Nutrition practices established during infancy underpin the development of taste preference, food acceptance, enjoyment of family-style foods and act as a foundation for future eating patterns( 1 , Reference Birch, Savage and Ventura 2 ). Beyond 1 year of age, once the transition to a mixed diet has been achieved, the focus shifts to increasing the variety of foods offered and allowing children the opportunity to develop their own food preferences( Reference Northstone and Emmett 3 ). Comparative research on the nutrient intake of children 1–2 years of age are limited( Reference Hilger, Goerig and Weber 4 ). In a cross-national comparison of four countries (Germany, Russia, USA and Brazil) inadequate intake of >20 % were seen for vitamins D, E and A and Ca( Reference Hilger, Goerig and Weber 4 ), however, data were not available for all nutrients in each country. In 16–24-month-old children from Australia, inadequate intake >5 % below the estimated average requirements (EAR) was reported for Fe (23 %), vitamin A (7 %), vitamin C (14 %) and Ca (8 %)( Reference Webb, Rutishauser and Knezevic 5 ).
The limitations inherent in dietary assessment tools, can result in misinterpretation of nutrient intake data, particularly in young children( Reference Livingstone, Robson and Wallace 6 ). The assessment of dietary patterns considers the synergistic effects of food that contribute to usual intake, nutrient combinations and habitual consumption( Reference Robinson, Marriott and Poole 7 , Reference Ambrosini 8 ), as it is likely there are additive effects of food constituents on health. Principal component analysis (PCA) is a multivariate, a posteriori, data-driven reduction technique commonly used to summarise dietary data( Reference Jolliffe 9 , Reference Hu 10 ); and in a recent systematic review, FFQ were found to be the most common method used to derive dietary patterns in children under the age of 5 years( Reference Smithers, Golley and Brazionis 11 ). Few studies have described the dietary patterns of children under 2 years( Reference Robinson, Marriott and Poole 7 , Reference Smithers, Brazionis and Golley 12 – Reference Kiefte-de Jong, de Vries and Bleeker 16 ) or the energy and nutrient intake during the dietary transition from complementary to family foods( Reference Kiefte-de Jong, de Vries and Bleeker 16 – Reference Shin, Oh and Park 18 ).
Growing Up Milk (GUM) or young child formula are a milk-based alternative to cows’ milk (CM) or breast milk for children 1–3 years of age, fortified with nutrients that are commonly low during the transition to family-based foods( Reference Hojsak, Bronsky and Campoy 19 ). Although not necessary for adequate nutrition, GUM may compensate for nutritional deficiencies during this period through improving nutritional status( Reference Przyrembel and Agostoni 20 ), but little is known about their effects on dietary patterns and nutrient intake over the entire second year of life. There are no reported safety issues associated with the use of GUM, however, concerns remain over its use to provide adequate nutrition in place of a varied diet, increasing dependence on a ‘liquid dietary intake’ over regular food consumption and disrupting satiety( Reference Hojsak, Bronsky and Campoy 19 , 21 , 22 ). In a recent position statement, the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) conclude that there is no necessity for routine use of GUM but suggest they may have a small role to play as a strategy to increase the intake of nutrients such as Fe and vitamin D during this period( Reference Hojsak, Bronsky and Campoy 19 ).
No studies have described the dietary patterns in New Zealand children aged 1–2 years, and data on Australian children remain limited( Reference Bell, Golley and Daniels 23 ). We aimed to evaluate the prospective whole-of-diet patterns in the children enrolled in the Growing Up Milk – Lite (GUMLi) trial using PCA, assess their energy and nutrient intakes and assess the impact of the GUMLi intervention over the 12-month trial period.
Methods
Study design
The GUMLi trial was a multicentre, two-arm, double-blinded, randomised controlled trial (RCT) consisting of 160 healthy children of 1-year of age, living in Auckland (n 108) and Brisbane (n 52). The main trial evaluated the effect of consuming GUMLi (reduced protein) compared with unfortified CM as part of a whole diet for 12 months, on body composition at 2 years of age (primary outcome)( Reference Wall, Hill and Lovell 24 ).
Secondary outcomes included measures of dietary intake; anthropometry; micronutrient status and cognitive development. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and ethical approval was obtained from the Northern B Health and Disability Ethics Committee, Ministry of Health, New Zealand (14/NTB/152) and the University of Queensland Medical Research Ethics Committee, Brisbane, Australia (2014001318). Written, informed consent was provided by the primary caregivers.
Study intervention
Participants were randomised 1:1 to GUMLi (intervention) or CM (control) (n 80 per group) for a period of 12 months. The study milks were provided at no cost to participants and were produced in powder form, packaged in plain, identical 900-g tins, with no additional nutrient information panels or nutrition-related statements. The study milks were independently allocated by Danone Nutricia Research. Both researchers and participants were blinded to treatment allocation until completion of the trial. Parents were not given any dietary advice during the intervention and breast-feeding continued throughout the trial, if desired. Any use of other infant formulas at baseline was discontinued after randomisation.
The compositional differences between the two study milks are described in Table 1. GUMLi had a reduced energy and protein content when compared with standard, commercial GUM on the market, 249kJ/100 ml v. 298kJ/100 ml and 1·7 g/100 ml protein v. 2·2 g/100 ml. An energy-matched, non-fortified CM was used as an active control, with a protein content of 3·1 g/100 ml. In addition, GUMLi was fortified with Fe and vitamin D. As with commercially available GUM, GUMLi was fortified with vitamin C to increase the bioavailability of non-heme Fe. The amount of vitamin C in GUMLi was higher than the standard GUM (17 mg/100 ml v. approximately 15 mg/100 ml).
Table 1 Nutritional composition of cows’ milk (CM) and Growing Up Milk – Lite (GUMLi) per 100 ml of prepared product
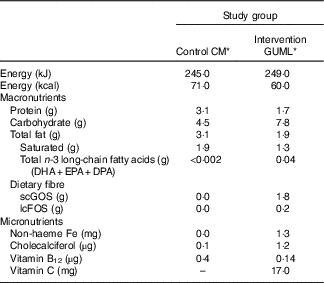
DPA, docosapentaenoic acid; scGOS, short-chain galacto-oligosaccharides; lcFOS, long-chain fructo-oligosaccharides.
* Obtained from manufacturer, values based on average totals from three batches produced for use in the GUMLi trial.
Child dietary assessment
Diet was assessed via a face-to-face interview, using the previously validated, interviewer-administered Eating Assessment in Toddlers (EAT) FFQ at baseline, 3, 6, 9 and 12 months post-randomisation. The EAT FFQ is designed to describe dietary intake over the previous 4 weeks, with good validity and high reproducibility for intake of macronutrients, key micronutrients( Reference Watson, Heath and Taylor 25 ) and dietary patterns( Reference Mills, Skidmore and Watson 26 ). The EAT FFQ contained ninety-nine food items that were collapsed into sixteen non-overlapping food groups based on nutrient profile and similarity of use, as previously described( Reference Watson, Heath and Taylor 25 , Reference Mills, Skidmore and Watson 26 ). Further information about the sixteen food groups and food items assigned to each group can be found in online Supplementary material (online Supplementary Table S1). A total of eleven nutrients were derived using the original EAT FFQ design and included nutrients such as Fe and vitamin D, identified by the European Food Safety Authority as key nutrients that may require specific attention in children 1–3 years of age( 27 ).
Nutrient intake
The amount (g) consumed per d for each food item was calculated by multiplying the frequency of consumption per d, volume (g) and the total amount eaten. Volume was calculated as natural food portions or using the child’s palm volume( Reference Watson, Heath and Taylor 25 , Reference Mills, Skidmore and Watson 26 , Reference Taylor, Heath and Galland 28 ). Nutrients were calculated as previously described( Reference Watson, Heath and Taylor 25 ). Nutrient analyses were performed in MATLAB® (MathWorks Inc.), using a custom written programme verified by hand calculations. Implausible energy intakes were identified using Tukey’s fences method of interquartile range (IQR) calculated as a lower cut-off of (Q1–(1·5×IQR)) and upper cut-off of (Q3+(1·5×IQR))( Reference Huang, Roberts and Howarth 29 ) and full dietary data excluded from the analysis( Reference Huang, Roberts and Howarth 29 ). Before identifying the baseline dietary patterns, three participants were excluded due to implausible energy intakes (n 157).
Identifying dietary patterns
Dietary patterns were derived using exploratory PCA of the baseline EAT FFQ administration (n 157). The frequency of intake per d for the sixteen food groups was used. The use of frequency of intake data allowed the inclusion of breast milk, as the portion size could not be estimated. The number of components was identified using the breakpoint on the scree plot( Reference Cattell 30 ), eigenvalues >1, components that account for at least 10 % of the total variance and component interpretability after varimax rotation. Varimax (orthogonal) rotation was performed to maximise the variance within components, resulting in uncorrelated factors( Reference Gorsuch 31 ). Food groups with an absolute factor loading ≥0·3 on a component were considered to have a strong association. The highest absolute loading score for each food group was considered the most informative in describing the dietary pattern. For each pattern identified at baseline, an individual score for each participant was created at each visit by multiplying the factor loadings by the corresponding standardised value for each food group and summing across the food groups. A positive factor loading score was associated with a higher standardised z score per food group consumption for an individual participant, and a negative factor loading score indicated an inverse association.
Statistical analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc.), following the principle of intention to treat, including all randomised participants who provided valid trial data for analysis. All statistical tests were two sided at a 5 % level of significance. Participant demographics and baseline characteristics were summarised by treatment group using descriptive statistics. Categorical variables were described as frequencies and percentages; continuous variables as means and standard deviations.
Dietary intakes for the sixteen food groups assessed by the EAT FFQ were summarised by intervention group at each visit. Dietary patterns were identified using PCA on the baseline dietary data. An individually standardised z score was calculated for each participant and dietary pattern at baseline and converted into quartiles. The differences in eleven key nutrients between participants in different quartiles were tested using the Kruskal–Wallis test.
Adherence to the dietary patterns identified at baseline was measured over time, using the scoring coefficients from baseline applied to each child’s food intake at later follow-up time points( Reference Twisk, Kemper and Mellenbergh 32 , Reference Luque, Escribano and Closa-Monasterolo 33 ). The impact of the GUMLi intervention on the dietary pattern individual z scores and average daily nutrient intake compared with CM was evaluated at each time point post-randomisation, using random effects mixed models with an interaction term between treatment group and time point, adjusting for baseline outcome value and study location. An unstructured correlation structure was applied to repeated measures on the same participant over time with a random cluster effect. Missing data at follow-up visits were taken into account in the model using the maximum likelihood method, assuming they were missing at random. Model-adjusted mean differences and 95 % CI were reported at each time point with associated P values.
Results
Population baseline characteristics
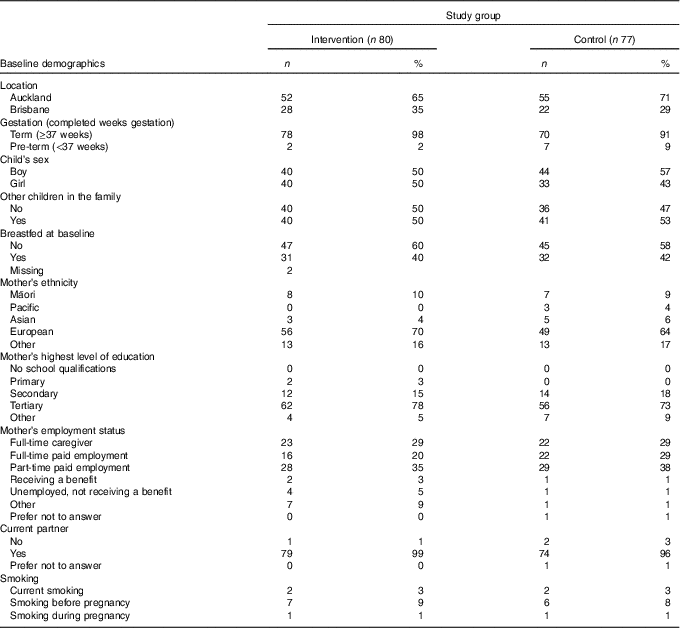
A total of 160 eligible children aged 1 year were randomly assigned 1:1 to receive the intervention (GUMLi) or non-fortified CM in the main trial. Characteristics of the two treatment groups included in the analysis of patterns and nutrients at 1 year of age are reported in Table 2 (n 157). A total of three participants (one GUMLi and two CM) were excluded from the analysis of dietary patterns and nutrients due to implausible estimated energy intake using the method described by Huang et al. ( Reference Huang, Roberts and Howarth 29 ) Tukey’s fences method of IQR were calculated as a lower cut-off (Q1–(1·5×IQR)) and upper cut-off (Q3+(1·5×IQR)). A flowchart of participants with complete dietary data and plausible estimated energy intake during the study period is reported in online Supplementary material (online Supplementary Fig. S1). There were no differences between treatment groups at baseline. Approximately half the participants were the only child, 40 % were breastfed at baseline and mothers were predominantly European and had achieved secondary education or higher.
Table 2 Characteristics of the cohort included in the analysis of patterns and nutrients at 1 year of age (Numbers and percentages)
Sixteen food groups
Mean (sd) daily intakes of the sixteen food groups expressed as frequency per d at each time point are displayed in online Supplementary Table S2. Over time, there were no differences in the frequency of intake between GUMLi or CM groups for baby/toddler food; bread/pasta/low-sugar-cereal; meat; eggs/beans; fruit; milk and milk products; breast milk; hot chips/roast potato/kumara and savoury snacks. There were no differences in the frequency of intake of toddler milk and infant formula, as from month 3 of the intervention this category recorded consumption of the study milk, that is, 300 ml/d of group A (CM) and group B (GUMLi). Children in the GUMLi group had a higher mean frequency of intake of processed meats at month 6, and consistently higher frequency of intake of vegetables following randomisation (i.e. from month 3). However, the GUMLi group also had a higher frequency of intake of sweet foods at baseline and month 9 and the CM group had a higher frequency of intake of sweet drinks at month 6 and spreads and savoury snacks at month 3.
Baseline dietary patterns
A total of three major dietary patterns were identified using PCA at baseline, accounting for 39 % of the variability within the sample, with the first pattern explaining 15 %, followed by 13 and 11 % in the second and third patterns, respectively. The first dietary pattern ‘junk/snack foods’ was characterised by positive loadings on hot chips/roast potato/kumara, processed meats, sweet foods, nutritive drinks, savoury snacks, sweet drinks and spreads (by descending order of PCA loadings), and with negative loadings on formula, breast milk, meat/fish, vegetables and fruit. The ‘healthy/guideline foods’ dietary pattern was positively associated with vegetables, fruit, bread/pasta, meat/fish, milk/milk products, eggs/beans and spreads, with negative loadings on sweet foods, savoury snacks, sweet drinks, fries/roast potato/kumara, breast milk, formula, nutritive drinks and baby/toddler food. The ‘breast milk/formula’ dietary pattern loaded with opposite strength on breast milk (0·84) and formula (including GUM) (–0·82) (Table 3).
Table 3 Food groups included in principal component analysis and varimax-rotated factor loading of the three dietary patterns at baseline
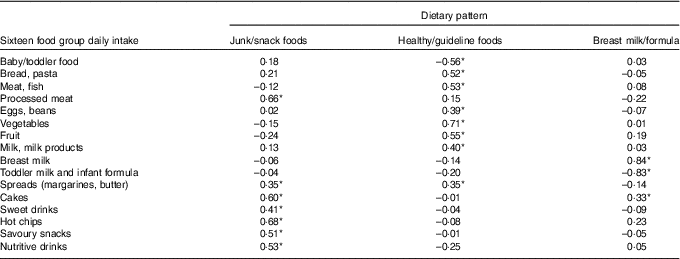
* Loading ≥0·30 to aid labelling of dietary patterns.
Construct validity of dietary patterns
Baseline nutrient intakes according to quartiles of dietary pattern scores were identified and quartiles 1 (Q1) and 4 (Q4) are displayed in online Supplementary Table S3. Those in Q1 had individual scores that were least associated with the dietary pattern, and those in Q4 had scores most associated with the dietary pattern. Higher scores on the ‘junk/snack food’ pattern were associated with higher total energy, protein, fat and carbohydrate intake, and higher scores on the ‘healthy/guideline foods’ pattern were associated with higher total energy, protein, total fat, fibre, Zn and vitamin B12 intake. Both the ‘junk/snack food’ pattern and the ‘healthy/guideline foods’ pattern showed significant trends for total energy, protein and total fat intakes, increasing from Q1 to Q4. To elucidate any differences in these trends, the difference in nutrient intake between Q4 and Q1 was calculated. Scores on the ‘junk/snack’ pattern and ‘healthy/guideline’ pattern were associated with 1143·9 v. 825·9 kJ difference in median energy, a 17·1 v. 19·2 g difference in median protein and 12·7 v. 15·2 g difference in median total fat intake between Q4 and Q1, respectively. Higher scores on the ‘breast milk/formula’ pattern were associated with lower intake of total energy, protein, fat, carbohydrate, Ca, Fe, Zn and vitamins B12, C and D compared with those in Q1 who were less adherent to the dietary pattern, thereby consuming more formula.
Effect of the intervention on dietary patterns over time
The effect of the intervention on dietary patterns at each time point was investigated. Standardised z scores of the dietary patterns at baseline, 3, 6, 9 and 12 months post-randomisation are presented in Table 4. No significant differences in the z scores were seen between CM and GUMLi groups for the ‘junk/snack foods’ or ‘healthy/guideline foods’ patterns over time. A significant group difference was observed in the ‘breast milk/formula’ dietary pattern z scores at 12 months post-randomisation (P=0·041), where those in the GUMLi group had more a positive loading on the dietary pattern.
Table 4 Effect of the intervention on dietary pattern scores at follow-up visits (Mean values and standard deviations; differences and 95 % confidence intervals)
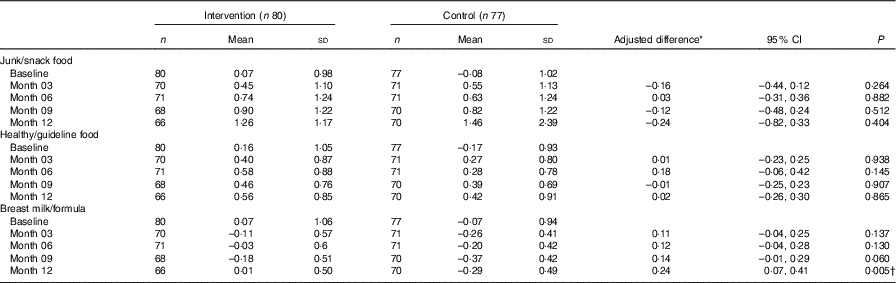
* Repeated-measures mixed model with an unstructured covariance structure, adjusting for baseline outcome and study location.
† P<0·05.
Nutrient intake
Mean (sd) nutrient intakes/d at baseline, 3, 6, 9 and 12 months post-randomisation are displayed in online Supplementary Table S4. There was no difference in the total intake of carbohydrates, fibre and Ca consumed per d between groups. At month 3, the GUMLi group had a lower total energy intake compared with the CM group (P=0·012). Total protein intake was significantly different at months 3 and 9 (P<0·0001 and P=0·0042, respectively), where the CM group consumed higher total protein intake. By 2 years of age, the GUMLi group consumed 65·8 g/d protein compared with 69·0 g/d in the CM group (P=0·451). The contribution of protein according to the sixteen EAT FFQ food groups was calculated (data not shown). Both groups had similar protein intake from milk and milk products (19·2 v. 19·9 g/d, CM and GUMLi, respectively), and the impact of a lower protein intervention milk was apparent (9·8 v. 4·9 g/d, CM and GUMLi, respectively). Total fat intake per d was similar between groups, except for month 3, where the CM group consumed a greater amount (P<0·0001). There was a significant difference in total Fe consumption between CM and GUMLi groups, with the GUMLi group consuming higher total Fe intake per d from month 3 (P<0·0001). No children exceeded the upper limit for Fe of 21·0 mg/d( 34 ). At month 6, the GUMLi group had a greater total Zn intake (P=0·0202). From month 3, those in the GUMLi group consumed significantly more vitamins C and D compared with the CM group (P<0·0001). From month 3, the CM group had significantly higher total vitamin B12 intake compared with the GUMLi group.
The EAR cut point method( Reference Carriquiry 35 , 36 ) was used to determine the number of participants that did not meet the nutrient reference values( 34 ) for the eleven nutrients specified by the EAT FFQ (data not shown). After 12 months of the intervention, 86 % (n 61) of the CM participants and 9 % (n 6) of the GUMLi participants had intake less than the EAR for vitamin C (GUMLi: 60·3 mg/d; CM: 19·3 mg/d; EAR: 25·0 mg/d; P<0·0001). In all, 24 % (n 17) of the CM participants and 1·5 % (n 1) for the GUMLi participants did not meet the EAR for Fe (GUMLi: 10·2 g/d; CM: 5·8 g/d; EAR: 4·0 g/d; P<0·0001) and 23 % (n 16) of the CM participants and 9 % (n 6) of the GUMLi participants did not meet the adequate intake (AI) for vitamin D (GUMLi: 7·2 µg/d; CM: 4·4 µg/d; AI: 5 µg/d; P<0·0001).
Discussion
In this RCT, we evaluated the effect of consuming a reduced protein, micronutrient-fortified GUM (GUMLi) compared with unfortified CM for 12 months on dietary patterns. This is the first study to use an applied dietary pattern z score on longitudinal trial data. Our findings suggest that consumption of GUMLi did not affect dietary patterns, however, lowered protein intakes and improved intake of Fe, vitamins D and C and Zn when compared with CM. We have also been able to identify trends in nutrient intake across dietary pattern scores identified at baseline, demonstrating that the EAT FFQ is able to describe dietary patterns and differences in both food and nutrient intake in children under 2 years of age.
Identified dietary patterns
A total of three clear patterns were identified at baseline and demonstrated similarities with cohorts of children at similar ages, including the Avon Longitudinal Study of Parents and Children (ALSPAC)( Reference Smithers, Brazionis and Golley 12 ), the Southampton Women’s Study (SWS)( Reference Robinson, Marriott and Poole 7 ), the Norwegian Mother and Child Cohort (MoBa)( Reference Ystrom, Niegel and Vollrath 37 ) and the French Etude des Déterminants du développement et de la santé de l'ENfant (EDEN) cohort( Reference Lioret, Betoko and Forhan 38 ). Our first pattern, ‘junk/snack food’ identified foods commonly found in an adult snack-type dietary intake, as seen in the ALSPAC ‘biscuits, sweets and crisps’ pattern( Reference Smithers, Brazionis and Golley 12 ), the SWS ‘adult foods’ pattern( Reference Robinson, Marriott and Poole 7 ), the MoBa ‘unhealthy’ pattern( Reference Ystrom, Niegel and Vollrath 37 ) and the EDEN ‘processed and fast foods’ pattern( Reference Lioret, Betoko and Forhan 38 ). Our second pattern, ‘healthy/guideline foods’ was similar to the ALSPAC ‘meat, vegetables and desserts’ pattern( Reference Smithers, Brazionis and Golley 12 ), the SWS ‘infant guidelines’ pattern( Reference Robinson, Marriott and Poole 7 ), the MoBa ‘wholesome’ pattern( Reference Ystrom, Niegel and Vollrath 37 ) and the EDEN ‘guidelines’ pattern( Reference Lioret, Betoko and Forhan 38 ) and was influenced by foods seen to conform to infant feeding guidelines. In New Zealand, dietary patterns have been described in older paediatric populations, where ‘junk’, ‘healthy’ and ‘traditional’ patterns were identified at 3·5 and 7 years of age( Reference Wall, Thompson and Robinson 39 ), and in 14- and 24-month-old Australian children, patterns that reflected core and non-core food intake were identified( Reference Bell, Golley and Daniels 23 ). Across both studies, the identified patterns consisted of similar food items to our ‘junk/snack foods’ and ‘healthy/guideline foods’ patterns.
Our third pattern, ‘breast milk/formula’ loaded with opposite strength on breast milk (0·84) and formula (including GUM) (–0·82), where the negative direction of the association indicates that lower scores are associated with a higher consumption of formula. Over time, the loadings were negatively associated with the pattern, indicating that formula consumption was increasing, but those in the GUMLi group were consuming more breast milk at these ages compared with the CM group. A similar relationship was identified in the ‘breast-feeding’ pattern in the 6-month-old infants from the ALSPAC study, where a high positive loading on breast milk (0·78) and high negative loading on infant formula (–0·76) were seen( Reference Smithers, Golley and Brazionis 17 ). Although identified in early infancy in the UK, children in our study still loaded on our ‘breast milk/formula’ pattern at months 9 and 12 of the intervention, when the children were 21 and 24 months of age, respectively. The relationship between breast-feeding and GUM intake has also been identified in the NutriBébé Survey in France, where a higher percentage of mothers still breast-feeding or who previously breastfed gave GUM to their children (32 %) compared with mothers that did not (25 %)( Reference Bocquet, Bresson and Briend 40 ).
Nutrient intakes
To date, only two studies described the construct validity of dietary patterns in terms of the underlying nutrient profiles in children under 2 years of age, confirming that dietary patterns are able to measure the differences in combinations of nutrient densities( Reference Smithers, Golley and Brazionis 17 , Reference Bell, Golley and Daniels 23 ). Using a similar approach, we were able to demonstrate trends in the underlying nutrient intake across quartiles of baseline dietary pattern scores. It is important to note that this analysis was performed on baseline data, before allocation into treatment group and cessation of any additional infant, follow-on formula or GUM, which would have contributed to both macro- and micronutrient intake.
The differences between ‘junk/snack’ and ‘healthy/guideline’ dietary patterns were difficult to interpret. Both patterns were positively associated with total energy, protein and total fat; however, in addition, the ‘healthy/guideline’ pattern was positively associated with fibre, Zn and vitamin B12, reflecting a more positive nutrient profile, with scope for further improvement. The EAT FFQ did not differentiate between types of fats, that is, saturated v. polyunsaturated, which may have contributed to both patterns having a positive association with total fat intake due to all fats contributing the same amount of energy (37·7 kJ) per g. In contrast, the highest quartile of the ‘breast milk/formula’ pattern had a negative association with total energy, protein, fat, carbohydrate, Ca, Fe, Zn and vitamins B12, C and D. Frequency (per d) of breast-feeding was used in the baseline PCA, however, volume and nutrient content estimates were not directly assessed. As described in the literature from this age group, the decision was made not to exclude participants that were breast-feeding as a large portion of the dietary data would be missed, reducing the ability of the FFQ to describe the total diet. Collecting accurate data on the amount of breast milk consumed and its nutrient content would have required breast milk collection and test weighing, both of which were not feasible in this study, as the data collection burden was significant for participants, with further measures increasing the likelihood of participant attrition and loss of statistical power. Therefore, associations between scores on the ‘breast milk/formula’ pattern and underlying nutrients may be less reliable but may still reflect an age-appropriate nutrient profile, also reported by Smithers et al. ( Reference Smithers, Golley and Brazionis 17 ) in an analysis of the relationship with nutrient intake at 8 and 15 months of age and dietary patterns at 6 and 15 months of age.
Effect of the intervention over time
The effect of GUM on energy and nutrient intakes has been assessed to a limited capacity in six cross-sectional studies( Reference Ghisolfi, Fantino and Turck 41 – Reference Virtanen, Svahn and Viinikka 46 ) and five RCT study designs( Reference Sazawal, Dhingra and Dhingra 47 – Reference Houghton, Gray and Szymlek-Gay 51 ), all performed in developed countries. Most studies focused on single-nutrient intake (i.e. Fe or vitamin D)( Reference Virtanen, Svahn and Viinikka 46 , Reference Atkins, McNaughton and Campbell 49 – Reference Houghton, Gray and Szymlek-Gay 51 ), with only one other RCT( Reference Sazawal, Dhingra and Dhingra 47 ) describing multiple-nutrient intakes according to CM or GUM, presented as a supplementary analysis. The compositional differences between the two intervention milks (GUMLi v. CM) resulted in significant differences in protein and key micronutrients (i.e. Fe and vitamin D) between groups. After 12 months, the children drinking GUMLi had a daily Fe intake 2·7 times the current EAR( 52 ). These increases were similar to those seen in another New Zealand study, where children in an Fe-fortified milk group had daily Fe intake 2·5 times the EAR compared with an unfortified control milk or red meat group after 20 weeks( Reference Szymlek-Gay, Ferguson and Heath 48 ). Consumption of GUMLi did not lead to excessive intake of nutrients compared with CM (online Supplementary Table S4). In addition to Fe, GUMLi participants were more likely to have vitamins C and D intake that were greater than the EAR and AI, respectively; however, no participants exceeded the upper limits for these nutrients( 34 ).
Greater mean protein intake compared with the literature was observed, with the CM group consuming larger quantities of protein at all time points and significant differences in intake at months 3 and 9. In a study of 12–24-month-old French children, those drinking CM had mean protein intake of 42 g/d and children drinking GUM had protein intake of 36 g/d (P<0·0001)( Reference Ghisolfi, Fantino and Turck 41 ). Supplementary analyses from a 12-month RCT in 1–4-year-old children in India( Reference Sazawal, Dhingra and Dhingra 47 ) described no differences in energy intake between children consuming a micronutrient fortified milk and a non-fortified control milk. No differences were seen in protein intake after 6 months of the intervention, but significant differences were seen after 12 months (P=0·01). However, as both milks had similar protein contents, this difference cannot solely be attributed to differences in milk intake. In our study, by 2 years of age, the children in the GUMLi group obtained larger amounts of protein from other food groups, that is, fruits, vegetables and meat/fish. Further research is indicated, but this may imply that the children in the GUMLi group experienced an earlier transition from a milk-based intake to an intake more reflective of family-based foods, with greater variety compared with the CM group.
Strengths and limitations
Strengths of our study include a data collection period of 52 weeks, spanning half the ‘at-risk’ toddler period of 1–3 years of age, and twice the time frame of studies with similar outcomes( Reference Szymlek-Gay, Ferguson and Heath 48 , Reference Akkermans, Eussen and van der Horst-Graat 53 ) and the use of a validate FFQ( Reference Watson, Heath and Taylor 25 ). To improve the accuracy of the use of self-reported data, three adjustments were made to the EAT FFQ before its validation. Cross-check questions were added to the fruits and vegetables question, in an attempt to calculate an adjustment factor for each consumption frequency within the group, a technique that has been shown to reduce overestimation when long itemised lists are included in FFQ( Reference Calvert, Cade and Barrett 54 ). In an effort to reduce the effect of portion size estimation leading to overestimation of intake, a unique methodology was adopted for the EAT FFQ, where the participants palm size was used as a reference and questions accounted for leftovers through asking for portion size information of the average amount offered each time and the amount actually eaten( Reference Watson, Heath and Taylor 25 ). Our novel approach of calculating applied dietary pattern z scores for five-time points using factor loadings from the baseline dietary patterns has allowed assessment of change in baseline dietary patterns over time and evaluation of the impact of the intervention. However, this methodology does not allow detection of any new dietary patterns that may arise at later time points( Reference Movassagh, Baxter-Jones and Kontulainen 55 ). Limitations of the study include the use of PCA which can be influenced by the way in which foods are grouped( Reference Tucker 56 ) and, as PCA is a data-driven method, results from this analysis cannot be extrapolated to other populations. The EAT FFQ included a complex method of reporting portion size where the child’s palm volume was used. This may have resulted in miss reporting portion sizes. As the intervention progressed, parents became more familiar with this method. n-3 PUFA (including DHA, EPA and docosapentaenoic acid (DPA)) were not included in the nutrient lines of the EAT FFQ. Although identified by the European Food Safety Authority( 57 ) and Food and Agriculture Organization/World Health Organization( 58 ) as a nutrient whose intake are below the recommended levels in 1–3-year-old children, we were not able to determine the estimated intake from food in our population. GUMLi was fortified with a total n-3 PUFA (including DHA, EPA, DPA) of 40 mg/100 ml. Therefore, consumption of the prescribed 300 ml/d would result in 120 mg of n-3 PUFA/d (DHA, EPA and DPA) in the GUMLi group. Therefore, achieving the recommended 100–150 mg/d( 57 , 58 ) for children 6–24 months of age, compared with those in the CM (control) group who received <6 mg/d (DHA, EPA and DPA) from the study milk.
Whether the consumption of GUM displaces energy intake, impacts dietary variety or inadvertently limits the foods offered to children by their parents due to providing a sense of ‘nutritional security’ remains an area of concern for parents, paediatric medical professionals and advisory groups( Reference Szymlek-Gay, Ferguson and Heath 48 ), and remains an area that has received less research attention. Young children’s feeding practices vary markedly and are influenced by cultural, social and economic factors, differing advice from healthcare professionals and availability of products( 22 ). Recommendations and parental perceptions for the use of GUM in European countries vary( 22 ), and our understanding as to what drives New Zealand and Australian parents to use GUM is less understood. In a recent position statement, ESPGHAN do not recommend the routine use of GUM in children from 1 to 3 years of age but do acknowledge that these milks can be used as one of several methods to increase intake of micronutrients such as Fe, vitamin D and n-3 PUFA and also as a strategy to reduce the total protein intake( Reference Hojsak, Bronsky and Campoy 19 ).
In conclusion, the consumption of a reduced protein GUM (GUMLi) fortified with Fe and vitamin D, for a period of 12 months did not affect dietary patterns compared with consumption of an unfortified CM. GUMLi resulted in improved intake of several key micronutrients, including Fe and vitamin D, and reduced intake of protein. It is possible that a whole diet, without the use of GUM, would provide sufficient nutrients, but in the case of increased susceptibility to nutrient inadequacy, the use of GUMLi in moderate amounts could be considered as a strategy to improve total nutrient intakes without affecting dietary patterns or displacing other foods in the diet throughout the second year of life.
Acknowledgements
Trial registration: Australian New Zealand Clinical Trials Registry (https://www.anzctr.org.au) number: ACTRN12614000918628. Date registered 27/08/2014. This trial received an investigator-initiated grant from Danone Pty Ltd. The funder had no role in data collection, analysis and interpretation of the study.
P. S. W. D., C. R. W. and C. C. G. developed the GUMLi trial. A. L. L., M. M., R. J. H., C. R. W. and P. S. W. D. wrote the study protocol. A. L. L., M. M., T. M. and R. J. H. conducted the study. A. L. L. and C. R. W. wrote the manuscript. Y. J. and R. X. C. conducted the statistical analyses of the data. All authors have read and approved the final manuscript.
C. R. W. has received honoraria for presentations and consultations from Danone, Nutricia, Pfizer and Fonterra. P. S. W. D. has received honoraria for presentations and consultations from Danone, Nurticia, Nestlé, Mead Johnson and Aspen Nutritionals. C. C. G. has received honoraria for consultations from Fonterra. R. J. H. has received honoraria for presentations and consultation from Danone Nutricia, Nestlé and Aspen Nutritionals. None of the remaining authors report a conflict of interest related to the study. A. L. L., T. M., M. M., Y. J. and R. X. C. have no conflicts of interest. The GUMLi trial received an investigator-initiated grant from Danone Nutricia Research. The funder had no role in the study design, data collection, analysis and interpretation of the study. There are no restrictions or delays on the timely publication of the results of the trial.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518003847







