, Barbara and Buurman97). Furthermore, protein consumption can modify the microbiota(Reference Moreno-Pérez, Bressa and Bailén98), favouring chronic systemic inflammation as a result of precipitating events, for which antigen-like peptides could be responsible(Reference Clària, Stauber and Coenraad99). Kiewiet et al. (Reference Kiewiet, Dekkers and Gros100) compared the immune effects and toll-like receptor activation and inhibition effects of whey and casein hydrolysates with different hydrolysis levels. These authors found that cells stimulated by intact WP and its hydrolysates were capable of inducing peripheral blood mononuclear cell cytokine secretion in healthy individuals, and the peptides of WP had a profound activating effect on toll-like receptor signalling, effects not observed for CA protein or its peptides. These effects were protein-type specific since none of the casein hydrolysates impacted toll-like receptor activation.
Systemic inflammatory diseases commonly cause alterations in a patient’s behaviour, including the development of fatigue, anxiety and loss of appetite and social interest, collectively termed sickness behaviour(Reference D’Mello and Swain101). In mice with liver inflammation, sickness behaviours were observed and the inhibition of cerebral monocytes in those animals was associated with the improvement of this condition(Reference D’Mello, Le and Swain102). Evidence suggests a link between neuroinflammation, predominantly modulated by microglia(Reference Liere, Sandhu and DeMorrow103), and impaired motor and cognitive functions during hepatic encephalopathy(Reference Görg, Karababa and Shafigullina104,Reference Wen, Schroeter and Klöcker105) . Additionally, recent studies have shown that eotaxin-1/CCL11 is able to influence neural progenitor cells and microglia(Reference Teixeira, Gama and Rocha106). Although the significant odds for encephalopathy among the severe CLD patients supplemented with WP was due to the reduction in this event among those supplemented with CA, we investigated brain alterations. Brain alterations are a consequence of both the immune response and increased protein consumption. By measuring neurotrophic factors and considering that WP increased MCP-1 and eotaxin-1/CCL11 levels, no differences were observed in either BDNF or GDNF levels. Both are important for central nervous system homoeostasis, particularly after damage and/or inflammation(Reference Allen, Watson and Shoemark107). Based on the evidence that dairy products may have beneficial outcomes for neurocognitive health(Reference Camfield, Owen and Scholey108,Reference Min, Kobayashi and Mogi109) , our expectation that milk protein supplementation could positively affect these neurotrophins was confirmed, not by an increase in their levels but by the observation that even under pro-inflammatory conditions, there was no reduction in their concentrations.
Although protein supplementation does not trigger gastrointestinal symptoms suggestive of protein intolerance, we cannot eliminate the possibility that WP may exacerbate pre-existing liver damage, but the increased IL-10 in patients with more severe disease supplemented with WP can contribute to preventing increased exacerbations of innate and adaptive immune responses, controlling pathological injury(Reference Sziksz, Pap and Lippai87). However, we could not determine the clinical meaning of this immunomodulation in liver disease or on the nutritional status of patients.
The present study has some limitations. The duration of the clinical trial was short. A relevant issue regarding clinical trials is the dropout rate, especially when enrolling patients with chronic severe diseases. Furthermore, diet and supplement intake were controlled basically by a retrospective data collection that required an extensive dependence on the recent memory of the study subject. In addition, patients’ energy intake did not reach the recommended values, which may further compromise the impact of protein supplementation. Regarding the immune investigation, although we evaluated a wide panel of immune molecules, we did not evaluate cell function, which could enlighten us to better understand the clinical impact of WP supplementation. To our knowledge, this is the first clinical trial investigating the effect of WP supplementation on end-stage CLD patients, and our results have raised the need for further investigations to establish the clinical significance of WP intake on the nutritional and immunomodulatory status of these individuals.
In conclusion, our results showed that WP consumption by patients with CLD impacted immunomodulatory responses when compared with the intake of casein by increasing pro- and anti-inflammatory protein synthesis with no impact on nutritional status.
Acknowledgements
This work was supported by the FAPEMIG (S. V. G., grant number APQ-02216-14) and CNPq (M. I. T. D. C., grant number 301593/2016-7).
FAPEMIG and CNPq had no role in the design, analysis or writing of this article.
Y. G. G. M. worked in study conception and design, data acquisition, analysis and interpretation, article writing and critical review of important intellectual content and final approval of the version to be published. T. A. S., M. O. d’A. and S. V. G. guided the collection, analysis and interpretation of data, and critical review of relevant intellectual content and final approval of the version to be published. A. L. T. collaborated in the relevant critical review of the intellectual content. A. S. L. collaborated with data acquisition. E. L. M. V. worked in study conception and design, data acquisition, analysis and interpretation, article writing and critical review of important intellectual content and final approval of the version to be published. M. I. T. D. C. worked in study conception and design, analysis and interpretation, article writing and critical review of important intellectual content and final approval of the version to be published.
All authors agree with all aspects of the work, ensuring that issues related to the accuracy or integrity of any part of the work have been properly investigated and resolved.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520003219







