Glycogen storage diseases (GSD) are inherited disorders of glycogen metabolism with several subtypes (types 0, Ia, Ib, IIIa, IIIb, VI and IX) affecting hepatic glycogenolysis and leading to predisposition to hypoglycaemia( Reference Özen 1 ). Carbohydrate ingestion is a simple yet effective therapy for improving glycaemia, but the clinical role of carbohydrates with different functional characteristics as a nutritional therapy for GSD patients has not been fully optimised, in part, due to incomplete knowledge of their metabolic effects.
Starches are comprised of amylose (glucose residues in unbranched α-1,4 linkages) and amylopectin (incorporates a branched α-1,6 linkage every thirty α-1,4 linkages). Endogenously, the α-1,4 and α-1,6 linkages of amylose and amylopectin are hydrolysed by salivary and pancreatic α-amylase to yield glucose( Reference Stryer 2 ). However, the usefulness of starch carbohydrates in improving blood glycaemia lies in their relative rate of availability. The ratio of amylose:amylopectin and entanglement of these polysaccharides determine the availability of starch for enzymatic hydrolysis and consequent influence on blood glucose (BG) concentrations. Modern industrial techniques can lower starch gelatinisation and alter conversion during processing with precise control of temperature, moisture, shear and time conditions( Reference Hoover 3 ). The end result is altered digestibility time and modification in starch-carbohydrate glycaemic properties.
Ingestion of uncooked maize starch (UCMS) by GSD patients aids in successful glycaemic management by lengthening the time between feedings compared with some other carbohydrate alternatives( Reference Lee, Dixon and Leonard 4 ). However, UCMS is reported to have a high glycaemic index (GI) value, and the development of lower-GI starch carbohydrates in recent years has been trialled in GSD cohorts with an aim of extending the duration between feedings even further. In one study comparing the glycaemic effects of ingestion of 2 g/kg body mass (BM) of an UCMS or a hydrothermally processed maize starch (HPMS), ten of twenty-one GSD (types Ia, Ib and III) patients displayed improved BG concentration, with five of the twenty-one individuals demonstrating equally well-maintained glycaemia after the ingestion of HPMS. The researchers demonstrated a trend (P= 0·05) for a slower rate of decline in BG concentrations following consumption( Reference Bhattacharya, Orton and Qi 5 ). In another study, twelve GSD patients displayed a significantly slower decline in BG concentrations after consuming HPMS than after consuming UCMS( Reference Correia, Bhattacharya and Lee 6 ). Thus, industrial refinement of starch structure that alters the functional characteristics may provide new avenues pertinent to clinical nutrition applications.
There is limited research underpinning the working mechanisms behind the potential improvement in glycaemia following the consumption of refined starch carbohydrates. It is difficult to compare data reported by Bhattacharya et al. ( Reference Bhattacharya, Orton and Qi 5 ) and Correia et al. ( Reference Correia, Bhattacharya and Lee 6 ) due to differing feeding protocols (100 g carbohydrate in 177 ml water v. 2 g/kg BM) delivering different quantities and in different concentrations. Though the glycaemic indices of both the carbohydrates were markedly different, no differences in peak insulin concentrations( Reference Bhattacharya, Orton and Qi 5 ) or blood lactate concentrations( Reference Correia, Bhattacharya and Lee 6 ) were reported after the consumption of UCMS or HPMS. However, hourly blood sampling periods may have obscured some insights into metabolic changes. Finally, there is some suggestion that the consumption of HPMS and UCMS evokes similar increases in resting glucose oxidation( Reference Bhattacharya, Orton and Qi 5 ). Many organs are susceptible to a mass-action effect exerted by raised BG concentrations and consequently increase the ratio of energy expenditure from carbohydrate oxidation. It is interesting to speculate as to how lower-GI starch carbohydrates might exert their glycaemic effects through a sparing of tissue carbohydrate use. Thus, more research is needed to explore the glycaemic and metabolic effects of ingestion of hydrothermally processed carbohydrates. Accessing patients with this relatively rare disease (general incidence of GSD in the population is 1:20 000–43 000( Reference Özen 1 )) is difficult, yet the characterisation of some of the metabolic responses to the ingestion of different starches can be gleaned from healthy individuals and it can provide clues to the underpinning mechanisms that aid the development of future studies in GSD-specific cohorts.
The aim of the present pilot study was to compare the glycaemic and whole-body fuel oxidation responses of healthy individuals to the ingestion of a HPMS, an UCMS and dextrose (DEX) at rest and during conditions of heightened tissue fuel demand.
Research design and methods
Participants
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the University Research Ethics Committee. A total of eight individuals (seven males and one female: age 20 (sem 0) years; BM 76·9 (sem 5·2) kg; height 1·73 (sem 0·02) m) volunteered to participate in the present study. Volunteers were recruited through local advertisements. All the participants gave written informed consent after being given a full explanation of the testing protocol. All the participants completed American Heart Association/American College of Sports Medicine medical history forms( 7 ) before partaking in the study. All the participants were currently regularly exercising for at least 6 months before the study and were receiving no medication. The female participant was taking a progestin-only oral contraceptive pill.
Experimental protocol
The participants initially visited the laboratory for a preliminary test for the quantification of peak rate of oxygen consumption (VO2peak) and peak heart rate (HRpeak). To obtain these variables, the participants were submitted to a continuous incremental treadmill assessment( Reference Winter, Jones and Davidson 8 ) to volitional exhaustion (ERGO ELG55, Woodway GmbH). Beginning at a velocity of 6–8 km/h, on a level gradient, the treadmill velocity was increased by 1 km/h every 3 min until the participants were unable to continue the exercise. VO2peak, HRpeak and RER were determined from the final minute of exercise. Peak cardiorespiratory characteristics of the participants in response to the continuous incremental treadmill run test were as follows: VO2peak – 51·8 (sem 5·0) ml/kg per min; HRpeak – 199 (sem 9) beats/min; RERpeak – 1·14 (sem 0·05.) The participants were randomised to their first treatment during the first visit using a web-based random number generator( 9 ).
The participants visited the laboratory on three further occasions 1 week after the preliminary visit, at the same time (between 06.00 and 09.00 hours) and at least 7 d apart. The participants avoided strenuous activity for 24 h before arriving to the laboratory after an overnight fast, having consumed similar evening meals before each trial (assessed via dietary recording sheets). On arrival to the laboratory, BM and height were recorded (Seca 770 Digital Scales, Seca; stadiometer, Holtain Limited).
The participants were then seated and asked to comment about their perceived feelings of hunger on a visual analogue scale, Satiety Labelled Intensity Magnitude( Reference Cardello, Schutz and Lesher 10 ). Thereafter, a resting 1 ml venous blood sample was collected for the determination of glucose, pH, lactate, Na+, K+ and haematocrit levels (GEM Premier 3000, Instrumentation Laboratories). Hb levels were determined using a haemocue-photometer (Haemocue AB). The remainder of the blood sample was used for later determination of plasma NEFA (Wako Chemicals), TAG (Cobas-Roche) and insulin (Invitron) concentrations using specific enzyme immunoassay or colorimetric assay kits. Blood haematocrit and Hb were used to estimate changes in plasma volume using the equations of Dill & Costill( Reference Dill and Costill 11 ).
In a randomised order, the participants were given a drink containing 1·0 g/kg BM of hydrothermally modified maize starch (HPMS, Glycosade™, Vitaflo Limited), UCMS (Argo, ACH Food Companies, Inc.) or DEX (MyProtein.com) mixed to a 10 % solution with distilled water and delivered in an opaque sports bottle. The carbohydrates demonstrated different glycaemic profiles: GI of Glycosade™ – 35 (sem 4); GI of UCMS – 41 (sem 1); GI of DEX – 100. The participants consumed the test solution within 5 min and remained in a rested, seated position for 2 h with venous blood samples being collected every 30 min during this rest period. Resting heart rate was monitored (RS400, Polar) with the participants in a supine position.
Exercise protocol: run test
The participants, still wearing a heart rate monitor (Polar RS400, Polar), mounted a motorised treadmill (Woodway GmbH) to perform a 1 h continuous run protocol at a speed determined to elicit a run intensity of 65 % VO2peak. Expired air samples were collected in Douglas bags for 60 s every 15 min of the run. Expired air was analysed for the fractional concentration of oxygen and fractional concentration of carbon dioxide by sampling through a paramagnetic transducer and an IR analyser (HiTech), respectively. The oxygen and carbon dioxide analysers were calibrated before each test using certified gases (gas 1: 100 % N; gas 2: 15·5 % oxygen and 4·7 % carbon dioxide; British Oxygen Company). The minute volume was determined for each Douglas bag using a dry gas meter (Harvard Limited) previously calibrated against a 3 litres gas syringe (Hans Rudolph). The temperature of the expired air was determined by an electronic thermometer (RS Supplies). Room temperature and barometric pressure were recorded at the time of the analysis with expired air volumes being later standardised against the temperature and pressure of dry air. The Haldane transformation method was used for the calculation of expired VO2 and VCO2. Non-protein RER were used to calculate the rates of carbohydrate and lipid oxidation, using the equations described by Jeukendrup & Wallis( Reference Jeukendrup and Wallis 12 ). Oxidative energy expenditure was calculated from the rates of carbohydrate and lipid use as unit energy release per g of carbohydrate or lipid. Ratings of perceived exertion were recorded using the Borg Scale( Reference Borg 13 ).
Blood samples and hypoglycaemia
Venous blood samples were collected at rest and 30, 60, 90 and 120 min following ingestion and then during the 2 h recovery period at 0, 5, 30 and 60 min following the cessation of exercise. Hypoglycaemia was defined as a BG value ≤ 4·0 mmol/l. The participants did not drink water during this trial.
Data analysis
Statistical analysis was carried out using the SPSS software (version 16; SPSS, Inc.), with significance set at P≤ 0·05. Data were tested for normal distribution (Shapiro–Wilk test) and subsequently analysed using repeated-measures ANOVA on two factors (treatment × time) with Bonferroni adjustment and dependent t tests being carried out where relevant. Preliminary statistical analysis revealed that the single female participant did not influence the outcomes of any finding. The data of the female participant were included in the analysis. Data are reported as means with their standard errors. Relative BG responses were calculated as a change from rest through the subtraction of fasting concentrations from further glucose values within each condition.
Results
2-h ingestion period at rest
Blood glucose responses
BG concentrations are shown in Fig. 1(a). Fasting BG concentrations were similar between the carbohydrate conditions. Peak BG concentrations were lower under the HPMS and UCMS conditions than under the DEX condition (HPMS 6·2 (sem 0·3) and UCMS 6·0 (sem 0·2) v. DEX 7·7 (sem 0·6) mmol/l; P< 0·05) and occurred later following ingestion (HPMS 60 (sem 6) and UCMS 30 (sem 6) v. DEX 30 (sem 0) min; P< 0·05). There were no observed differences in peak BG concentrations or time to reach peak concentrations between the HPMS and UCMS conditions (NS). Although there were no significant differences in the minimum BG concentration attained during the 2 h ingestion period under any carbohydrate condition (HPMS 4·7 (sem 0·1), UCMS 4·5 (sem 0·2), DEX 4·1 (sem 0·3) mmol/l; NS), the drop from peak to minimum values was less under the HPMS and UCMS conditions compared with that under the DEX condition (HPMS − 1·5 (sem 0·2) and UCMS − 1·5 (sem 0·3) v. DEX − 3·6 (sem 0·4) mmol/l; P< 0·001), with no differences being observed between the HPMS and UCMS conditions. BG concentrations under the HPMS condition were still significantly elevated from resting values at 90 min (P= 0·02) after ingestion compared with those under the UCMS (60 min; P= 0·02) and DEX (30 min; P= 0·001) conditions. Of clinical note, three individuals experienced hypoglycaemia ( ≤ 4·0 mmol/l) during the ingestion period under the DEX condition, while there were no occurrences of hypoglycaemia in either the HPMS or UCMS condition.

Fig. 1 (a) Blood glucose, (b) plasma insulin and (c) plasma NEFA responses to the ingestion of dextrose (DEX, ![]() ), hydrothermally processed maize starch (HPMS,
), hydrothermally processed maize starch (HPMS, ![]() ) and uncooked maize starch (UCMS,
) and uncooked maize starch (UCMS, ![]() ) at rest and 2 h following a submaximal run (Ex). Values are means, with their standard errors represented by vertical bars. Hollow sample points indicate significant changes from rest within each condition (P≤ 0·05). * Mean value was significantly different between the HPMS and UCMS conditions (P≤ 0·05). † Mean values were significantly different between the HPMS and DEX conditions (P≤ 0·05). ‡ Mean values were significantly different between the UCMS and DEX conditions (P≤ 0·05).
) at rest and 2 h following a submaximal run (Ex). Values are means, with their standard errors represented by vertical bars. Hollow sample points indicate significant changes from rest within each condition (P≤ 0·05). * Mean value was significantly different between the HPMS and UCMS conditions (P≤ 0·05). † Mean values were significantly different between the HPMS and DEX conditions (P≤ 0·05). ‡ Mean values were significantly different between the UCMS and DEX conditions (P≤ 0·05).
Plasma insulin
Plasma insulin concentrations are shown in Fig. 1(b). Resting insulin concentrations were similar across the carbohydrate conditions (HPMS 26 (sem 5), UCMS 27 (sem 2), DEX 25 (sem 3) pmol/l; NS). Peak insulin responses to carbohydrate ingestion were 3-fold lower under the HPMS and UCMS conditions compared with those under the DEX condition (HPMS 105 (sem 15) and UCMS 108 (sem 17) v. DEX 391 (sem 51) pmol/l; P< 0·001), with the drop from peak to minimum values being smaller under the HPMS and UCMS conditions compared with that under the DEX condition (HPMS 52 (sem 10) and UCMS 54 (sem 12) v. DEX 331 (sem 45) pmol/l; P< 0·001).
Plasma NEFA and TAG responses
Plasma NEFA concentrations are shown in Fig. 1(c). Resting NEFA concentrations were similar across the carbohydrate conditions (HPMS 0·56 (sem 0·10), UCMS 0·50 (sem 0·10), DEX 0·57 (sem 0·08) mmol/l; NS). Carbohydrate ingestion led to a reduction in plasma NEFA and TAG concentrations over the rest period. By 2 h, NEFA concentrations were greater under the HPMS and UCMS conditions compared with those under the DEX condition (HPMS 0·16 (sem 0·04) and UCMS 0·17 (sem 0·07) v. DEX 0·05 (sem 0·01) mmol/l; P< 0·01). Resting TAG concentrations were similar across the carbohydrate conditions (HPMS 0·88 (sem 0·09), UCMS 0·74 (sem 0·08), DEX 0·71 (sem 0·08) mmol/l; NS). There was a significant reduction in plasma TAG concentrations by the end of the ingestion period under all the carbohydrate conditions (HPMS 0·76 (sem 0·09), UCMS 0·66 (sem 0·09), DEX 0·56 (sem 0·06) mmol/l; P< 0·05), but there were no differences between the carbohydrate conditions (NS).
Blood acid–base and electrolyte balance
Blood acid–base responses are shown in Fig. 2. Resting blood pH was similar between the carbohydrate conditions and did not change under the HPMS or UCMS condition over the 2 h ingestion period, but there was a significant reduction under the DEX condition 2 h after ingestion (rest 7·36 (sem 0·01) v. 120 min 7·34 (sem 0·01); P= 0·03; Fig. 2(a)). Resting blood lactate concentrations increased under all the carbohydrate conditions (P< 0·05; Fig. 2(b)), but the peak blood lactate concentration was lower under both the HPMS and UCMS conditions compared with that under the DEX condition (HPMS 1·0 (sem 0·1) and UCMS 0·9 (sem 0·1) v. DEX 1·3 (sem 0·1) mmol/l; P< 0·01). There were significant reductions in blood K+ concentrations compared with resting values over the 2 h ingestion period under all the carbohydrate conditions (P< 0·001), but there were no differences between the carbohydrate conditions (NS). Resting blood Na+ concentrations were reduced under both the HPMS and UCMS conditions (P< 0·05), but there was no change from resting values under the DEX condition (NS).

Fig. 2 (a) Blood pH and (b) lactate responses to the ingestion of dextrose (DEX, ![]() ), hydrothermally processed maize starch (HPMS,
), hydrothermally processed maize starch (HPMS, ![]() ) and uncooked maize starch (UCMS,
) and uncooked maize starch (UCMS, ![]() ) at rest and 2 h following a submaximal run (Ex). Values are means, with their standard errors represented by vertical bars. Hollow sample points indicate significant changes from rest within each condition (P≤ 0·05). * Mean values were significantly different between the HPMS and DEX conditions (P≤ 0·05). † Mean values were significantly different between the UCMS and DEX conditions (P≤ 0·05).
) at rest and 2 h following a submaximal run (Ex). Values are means, with their standard errors represented by vertical bars. Hollow sample points indicate significant changes from rest within each condition (P≤ 0·05). * Mean values were significantly different between the HPMS and DEX conditions (P≤ 0·05). † Mean values were significantly different between the UCMS and DEX conditions (P≤ 0·05).
Estimated changes in plasma volume
Estimated changes in plasma volume increased significantly from rest under all the carbohydrate conditions during the 2 h ingestion period (peak increase in plasma volume for HPMS 13 (sem 3), UCMS 9 (sem 2), DEX 7 (sem 2) %; P< 0·05) before returning to basal levels by 2 h. The magnitude of change in plasma volume was similar between the carbohydrate conditions (NS).
Perception-of-hunger scores
Peak subjective measures of fullness increased from rest across the carbohydrate conditions (HPMS 26 (sem 11), UCMS 15 (sem 10), DEX 25 (sem 16) units; P< 0·05). This reduction in hunger was similar under each carbohydrate condition (NS) and corresponded to a qualitative rating of ‘slightly full’.
Physical exercise
Physiological cost of running
The physiological responses to submaximal running are detailed in Table 1. Mean exercising heart rates were similar across all the carbohydrate conditions (HPMS 160 (sem 5), UCMS 157 (sem 4), DEX 162 (sem 4) beats/min; NS). The mean rate of carbon dioxide production was significantly lower under the HPMS condition compared with that under the DEX condition (HPMS 30·0 (sem 1·2) v. DEX 30·6 (sem 1·3) ml/kg per min; P= 0·02). The rate of oxygen consumption was significantly greater under the HPMS condition compared with that under the UCMS condition (HPMS 33·5 (sem 1·1) v. UCMS 32·5 (sem 1·0) ml/kg per min; P< 0·01); thus, participants were exercising at a higher percentage of VO2max under the HPMS condition than under the UCMS condition (HPMS 65·1 (sem 2·9) v. UCMS 63·1 (sem 2·5) %; P< 0·01). There were no differences in the rate of oxygen consumption between the DEX condition and either the HPMS or UCMS condition in absolute (ml/kg per min) or relative (%VO2max) units (NS). The ratings of perceived exertion were similar across the carbohydrate conditions (HPMS 12 (sem 1), UCMS 12 (sem 1), DEX 12 (sem 2); NS), corresponding to a subjective intensity between ‘fairly light’ and ‘somewhat hard’.
Table 1 Physiological responses and perception of intensity during run exercise (Mean values with their standard errors)
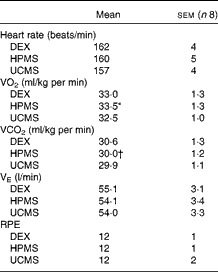
DEX, dextrose; HPMS, hydrothermally processed maize starch; UCMS, uncooked maize starch.
* Mean value was significantly different between the HPMS and UCMS conditions (P≤ 0·05).
† Mean value was significantly different between the HPMS and DEX conditions (P≤ 0·05).
Fuel oxidation
Mean RER values under the HPMS condition (0·89 (sem 0·01) units) were significantly lower compared with those under the UCMS condition (0·92 (sem 0·01) units; P= 0·049), with no differences being observed between the HPMS and DEX or DEX and UCMS conditions (NS). The mean rates of aerobic energy expenditure under the HPMS condition (49·4 (sem 2·9) kJ/min) were significantly higher compared with those under the UCMS condition (48·1 (sem 3·0) kJ/min; P= 0·01); thus, total aerobic energy expenditure under the HPMS condition was greater than that under the UCMS condition (HPMS 2·96 (sem 0·2) v. UCMS 2·89 (sem 0·2) MJ; P= 0·01). There were no differences in average energy expenditure rate or total aerobic energy expenditure between the HPMS and DEX or DEX and UCMS conditions (NS).
The mean rate of aerobic energy expenditure derived from carbohydrates was lower under the HPMS condition (32·3 (sem 3·6) kJ/min) compared with that under the UCMS or DEX condition (UCMS 35·4 (sem 3·0); P= 0·04; DEX 36·4 (sem 4·3) kJ/min; P= 0·07). The percentage of energy derived from carbohydrate oxidation under the HPMS condition (65 (sem 4) %) was lower than that under the UCMS (73 (sem 3) %; P= 0·039) and DEX (72 (sem 6) %; P= 0·074) conditions. Finally, the total amount of oxidised carbohydrate tended to be lower under the HPMS condition (124·4 (sem 13·6) g) compared with that oxidised under the UCMS condition (136·1 (sem 11·5) g; P= 0·09) or DEX condition (140·1 (sem 16·7) g; P= 0·054; Fig. 3(c)). The mean rate of energy expenditure derived from lipids was greater under the HPMS condition (17·0 (sem 1·74) kJ/min) compared with that under the UCMS and DEX conditions (UCMS 12·7 (sem 1·8); P= 0·04; DEX 13·4 (sem 2·4) kJ/min; P= 0·07). Thus, the percentage of energy derived from lipid oxidation under the HPMS condition (35 (sem 4) %) was greater than that under the UCMS (27 (sem 3) %; P= 0·039) and DEX (28 (sem 6) %; P= 0·074) conditions. The total amount of lipids oxidised was greater under the HPMS condition (26·2 (sem 2·8) g) compared with that oxidised under the UCMS condition (19·6 (sem 2·7) g; P= 0·04) or DEX condition (20·6 (sem 3·6) g; P= 0·07; Fig. 3(c)).

Fig. 3 (a) Carbohydrate (CHO) oxidation, (b) lipid oxidation and (c) percentage of energy expenditure derived from carbohydrates (grey) and lipids (black) during the submaximal run protocol. Values are means, with their standard errors represented by vertical bars. ■, Dextrose (DEX); □, hydrothermally processed maize starch (HPMS); ![]() , uncooked maize starch (UCMS). * Mean values were significantly different between the HPMS and DEX conditions (P= 0·07). † Mean values were significantly different between the HPMS and DEX conditions (P≤ 0·05). (A colour version of this figure can be found online at
http://www.journals.cambridge.org/bjn).
, uncooked maize starch (UCMS). * Mean values were significantly different between the HPMS and DEX conditions (P= 0·07). † Mean values were significantly different between the HPMS and DEX conditions (P≤ 0·05). (A colour version of this figure can be found online at
http://www.journals.cambridge.org/bjn).
2-h recovery
Blood glucose responses
BG concentrations peaked at 5 min after exercise under all the carbohydrate conditions, but values were greater under the HPMS and UCMS conditions compared with those under the DEX condition (HPMS 5·4 (sem 0·2) and UCMS 5·4 (sem 0·1) v. DEX 4·9 (sem 0·2) mmol/l; P< 0·05). This effect persisted for 30 min into the recovery period and then the values were similar across the carbohydrate conditions. In addition to the three episodes of hypoglycaemia before exercise, there were a further two episodes of hypoglycaemia under the DEX condition in two more individuals during the first 5 min of recovery (0 min after exercise 3·9 mm; 5 min after exercise 3·7 mm). There were no occurrences of hypoglycaemia under the HPMS or UCMS condition.
Plasma insulin
Peak insulin concentrations during the recovery period were higher under the HPMS and UCMS conditions compared with those under the DEX condition (HPMS 59 (sem 13) and UCMS 77 (sem 10) v. DEX 27 (sem 6) pmol/l; P< 0·01). Although concentrations dropped under all the carbohydrate conditions over the remainder of the recovery period, insulin concentrations remained higher under the HPMS and UCMS conditions compared with those under the DEX condition (P< 0·05).
Plasma NEFA and TAG responses
Plasma NEFA concentrations peaked in the first 5 min of recovery to a similar degree across the carbohydrate conditions (HPMS 1·33 (sem 0·15), UCMS 0·99 (sem 0·19), DEX 1·27 (sem 0·19) mmol/l; NS). However, from this point onwards, NEFA concentrations were lower under the HPMS and UCMS conditions compared with those under the DEX condition (e.g. 120 min: HPMS 0·55 (sem 0·06) and UCMS 0·51 (sem 0·06) v. DEX 0·93 (sem 0·05) mmol/l; P< 0·01). TAG concentrations increased modestly with exercise, peaking in the first 5 min and thereafter decreasing to pre-exercise resting levels from 30 min onwards similarly across the carbohydrate conditions.
Blood acid–base and electrolyte balance
Compared with resting values, blood pH was significantly elevated in the immediate recovery period similarly across the carbohydrate conditions (HPMS 7·42 (sem 0·00), UCMS 7·42 (sem 0·01), DEX 7·42 (sem 0·01) units; NS). Thereafter, there was a progressive decline in blood pH such that values returned to resting values by 2 h. Peak blood lactate concentrations were achieved immediately after exercise (HPMS 1·6 (sem 0·03), UCMS 1·3 (sem 0·2), DEX 1·9 (sem 0·3) mmol/l; NS), gradually returning to resting values by 30–60 min under each condition.
Immediately after exercise, there were significant increases in blood K+ concentrations and decreases in blood Na+ concentrations compared with resting values under all the carbohydrate conditions (P< 0·01). Blood Na+ concentrations gradually returned to resting levels, whereas there was a significant increase in blood K+ concentrations by 2 h (P< 0·05). However, there were no significant differences between the carbohydrate conditions at any time point (NS).
Estimated changes in plasma volume
Plasma volume decreased significantly from rest under all the carbohydrate conditions during the 2 h ingestion period (HPMS − 2·7 (sem 2·5), UCMS − 4·4 (sem 2·9), DEX − 6·2 (sem 1·9) %; P< 0·05) before returning to basal levels by 2 h. The magnitude of change in plasma volume did not differ between the carbohydrate conditions (NS).
Perception-of-hunger scores
The mean hunger scores of the participants gradually declined from peak values, reaching a nadir 2 h after exercise (HPMS − 33 (sem 9), UCMS − 37 (sem 9), DEX − 33 (sem 10) units; P< 0·05) across the carbohydrate conditions. This increase in hunger sensation corresponded to feelings of being ‘slightly hungry’ to ‘moderately hungry’.
Discussion
The aim of the present study was to compare the metabolic responses to the ingestion of a HPMS with those to the ingestion of UCMS or maize starch-derived DEX at rest and during and after physical exercise in healthy individuals. The results demonstrate both HPMS and UCMS to be equally effective in improving blood glycaemia compared with DEX, yet BG concentrations remained elevated from resting values for a longer duration under the HPMS condition compared with those under the UCMS or DEX condition. In addition, there was a 7–9 % reduction in carbohydrate oxidation after the ingestion of HPMS compared with that observed after the ingestion of UCMS or DEX due to a lesser suppression of endogenous lipid use with the lower-GI starch.
Ingestion
All the carbohydrates increased BG concentrations, but the peak BG concentration was 50 % lower and occurred approximately 30 min later under the HPMS condition compared with that occurring under the DEX condition. From a glycaemic management point of view, this is a beneficial acute effect of the consumption of either HPMS or UCMS. Stimulation of pancreatic insulin release was lower as a result of a smaller and slower release of carbohydrates into the circulation, with values under the HPMS and UCMS conditions being 3-fold lower compared with those under the DEX condition. There were no incidences of BG values decreasing below the ≤ 4 mmol/l threshold under either the HPMS or UCMS condition, but three individuals experienced hypoglycaemia when consuming DEX. Taken together, these findings suggest that the ingestion of HPMS and UCMS may contribute to smaller changes in BG and plasma insulin concentrations, which may positively affect good glycaemic management.
Glycaemic responses to the ingestion of approximately 77 g of HPMS as a 10 % solution were similar to those observed for an equivalent dosing of UCMS whether comparing peak concentrations, time to reach peak values, minimum values or drop from peak to nadir. However, within each trial, there was some evidence of differences in BG concentrations under the HPMS condition compared with those under the UCMS condition, similar to the findings of other studies in GSD patients( Reference Bhattacharya, Orton and Qi 5 Reference Correia, Bhattacharya and Lee 6 ). We demonstrated significantly elevated BG concentrations from resting values for 90 min following ingestion under the HPMS condition compared with those for 60 min under the UCMS condition and for 30 min under the DEX condition. Given that the available data on GI of these carbohydrates demonstrate a GI value of 35 (sem 4) (low GI) for HPMS and of 41 (sem 1) (low GI) for UCMS, this finding is a little surprising. In the study carried out by Bhattacharya et al. ( Reference Bhattacharya, Orton and Qi 5 ), although there were no differences in peak, AUC or rate of glucose increase between the HPMS and UCMS conditions in GSD type Ia, Ib and III patients, a betterment of glycaemia was inferred from the slower decline in BG concentrations from peak to trough (P= 0·05) – a finding similar to our findings. Taken together, there appears to be some merit in support of hydrothermally modified maize starch ingestion over UCMS ingestion in improving the glycaemic profile of patients and healthy individuals despite the equivalent ‘low’ GI rating.
A reduced BG response to the ingestion of HPMS or UCMS compared with the response to the ingestion of DEX resulted in lower insulin responses and greater circulating NEFA concentrations. This suggests a proportionally better balanced mix of circulating glucose and NEFA for tissue uptake in contrast to the greater mass-action effect of higher BG concentrations under the DEX condition( Reference Frayn 14 ). Indeed, the greater increase in blood lactate concentrations under the DEX condition is suggestive of an increased tissue glycolytic rate. Finally, circulating insulin increases K reuptake into tissue cells, so we explored the influence of carbohydrate-induced insulin release on blood K+ disappearance for different carbohydrates. Although there were differences in insulin secretion between the carbohydrate conditions, we found no differences in the magnitude of changes in blood K+ or K+/Na+ concentrations.
Physical exercise
Aerobic running increases tissue fuel requirements and presents a useful modality for exploring the impact of carbohydrate ingestion on muscle metabolic need. There was a 2 % greater rate of oxygen use during running at the same speed for 1 h under the HPMS condition compared with that under the UCMS condition. Given that the oxygen cost of burning lipids is greater than that of burning carbohydrates, the greater rate of aerobic energy expenditure and lower RER value under the HPMS condition compared with those under the UCMS condition (RER values of 0·89 v. 0·92) together support an increased contribution of lipids to exercising oxidative energy metabolism. Indeed, using principles of indirect calorimetry, we were able to demonstrate a greater rate of lipid oxidation of exercising participants whether expressed as g/min or as a percentage of total energy. Thus, our data suggest a lesser suppressive effect of starch carbohydrates on lipid oxidation when the source of the carbohydrate has been hydrothermally modified. Conversely, the data suggest lower carbohydrate use during exercising, indicating better preservation of carbohydrate stores. Interestingly, in ten GSD patients who had fasted for ≥ 2 h before the ingestion of 2 g/kg BM starch, resting glucose oxidation values tended to be lower (but did not reach statistical significance) during the HPMS ingestion trial compared with those during the UCMS trial( Reference Bhattacharya, Orton and Qi 5 ). The lower carbohydrate oxidation rate during physical activity despite a longer duration of raised BG concentrations above resting values under the HPMS condition compared with that under the UCMS condition appears to be a paradox. Moreover, this tissue oxidation difference persisted despite similar changes in insulin, NEFA and blood lactate concentrations in response to physical activity. However, available data reported in the study of Bhattacharya et al. ( Reference Bhattacharya, Orton and Qi 5 ) demonstrate subtle differences in the fraction of resistant starch of both starch carbohydrates. Using the methods of Englyst et al. ( Reference Englyst, Kingman and Cummings 15 ), a 7·2 % greater fraction of resistant starch was observed in HPMS compared with UCMS. Thus, the magnitude of difference in resistant starch is equivalent to the percentage differences in carbohydrate use and could explain the preference for lipid combustion under the HPMS condition than under the UCCS condition. So, a lower digestibility of a lower-GI HPMS presented a smaller amount of carbohydrate to the circulation (and remained for a longer duration) than a larger amount of more digestible low-GI UCMS carbohydrate that disappeared quicker.
Recovery
BG concentrations under the HPMS or UCMS conditions were greater for 30 min of the 2 h recovery period compared with those under the DEX condition. Less pancreatic β-cell insulin release before exercise combined with more available circulating NEFA may have contributed to less glucose disposal during exercise and higher residual glucose concentrations in the recovery period. That between-carbohydrate differences disappeared after 30 min of recovery suggests a greater uptake of circulating glucose by GLUT-4 transporters to replenish muscle glycogen stores under the HPMS and UCMS conditions despite a lower rate of carbohydrate use during exercise. It has been shown that maltodextrin that is ingested tends not to be stored in the liver, favouring appearance in the circulation after ingestion( Reference Décombaz, Jentjens and Ith 16 ). That there were lower glucose concentrations in the circulation under the DEX condition might suggest greater absorption and more use of exogenously ingested DEX compared with HPMS or UCMS and might contribute to an explanation for the additional two occurrences of exercise-induced hypoglycaemia in the present study. Finally, the present pilot study was useful in determining a metabolic advantage of HPMS over UCMS and DEX in eight participants in a similar age range. Post-priori power analysis revealed a 58·4 % statistical power (5 % α-level; 95 % CI) to be associated with clinically meaningful alterations in carbohydrate oxidation. Based on the present study, fifteen participants would be required to achieve 80 % statistical power in significant findings.
Conclusions
The present experiment demonstrated a small glycaemic advantage to ingestion of HPMS over other carbohydrate alternatives with BG concentrations remaining significantly elevated from resting values for longer than UCMS or DEX. In addition, there was a reduction in carbohydrate oxidation after the ingestion of the HPMS compared with that after the ingestion of UCMS most probably due to small differences in resistant starch. These results warrant a study to determine whether similar magnitudes of change in blood glycaemia and fuel oxidation occur and may be important in improving the quality of life of GSD patients (e.g. reduced overnight feeding frequency, improved sleep patterns and resistance to glycaemic imbalances).
The authors thank Vitaflo International for the supply of carbohydrates and financial support to facilitate metabolite analysis. Vitaflo contributed to the preparation of the manuscript.
The authors' contributions are as follows: R. M. B. contributed to the conception and design of the study, researching and analysing data, and wrote the manuscript; B. J. G. contributed to the design of the study, researching data, and reviewed/edited the manuscript; D. T. contributed to the design of the study, researching data, and biochemical analysis and reviewed/edited the manuscript.
There are no known potential conflicts of interest relevant to this article for any author.






