Obesity is a growing global health issue requiring more attention and action to combat and control it worldwide(Reference Henriques, O’Dwyer and Dias1,Reference Trandafir and Temneanu2) . Recent research has shown that several risk factors may play significant roles in the development of obesity, starting from the early stages of life. If weight gain occurs in the early stages of life, the number of years a person lives with overweight and obesity will increase as well. Subsequently, the development of obesity in the early stages of life will lead to serious health consequences and a greater burden of diseases(Reference Mirza and Yanovski3). According to the WHO’s report, in 2019, about 38 million children aged < 5 years were suffering from overweight and obesity, whereas in 2016 more than 380 million children and adolescents aged 5–18 years were overweight or obese(Reference Cheater4). In middle-income countries, such as Iran, the coexistence of malnutrition and an upward growth of obesity and overweight have been observed. The prevalence of obesity and overweight in Iranian children has reported to 8 % and 9 %, respectively, in 2018(Reference Khateri, Moradi and Khazaei5). Obesity is associated with a myriad of complications and comorbidities, i.e. insulin resistance and other metabolic disorders, CVD, high risk of bone fractures, etc., all of which influence the children’s health status(Reference Catalano and Shankar6). Recent research has shown that governments face excessive costs, either directly or indirectly, for the treatment of obesity(Reference Esdaile, Thow and Gill7). For example, the cost of medication during the life of a 10-year-old American child with obesity, compared with a child with a normal weight, was about $12 660–$19 630 in 2016(Reference Di Cesare, Sorić and Bovet8).
Fortunately, the development of overweight and obesity can be prevented(Reference James9). Researchers have recommended that there are time spans during childhood when the prevention of overweight and obesity seems to be efficient. Such ‘golden times’ when the development of these disorders can be prevented include the prenatal period, at 5–7 years of age and at puberty. There is a great deal of evidence suggesting a remarkable chance for preventing overweight and obesity in the first 1000 d of life, i.e. from conception to 24 months of age(Reference Blake-Lamb, Locks and Perkins10). Over the past two decades, researchers have tried to assess the role of this critical period in children’s health in order to identify the risk factors associated with the development of childhood overweight and obesity(Reference Baidal, Locks and Cheng11,Reference Mameli, Mazzantini and Zuccotti12) . The modifiable risk factors in the first 1000 d of a child’s life can be divided into three groups: maternal, paternal and child-related factors. The maternal BMI before pregnancy(Reference Heslehurst, Vieira and Akhter13), the gestational weight gain(Reference Heslehurst, Vieira and Akhter13,Reference Hillier, Pedula and Vesco14) , the presence of gestational diabetes(Reference Skrypnik, Bogdański and Zawiejska15) and smoking during pregnancy(Reference Rayfield and Plugge16) are risk factors pertaining to the ‘maternal group’. The factors related to the child include the birth weight, the period of exclusive breast-feeding, the time of food introduction and the child’s weight at 24 months(Reference Baidal, Locks and Cheng11). Recently, paternal risk factors, e.g. BMI and parental smoking, have also been addressed; however, only a few studies have discussed them comprehensively(Reference Baidal, Locks and Cheng11). It is noteworthy that these factors have been evaluated individually in many studies, and to the best of the authors’ knowledge, there are only two large studies in which these risk factors have been investigated. In the first study, based on the data from a cohort from Singapore, six factors related to the development of childhood overweight and obesity, namely the paternal BMI, the presence of pre-gestational hyperglycaemia, the BMI during pregnancy, the duration of breast-feeding, the gestational weight gain and the timing of solid food introduction in two-year-olds, were examined(Reference Soh, Tint and Gluckman17). In the second study, based on a cohort from the UK, five risk factors, including low maternal serum vitamin D concentrations, smoking during pregnancy, excessive gestational weight gain, breast-feeding < 1 month and pre-pregnancy obesity were examined in children at ages 4 and 6(Reference Robinson, Crozier and Harvey18).
Due to the prevalence of adulthood obesity and its related complications, as well as its emergence as a global public health issue, it is essential that children at risk be identified in the early stages of overweight and obesity development via screening measures. In order to identify measures to combat these comorbidities, we sought to discover the most relevant modifiable risk factors for overweight and obesity in children during their first 1000 d of life.
Materials and method
Study population
This case–control study was conducted in Tehran, the capital and the most populated city of Iran, from January 2019 to February 2020. The participants of the study were preschool children and their parents who were living in Tehran. According to the educational system of Iran, children start school at the age of 6. Using a simple random sampling technique, we randomly selected five regions out of the twenty-two districts of Tehran, representing the population of the northern, southern, western, eastern and central regions. Afterwards, four schools, consisting of two all-girls schools and two all-boys schools, were identified in each region. Ultimately, a total of twenty schools were selected randomly. The sample size of the study was calculated according to the study of Bammann et al. (Reference Bammann, Peplies and De Henauw19), on the basis of the occurrence or non-occurrence of obesity and overweight in children, and given that the probability of obesity in children with birth weight less than 4 kg was 49 % and above 4 kg was 64 %. Considering the error of the first type 0·05 and the study’s power of 90 %, about 245 obese and overweight children (the case group) and 245 normal-weight children (the control group) were included in the study. The ratio of case to control was considered equal and matching was performed on the basis of gender and selected schools. Twins or multiple twins, children born prematurely or suffering from developmental disorders, specifically failure to thrive, metabolic disorders or cardiovascular diseases, were excluded. In order to achieve the representing sample size, a total of twenty schools and the mothers of 600 children were invited to participate in the research, and 509 mothers accepted the invitation and referred to the school on a scheduled day.
Study setting and ethical consideration
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving patients were approved by the Regional Ethical Committee for Medical research. Then, the objectives and methodology of the study were explained to them and after accepting to join the study, they signed the written informed consent forms. All mothers accepted to participate in the study voluntarily, and a response rate of 100 % was achieved. This study was approved by the local ethics committee (approval number: IR.IUMS.REC.1397·833).
Parental and child data in school
The parents of the children involved in the study were questioned according to a checklist regarding several demographic data, i.e. age, level of education (< 12 years,12–16 years, > 16 years), parents’ occupation (unemployed, employed, worker, self-employed), history of smoking during the pregnancy (smoker, non-smoker), the father’s smoking status (smoker, non-smoker), the marital status (married, without husband), the child’s birth order and the family size (the persons living together in one household). Moreover, using the existing health cards of the children, we were able to retrieve their weight (in grams) and height (in meters) at birth. The maternal weight and the height before pregnancy were obtained from the mothers of the children enrolled in our study.
Anthropometric measurement
A trained nutritionist measured the height and weight of the children at the school, using calibrated instruments. The height measurement was performed by using a stadiometer while standing in a fixed standard position, with an accuracy of 0·5 cm. The weight measurement was carried out with light clothing, without shoes and by using a Seca scale (model 762, made in Germany), with an accuracy of 100 g. The BMI was calculated by dividing the weight in kilograms by the squared-height in metres, which was then compared with the age and sex growth reference chart of the WHO to identify the BMI status.
Physical activity questionnaire for children and sleep duration
The Children’s Physical Activity Questionnaire, which was modified by Faqih Imani et al.(Reference Faghihimani, Nourian and Nikkar20), was used to obtain a general estimate of the physical activity level. The questions were asked by a trained interviewer and were related to the physical activity of the children during their leisure time, after school, after lunch, in the evening and at weekends, in the past 7 d. The questions also evaluated the practice of physical exercise causing heavy sweating, increased respiratory function and heart rate, e.g. climbing. In addition, the child’s total sleep time throughout the day was assessed by self-reporting.
Parental and child data in healthcare centres
Iranian children’s information is routinely registered in the Healthy Child Care Registration Form by healthcare centres. Maternal information, including the type of delivery (caesarian or vaginal), gestational diabetes (yes or no), maternal weight at delivery, exclusive breast-feeding duration, time of solid food introduction and weaning time, was extracted. Gestational diabetes was diagnosed in fasting women receiving 75 g of oral glucose at 26–28 weeks of gestation based on the WHO criteria, including FPG 7·0 mmol/l or 2-h glucose 11·1 mmol/l(21). Exclusive breast-feeding was defined as infants who were only breastfed (including milk expressed or from a wet nurse) and had no restrictions on receiving ORS solutions, multivitamins, minerals and medications. Weaning was defined as the process of breast-feeding cessation. The total duration of breast-feeding was evaluated. The gestational weight gain was obtained by calculating the difference between the weight before delivery and the weight before pregnancy(22). The BMI of the parents was calculated by dividing their weight in kilograms to their squared height in metres.
Risk factors
Nine independent risk factors, all of which are associated with a higher risk of childhood obesity, were evaluated. We investigated four risk factors related to the mother’s status of health, namely the maternal BMI before pregnancy, the gestational weight gain, the presence of gestational diabetes and smoking during pregnancy and three risk factors related to the child, including the birth weight, the duration of breast-feeding and the time of solid food introduction. The paternal BMI and the smoker/non-smoker status at the time of the birth of the infant were also assessed. The following variables were investigated as risk factors for paediatric obesity: maternal BMI before pregnancy and paternal BMI ≥ 25 kg/m2, excessive maternal gestational weight gain, based on the Institute of Medicine 2009 guidelines, the presence of gestational diabetes, birth weight >4000 g, introduction of solid food at an age < 4 months, exclusive breast-feeding of < 4 months and maternal and paternal smoking during the pregnancy.
Statistical analysis
The χ 2 test was used for the statistical analysis of two-state variables, and the t test was used for quantitative variables. The logistic regression model was used to investigate the effect of each factor on the risk of overweight and obesity. After controlling the confounding factors such as physical activity and sleep rate, the OR of overweight and obesity was estimated in the logistic regression model at a 95 % CI. We fitted the univariate regression model and defined the significant values at the 20 % level in the multiple regression models. The modelling was based on a step-by-step logistic regression, and the selection criterion was the R2 Cox Snell. It should be noted that both overweight and obese children were measured in a group relative to the control group and were examined as obese children in this study. The hypothesis of normality of variables was made by using Shapiro–Wilk test and homogeneity of variance based on Levene’s test. Multicollinearity among continuous independent variables was performed. Also, receivers operating characteristic curve, or ROC curve, was drawn to evaluate the sensitivity and specificity for each of the quantitative variables (online Supplementary Fig. 1–3).
Results
In this case–control study, 509 participants were included, 259 normal-weight and 250 obese preschool children. The characteristics of the children and their parents are presented in Table 1. There was a significant difference between the BMI of the offspring, based on the parental characteristics, including parental age, level of education, job, parity, marital status, mode of delivery, height, maternal BMI pre-pregnancy (overweight or obese mothers before pregnancy), excessive gestational weight gain, the presence of gestational diabetes and smoking during pregnancy (P < 0·001). In addition, we extracted information regarding the paternal smoker/non-smoker status and the weight and height at the time of the birth of the offspring. Among the included children, 51·1 % were girls and 48·9 % were boys. The mean BMI of the obese children and the normal-weight children was 20·82 (2·87) kg/m2 and 15·79 (0·88) kg/m2, respectively. Obese children were taller and had a higher likelihood of having higher birth weight, exclusive breast-feeding before 4 month of age (2·98 v. 3·42 months), shorter total breast-feeding duration (12·8 v. 14·47 months) and introduction of solid food before 4 month of age (28 % v. 11·2 %) compared with their normal-weight counterparts. While there were no differences between the two groups regarding sleep time and family size, low levels of physical activity was observed in 51·2 % of children with obesity and 30·1 % of children with normal weight. In addition, compared with fathers of normal weight children, fathers of obese children had higher smoking rates (29·6 % v. 17·4 %).
Table 1. Socio-demographic and clinical characteristics of the overweight/obese v. normal-weight children
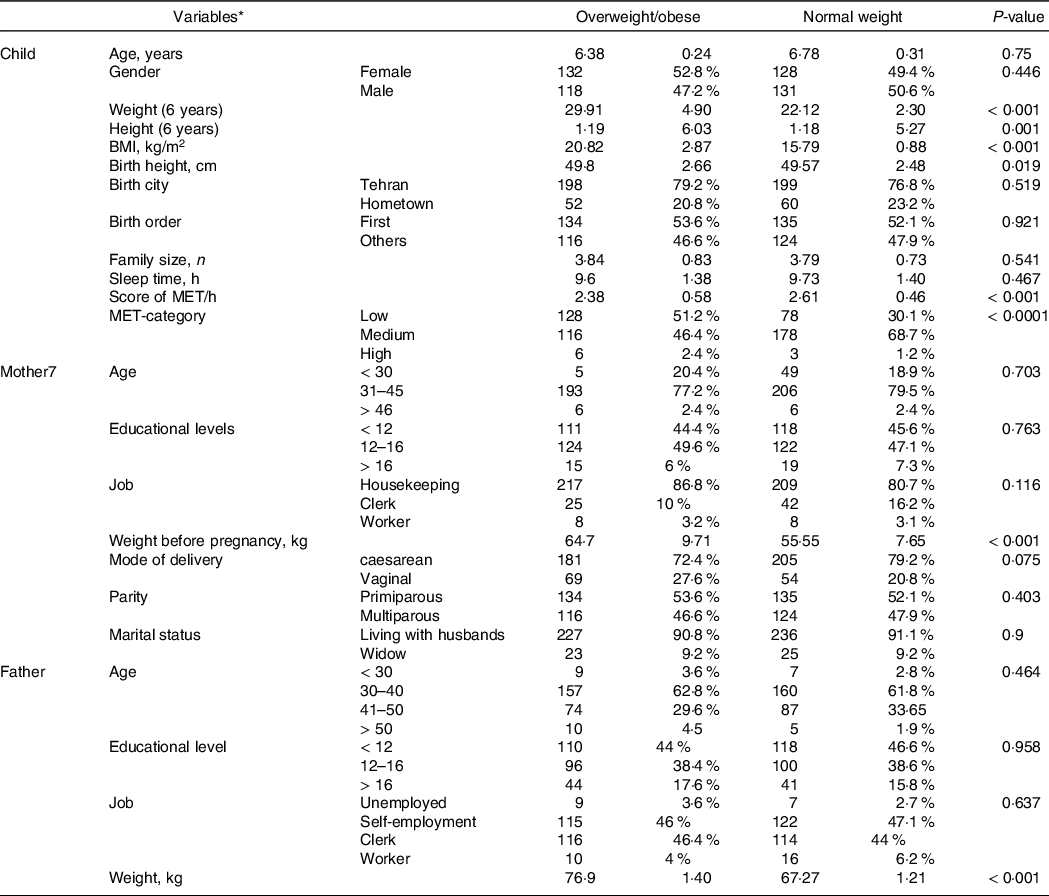
* Qualitative variables are reported as numbers and percentages, and quantitative variables are reported as mean and sd.
As presented in Table 2, in children with obesity, the maternal weight and BMI were significantly higher compared with normal-weight children (P < 0·001). Moreover, 11·2 % of the mothers of overweight children had gestational diabetes, while only 3·9 % of the mothers of normal-weight children were diagnosed with the same disease. Our results show that 17·7 % of the mothers with obese offspring and 2·2 % of the mothers with normal-weight offspring smoked during pregnancy. It is noteworthy that excessive gestational weight gain was more frequent in the mothers of these children. The mothers of normal-weight children had an excessive gestational weight gain of 37·7 %, whereas it was 69·5 % in the mothers of obese children.
Table 2. Risk factors for paediatric overweight and obesity in the study participants
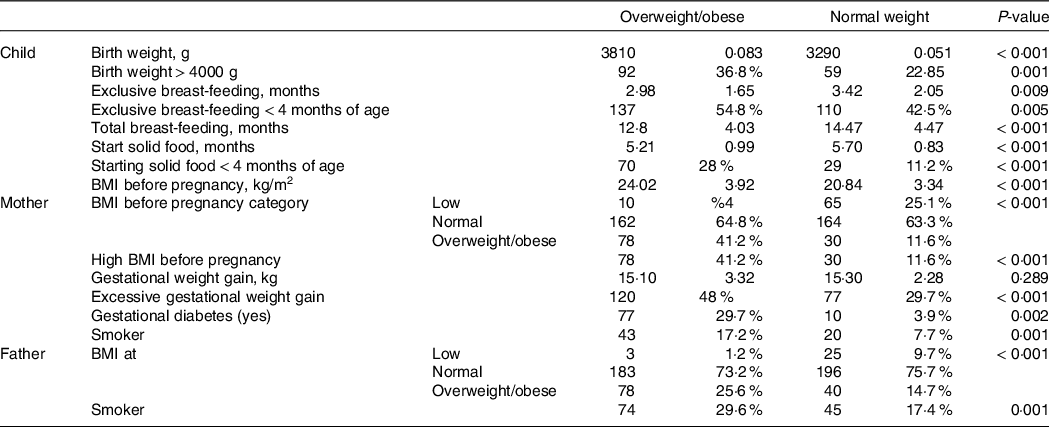
Table 3 shows the adjusted predicted possibility of overweight and obese children for each risk factor. Our results show that children born from mothers with gestational diabetes and starting eating solid food at an early age (< 4 months of age) had increased odds of obesity and overweight, 4·36 (1·94, 9. 80) and 2·97 (1·77, 4·97), respectively.
Table 3. Estimated multivariate regression coefficients and odds ratio (95 % CI) of the associations between the investigated risk factors and the risk of paediatric overweight or obesity
(Odds ratio and 95 % confidence intervals)
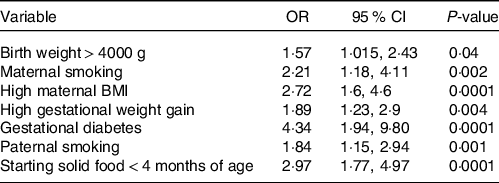
The criteria of multiple regressions logistic with Cox Snell.
Odds ratio was adjusted by MET-category.
The odds of obesity in the children whose mothers or fathers smoked during pregnancy were 2·21 (1·17, 4·11 %) and 1·84 (1·15, 2·94 %) times, respectively, compared with children whose mothers or fathers had no history of smoking. In the families in which mothers experienced an excessive gestational weight, the risk of obesity was 1·89 times (1·23, 2·91) higher than otherwise. Furthermore, the risk of obesity in children whose mothers had a high BMI before pregnancy was 2·72 times higher (1·60, 4·60 %) than their counterparts.
Discussion
Our results showed that gestational diabetes, a high maternal BMI before pregnancy, a high gestational weight gain, maternal smoking during pregnancy, paternal smoking, a high birth weight and an early start of solid food increased the odds of developing obesity in preschool children. However, a high paternal BMI and a short exclusive breast-feeding time had no effect on obesity in the aforementioned subjects. Moreover, the most important predictor in the first 1000 d of life for the risk of obesity was the presence of gestational diabetes. The children whose mothers had gestational diabetes had an increased risk of obesity compared with those whose mothers were healthy.
We found that all maternal-related factors had a significant effect on the child’s risk of developing obesity. To the best of our knowledge, no studies have so far identified the most important predictors of obesity in the 1000 d of life in preschool children in the past 10 years. In fact, the age of 5–7 years, also called the period of adipose rebound, is the period in which the child’s weight can impact the weight of the individual during adulthood. Recently, in line with our study, several studies conducted in different age groups, e.g. an UK cohort(Reference Ziauddeen, Wilding and Roderick23), an USA cohort(Reference Gillman and Ludwig24) and a Singapore cohort(Reference Aris, Bernard and Chen25), have examined several predictors of childhood obesity. Ziauddeen et al. (Reference Ziauddeen, Wilding and Roderick23), based on the data from a large UK cohort, discovered that the maternal pre-pregnancy BMI, the presence of gestational diabetes mellitus and maternal smoking were predictors of obesity in children aged 4–5 years old, whereas the period of breast-feeding and the birth weight did not affect the risk of obesity during childhood. However, they did not report any data regarding the impact of gestational weight gain, the time of solid food introduction, the period of exclusive breast-feeding, the paternal BMI or the paternal smoking on the risk of childhood obesity. In addition to the aforementioned studies, the Southampton Women’s Survey in 2015, conducted by Robinson et al. (Reference Robinson, Crozier and Harvey18), reported that the presence of maternal pre-pregnancy obesity, an excessive gestational weight gain, the smoking status, low maternal vitamin D levels and breast-feeding for less than one month were the predictors of childhood obesity. The findings of their study suggest that the gradual addition of risk factors results in an increased risk of obesity in children aged 4–6 years. Another study conducted in Singapore by Aris et al. (Reference Aris, Bernard and Chen25) showed that the maternal BMI before pregnancy, the gestational weight gain, breast-feeding for less than 4 months, the early introduction of solid food, fasting glucose levels during pregnancy and the paternal weight when the child is two years old were related to the development of obesity in four-year-old children. However, although they concluded that the risk of childhood obesity could be increased by any of those factors, parental smoking was not taken into consideration in their models. In a USA cohort study, Gillman et al. (Reference Gillman and Ludwig24) illustrated that there was a significant positive relationship between an excessive gestational weight gain, maternal smoking, breast-feeding for 12 months and childhood obesity. Although they reported that short sleep duration during infancy was related to the development of obesity in children aged 7 to 10 years, they did not assess other modifiable risk factors such as the birth weight, early introduction of solid food, parental BMI, presence of gestational diabetes or paternal smoking. In a another cohort study, Eales et al. (Reference Eales, Reynolds and Ou26) showed that birth weight was a predictor of BMI in 35-year-old adults. Moreover, they examined the relationship between the human capital of neighbourhood, psychosocial skills and the contexts of family/school, demonstrating that higher school quality, the parents’ involvement and the human capital of neighbourhood were related to a lower risk of obesity at the age of 35. Predicting obesity requires a life-course approach, including the evaluation of parental and childhood variables that have not been fully addressed by researchers yet. In our research, we analysed factors such as the parental BMI, the presence of gestational diabetes mellitus and information regarding the duration of breast-feeding.
It is worthy of consideration that the risk factors investigated in our study are potentially modifiable by behavioural changes that can prevent the occurrence of high maternal blood glucose concentrations, the development of gestational diabetes or macrosomia, as well as an excessive gestational weight gain. Interventional studies are warranted to elucidate the most important risk factors for childhood obesity in different communities in order to prevent its development via effective and timely measures. In terms of risk factors for obesity, the results of our study showed that the presence of gestational diabetes was associated with the highest odds of obesity in preschool children. In line with our findings, Zhao(Reference Zhao, Liu and Qiao27), Dabelea(Reference Dabelea28), Josey(Reference Josey, McCullough and Hoyo29) and Babu et al. (Reference Babu, Deepa and Lewis30) also demonstrated that gestational diabetes is an important risk factor for the development of childhood obesity. There is evidence that pregnancy, similar to obesity, disrupts the normal circulation of fat in the blood and leads to insulin resistance. Therefore, maternal insulin resistance and hyperglycaemia with subsequent fetal hyperinsulinaemia lead to overgrowth and fat gain(Reference Dahlgren31). It is noteworthy that the severity of this effect varies, depending on the maternal weight status. Pregnant overweight and obese women with gestational diabetes are more susceptible to insulin resistance than a mother with normal weight who also suffers from gestational diabetes(Reference Schack-Nielsen, Sørensen and Mortensen32). Therefore, there is a high likelihood that a child born to an obese mother with gestational diabetes will also develop obesity. Similar to the findings of our study, in a cross-sectional study, Jiménez-Cruz et al. (Reference Jiménez-Cruz, Wojcicki and Bacardí-Gascón33) have remarked that a higher maternal BMI increases the risk of becoming overweight or obesity in 10-year-old Mexican children. We suggest that the maternal weight status before pregnancy and the pregnancy weight gain be assessed subsequently due to their impact on the risk of developing childhood obesity in the offspring. It has been reported that the genetic predisposition of the mother, the presence of low-grade inflammation and the nutritional status are all determinants of maternal pre-pregnancy weight(Reference Sen, Carpenter and Hochstadt34–Reference Ajslev, Andersen and Gamborg36). Similar to our results, Olson et al. discovered that an excessive gestational weight gain in mothers with a higher BMI compared with a normal BMI could increase the risk of developing overweight and obesity at the age of 3 in a significant manner. In addition, Oken et al. (Reference Oken, Taveras and Kleinman37), based on data derived from the Nurses’ Health Study II, demonstrated that a higher maternal gestational weight gain increased the risk of overweight and obesity during adolescence. Although no clear mechanism has been depicted to explain this finding, various studies have suggested that intrauterine programming might be responsible for the risk of overweight and obesity(Reference Branum, Parker and Keim38). Thus, we may assume that the overfeeding of the fetus caused by the access of the fetus to more nutrients is influenced by the adipose tissue, resulting in a disruption of the control of the appetite, thereby increasing the susceptibility to paediatric obesity via disruptions in the metabolism of energy(Reference Budge, Gnanalingham and Gardner39,Reference Symonds, Pearce and Bispham40) .
Our findings also support the father’s role in the weight status of the offspring. The relationship between the paternal overweight and obesity and the development of paediatric obesity was significant in the univariate regression analysis. However, in the stepwise logistic regression, due to multiple linear correlations with the high weight at birth and its prominent impact, the paternal BMI was excluded from the model. Nevertheless, this does not imply that the paternal BMI does not influence the weight status of the offspring. Moreover, according to our results, the father has a specific role in determining the child’s BMI at the age of six. In a longitudinal study, Freeman et al. (Reference Freeman, Fletcher and Collins41) showed that the children whose fathers were overweight or obese were more likely to be obese by the age of four which has also been confirmed by our data. In another study, Chen et al. (2012) showed that the paternal BMI is significantly correlated with the birth weight in the male but not in the female offspring(Reference Chen, Xiao and Li42). It seems that the higher paternal BMI affects the progeny in a gender-dependent manner possibly because sex hormones lead to differences in the organ growth. The rather complex effect of the presence of paternal obesity on the child’s risk of becoming obese has been evaluated in a limited number of studies(Reference Whitaker, Jarvis and Beeken43). Factors such as the paternal genetics, parenting style, paternal knowledge, feeding style of the offspring, physical activity, patriarchal pressures and social status are all determinant factors of childhood obesity of paternal origin(Reference Milliken-Smith and Potter44–Reference McMillen, Adam and Mühlhäusler46). One of the risk factors affecting the weight gain and the risk of paediatric obesity is the high birth weight. Sparano et al. (Reference Sparano, Ahrens and De Henauw47) conducted a prospective study and reported that children with macrosomia had a higher weight, height, BMI, sum of skinfold thickness and waist circumference compared with children with an adequate weight for gestational age at the age of 6. Based on the data from a cohort study from Western China, Pan et al. (Reference Pan, Tang and Lee48) demonstrated that macrosomia can increase the risk of developing obesity in three-year-olds. Although the weight-for-height index was related to the presence of macrosomia, the same results did not apply to the BMI for age. This finding is also supported by our results(Reference Oldroyd, Renzaho and Skouteris49), and we may assume that the weight at birth is affected by the maternal weight before pregnancy, the maternal weight gain during pregnancy and the presence of gestational diabetes, all of which can effectively increase the risk of overweight and obesity. Although the mechanism is not clear, it seems that factors such as epigenetic changes(Reference Bouchard50), insulin resistance(Reference Li, Leng and Li51) and fetal hyperleptinaemia(Reference Ornoy52) lead to the birth of a macrosomic infant. Another risk factor for childhood obesity is parental smoking. In line with our study, Mamun et al. (Reference Mamun, Mannan and Doi53) and Chen et al. (Reference Chen, Xiao and Li42) demonstrated that maternal smoking during pregnancy increased the risk of obesity in the offspring. Scientists have reported that nicotine can reduce the appetite or result in starvation, leading to cognitive dysfunction, thereby causing a fetus-induced abnormal cell proliferation and differentiation, and ultimately altering the total energy expenditure(Reference Cole54). It was also suggested that nicotine can induce the shrinkage of the amygdala and influence children to select diets with a higher fat content(Reference Haghighi, Schwartz and Abrahamowicz55).
Given that most obese adults have gained weight during adulthood, individuals with a family history of obesity should be evaluated from an early age to prevent the development of obesity and its related complications. Recent studies have suggested that the effect of paternal smoking on the risk of childhood obesity, independent of maternal smoking, could be explained by the fact that nicotine, due to its effect on the epigenome, i.e. dose-dependent effects on DNA methylation changes, can induce obesity in children. This effect will persist even after the cessation of smoking(Reference Milliken-Smith and Potter44).
The results of our analysis, based on univariate regression, showed that although exclusive breast-feeding increased the risk of childhood obesity, this variable was excluded from the final model due to its correlation with high birth weight. Some previous studies have reported that exclusive breast-feeding could protect infants against future risks of overweight and obesity. For example, on the basis of the data from a large cohort study, Moss et al. (Reference Moss and Yeaton56) found that breast-feeding and delaying solid food for four months could decrease the likelihood of obesity in four-year-old children. Similarly, Zhao et al. (Reference Zhao, Liu and Qiao27) showed that Chinese children who were breastfed exclusively for three months, and were born to mothers with gestational diabetes, were protected from becoming overweight. In agreement with our results, the cross-sectional study of Vafa et al. (Reference Vafa, Moslehi and Afshari57), conducted in Iran, reported that there was no link between exclusive breast-feeding or the- total breast-feeding duration and the childhood BMI. Schack-Nielsen et al. illustrated that a longer breast-feeding duration was not associated with paediatric obesity, whereas a delay in the introduction of complementary feeding, e.g. after 6 months, could protect against paediatric obesity(Reference Schack-Nielsen, Sørensen and Mortensen32). Another cross-sectional study, conducted by Burdette et al. (Reference Burdette, Whitaker and Hall58), concluded that breast-feeding and complementary feeding were not related to adiposity levels in 5-year-old children. Some of the discrepancies across various studies might be explained by environmental or genetic factors, as well as sample sizes(Reference Milliken-Smith and Potter44). It is worth mentioning that the majority of studies that have depicted a protective effect of exclusive breast-feeding on the weight of the infant have been performed in communities with a long history of development. In other words, in a socially patterned population, confounders can lead to different outcomes in diverse populations.
According to previous studies, a short-term sleep duration in children can be an important environmental confounder in the development of paediatric obesity. It has been reported that sleep disorders can lead to an impaired energy metabolism(Reference Jiang, Zhu and Yan59,Reference Baranowski, Motil and Moreno60) . In a recent study, it was shown that the relationship between sleep duration and the risk of childhood obesity is U-shaped. In other words, short or excessive sleep durations result in an increased risk of childhood obesity(Reference Baranowski, Motil and Moreno60). However, due to the importance of this factor that was dismissed in previous studies, we examined this variable in addition to physical activity as a confounder. Unexpectedly, it did not appear to have a significant effect on the risk factors included in the stepwise model.
There are several limitations that should be considered in this study. First, our study could not explain the cause and effect relationship due to the observational approach. Second, nutritional assessment questionnaires such as the FFQ were not employed. Third, we did not collect data about the paediatric weight at the age of 2 which is a determinant of the weight gain of the child at the age of 6. Fourth, the paternal information was self-reported, and recall biases might have distorted our findings. Finally, we included both obese and overweight children in one group that might have affected the interpretation of the results.
There are several strengths associated with our study. To the best of our knowledge, this is the first case–control study aiming to predict the main predictors of paediatric obesity in preschool children in the Asian population via an assessment of factors that originate in the first 1000 d of life. We examined some confounders, including physical activity and the sleep time. Moreover, our prediction was made by using a stepwise modelling strategy and multiple logistic regressions.
Finally, we recommend that healthcare officials be given the opportunity to identify the children at risk for overweight and obesity via screening initiatives in the early stages of life. During this critical period (the first 1000 d of life), it is possible to perform practical and special interventions in the mother–father–child triumvirate and considerably reduce the burden of obesity and its related complications and comorbidities.
Conclusion
Paediatric obesity remains a public health challenge, and the evaluation of the relevant predictors in the early days of childhood is warranted. The findings of the current study show that gestational diabetes is a critical factor in the development of paediatric obesity in preschool children. Risk factors such as starting solid foods before 4 months of age, excessive weight gain during pregnancy, obesity and overweight of the mother before pregnancy, smoking by the mother and the father are important risk factors for obesity and overweight in children at 6 years of age.
Acknowledgements
We thank all children and parents who contributed to the completion of the present study.
The authors have no support or funding to report.
The named authors have no conflict of interest, financial, or otherwise.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114521003937





