Epidemiological studies within populations have indicated that a high dietary intake of fruit and vegetables is associated with decreased CVD risk. The WHO has, in its Global Burden of Disease 2000 Study, conducted a comparative risk assessment to estimate the health effects of low fruit and vegetable intakes. The WHO concludes that the total global burden of disease would be reduced 1·8 % by increasing individual fruit and vegetable consumption up to 600 g/d. Within the total burden, 31 and 19 % are accounted for by a decreased risk of IHD and ischaemic stroke, respectivelyReference Lock, Pomerleau, Causer, Altmann and McKee1. This is confirmed in prospective studies showing a direct inverse association between fruit and vegetable intake and CVDReference Rimm, Ascherio, Giovannucci, Spiegelman, Stampfer and Willett2–Reference Hung, Joshipura, Jiang, Hu, Hunter, Smith-Warner, Colditz, Rosner, Spiegelman and Willett8. The biological mechanisms whereby fruit and vegetables may exert their effects are not entirely clear and are likely to be multiple. Many nutrients, like folate, K, glucosinolates, plant sterols and phytochemicals might be involvedReference Ignarro, Balestrieri and Napoli9.
A limited number of intervention studies have investigated the relationship between increased fruit and vegetable consumption and CVD. These studies have severe shortcomingsReference Horton10 or have studied the effect of multiple dietary changes simultaneouslyReference de Lorgeril, Renaud, Mamelle, Salen, Martin, Monjaud, Guidollet, Touboul and Delaye11. Carotid intima-media thickness (IMT) has been used in a wide range of lifestyle interventionsReference Persson, Israelsson, Stavenow, Holmstrom and Berglund12–Reference Bemelmans, Lefrandt, Feskens, Broer, Tervaert, May and Smit14 and in observational studies for nearly two decades as a marker of atherosclerosis and as a surrogate CVD endpoint, not only of stroke but also of IHDReference O'Leary and Polak15. Several studies have indicated an inverse relationship between plasma levels of antioxidant vitamins that are found in fruit and vegetables such as vitamin C, carotenoids and lutein and carotid IMTReference Iribarren, Folsom, Jacobs, Gross, Belcher and Eckfeldt16–Reference Dwyer, Paul-Labrador, Fan, Shircore, Merz and Dwyer18. However, studies directly relating consumption of fruit and vegetables to atherosclerosis in the carotid arteries as assessed by IMT seem to be lacking.
The Diet and Omega-3 Fatty Acid Intervention Trial on Atherosclerosis (DOIT) was initiated to determine the effect of a dietary and/or fish oil supplementation intervention on the progression of atherosclerosis as measured by the carotid IMTReference Hjerkinn, Abdelnoor, Breivik, Bergengen, Ellingsen, Seljeflot, Aase, Klemdal, Hjermann and Arnesen19 in a cohort of elderly male survivors of an earlier trialReference Hjermann, Velve, Holme and Leren20. The aim of the present investigation was to assess whether the baseline level of atherosclerosis in these men as measured by the carotid IMT was associated with their dietary intake of putative cardioprotective foods, in particular, fruit, berries and vegetables. Though limited research has been performed on the association between berries and CHD, berries are rich in antioxidants and other micronutrientsReference Blomhoff21 and are often categorised together with fruit when dietary intakes are assessedReference Johansson, Solvoll, Bjørneboe and Drevon22.
Subjects and methods
The Oslo Diet and Antismoking Trial was initiated in 1972 as a primary prevention study that sought to establish whether lowering of serum cholesterol and cessation of smoking reduced the incidence of CHD. The study and its outcome have been extensively described previouslyReference Hjermann, Velve, Holme and Leren20. Briefly, 1232 healthy men aged 40–49 years with elevated serum total cholesterol or a high coronary risk score, invited from a pool of 16 202 screened men (65 % of all men aged 40–49 years in Oslo), were included in 1972–3. All subjects had a normal electrocardiogram at rest, reported no chest pain at exercise testing, and were free of CVD, hypertension, diabetes mellitus, cancer, disabling or psychopathological conditions and alcoholism. Subjects in the intervention group (n 604) were counselled by the physician and dietitian every 6 months during 5 years, while controls (n 628) were seen at 12-month intervals and were not given dietary or anti-smoking advice. The decrease in CHD in the intervention group after 5 years was closely related to change in total cholesterol concentration but less so to smoking cessationReference Hjermann, Velve, Holme and Leren20.
Vital status was gathered for every participant in the Oslo Diet and Antismoking Trial up to 31 December 1996. This was made possible by linkage to Statistics Norway and based on the unique eleven-digit identification number of all Norwegian citizens. The procedure was approved by the Norwegian data inspectorate. In 1997–9 survivors (n 910) of the study were invited to participate in a new study, the DOIT, a randomised factorial clinical trial of dietary change and/or fish oil supplementation on the progression of atherosclerosis in the carotid arteryReference Hjerkinn, Abdelnoor, Breivik, Bergengen, Ellingsen, Seljeflot, Aase, Klemdal, Hjermann and Arnesen19. Of the 910 men, 255 did not respond to the invitation, and ninety-two responders were excluded because of unwillingness to participate, serious illness or difficulties with transportation; thus 563 were included in the DOIT. The regional ethics committee approved the study, and all participants gave their informed written consent.
Physical assessments
Data regarding medical history including history of established CVD, use of cholesterol-lowering and other drugs and lifestyle factors were obtained by medical examination at screening for the DOITReference Hjerkinn, Abdelnoor, Breivik, Bergengen, Ellingsen, Seljeflot, Aase, Klemdal, Hjermann and Arnesen19. Body weight and height were measured with the subject barefoot and wearing light clothing. BMI was calculated as kg/m2.
Assessment of educational level and smoking
The participants' former and present work or profession was self-reported. Based on this information each participant was given a score for his educational level according to data from the Standard Classification of Occupations (Statistics Norway; www.SSB.no/emner/06/yrke). Thus occupations that require no more than 9 years of primary education and occupations that require 1–3 years of secondary education (9–12 years in total) were equivalent to educational level 1. Occupations that normally require 1–3 years of college education (13–15 years in total) and occupations that normally require education equivalent to a first or postgraduate university degree (16–18 years in total) were equivalent to educational level 2.
Subjects were classified as smokers if they smoked daily or occasionally and if they had quit smoking less than 6 months before baseline examination. Pipe and cigar smokers were classified as smokers.
Dietary data
Of the 563 men included in the study, 558 supplied dietary information and five participants refused to do so. Participants completed an optically read quantitative FFQ designed to cover the whole diet, They were asked to have the last 6 months in mind when filling it out. The FFQ was developed and evaluated at the Section for Dietary Research, Institute for Nutrition Research, University of Oslo, NorwayReference Solvoll23. The FFQ included 180 food items which were selected on the basis of Norwegian dietary patterns obtained in previous dietary surveys. The food items represented in the FFQ followed a traditional meal pattern consisting of bread-based meals and a main meal (dinner). The food items were grouped in fourteen sections based on these food items and meal type. The questions were grouped according to the frequency and portion size of a single food or dish. There were additional sections for dietary supplements and regarding attitudes towards diet and health. These included a questionnaire regarding how attentive they were to following a healthy diet and whether they had changed their diet during or after their participation in the Oslo Diet and Antismoking TrialReference Ellingsen, Hjerkinn, Arnesen, Seljeflot, Hjermann and Tonstad24. The FFQ has been validated against plasma fatty acids and α-tocopherol levels in adipose tissue and serumReference Andersen, Solvoll, Johansson, Salminen, Aro and Drevon25, and the questions regarding intake of fruit and vegetables have been validated against serum carotenoid levelsReference Andersen, Veierød, Johansson, Sakhi, Solvoll and Drevon26.
The FFQ and a written instruction formula were handed out to participants to complete at home and return at the following visit. Participants were asked to fill in the food items as number of portions and frequency of consumption. To avoid inaccuracy, a nutritionist (I. E.) or a trained nurse checked and corrected missing information together with the participant (for example, forgotten foods, added phantom foods, inaccurately reported food frequencies, incorrect quantification of consumed portions and overestimation of seasonal foods). Portion size was estimated by models or photographs (Matmallen, Livsmeldelsverket, Uppsala, Sweden). Daily intakes of food and nutrients were computed using food database and software systems developed at the Section for Dietary Research, University of Oslo. The nutrient calculation did not include the use of cod liver oil or other vitamin and mineral supplements. The FFQ interviews were conducted between April 1997 and January 1999 during all months of the year, except July.
Assessment of carotid intima-media thickness
IMT was measured to detect structural changes in the carotid artery as described previouslyReference Hjerkinn, Abdelnoor, Breivik, Bergengen, Ellingsen, Seljeflot, Aase, Klemdal, Hjermann and Arnesen19. The subjects were examined in a supine position with an ultrasound scanner (Acuson 128; Acuson, Mountain View, CA, USA) with a 7·0 MHz linear array transducer as previously described in detailReference Persson, Stavenow, Wikstrand, Israelsson, Formgren and Berglund27. All scans were performed by the same sonographer (Lise Bergengen). In brief, at the position of the thickest part of the far wall common carotid IMT (visually judged) three end-diastolic images were captured and recorded on videotapes for off-line analysis. The ultrasound images from the videotape were analysed blindly at the Ultrasound Laboratory, Clinical Research Unit, Department of Medicine, Malmø University Hospital, SwedenReference Persson, Stavenow, Wikstrand, Israelsson, Formgren and Berglund27. The mean IMT value in a 10 mm long segment in the common carotid artery was used for statistical analyses. Intra-observer reproducibility tests yielded a CV of 3–9 %. Twelve IMT measurements were not interpretable due to technical problems.
Statistics
A univariate linear regression model was initially used to examine the relationship of the IMT to possible correlates including clinical and dietary variables. The χ2 test was used to compare categorical variables. One-way ANOVA including a Bonferroni post hoc test was used to test mean differences and differences between continuously distributed variables with the consumption of fruit and berries (divided into quartiles) as the dependent variable. The natural logarithm of continuous variables was used because of skewed distributions. Fruit and berry consumption (g/d) was the primary exposure variable and IMT (mm) was the dependent variable. Dietary variables that were related both to IMT and fruit and berry consumption were chosen as potential intermediate confounders, while age (years), smoking (yes or no) and energy intake (MJ) were considered as confounders at baseline (Fig. 1). Energy intake was included to ensure that any association between fruit and berry consumption and IMT was not because of differences in energy intake as recommended by Willett & StampferReference Willett, Stampfer and Willett28. Other risk factors such as systolic blood pressure (mmHg) and HDL-cholesterol (mmol/l) were not included in the multivariate model because these factors are strongly influenced by the diet and may be in the causal pathway relating fruit and berry consumption to atherosclerosisReference Willett, Stampfer and Willett28. Partial correlation analyses with and without the intermediate exposure variables were performed. In addition a multivariate linear regression model was used in order to estimate the reduction in IMT associated with the increase in fruit and berry consumption. Two-sided P values < 0·10 were used to choose possible confounders, while P values < 0·05 were considered statistically significant for the main results. Analyses were done using SPSS software version 14.0 (SPSS Inc., Chicago, IL, USA).

Fig. 1 Model showing the theoretical association between the dietary intake of fruit and berries and carotid intima-media thickness.
Results
One subject was missing both dietary and ultrasound data. Thus, dietary data and ultrasound measurements were available for 547 of the 563 subjects. Table 1 shows the baseline characteristics of the participants. The univariate regressions between IMT and clinical and dietary variables are shown in Table 2. IMT was positively correlated with age, smoking, systolic blood pressure, dietary energy, consumption of milk, cream and ice cream, dietary saturated fat and dietary cholesterol, and inversely correlated with HDL-cholesterol and consumption of fruit and berries. No relationship was found between IMT and vegetable consumption. Vegetable consumption did not include potatoes, and the intake of potatoes was not related to IMT (data not shown).
Table 1 Baseline characteristics (n 547)
(Mean values and standard deviations)

* n 546.
† n 543.
‡ n 544.
§ n 541.
Table 2 Univariate linear regression between intima-media thickness (IMT) (mm; dependent variable) and clinical and dietary variables (n 547)

B, regression coefficient; R 2, percentage variability of IMT.
* n 546.
† n 543.
‡ n 544.
§ n 541.
Clinical characteristics according to quartiles of intake of fruit and berries are shown in Table 3. Consumption of fruit and berries was related to age, less smoking, high educational level, and to the presence of CVD and statin use.
Table 3 Characteristics according to quartiles of daily consumption of fruit and berries (n 558)*
(Mean values and standard deviations)

* An ANOVA test with ln-transformed variables was used for continuous variables. The χ2 test was used for categorical variables. Data on statin use and CVD was missing for five subjects. Glucose values were missing for six subjects, total cholesterol and TAG values were missing for four subjects, and HDL-cholesterol values were missing for three subjects.
The percentage of subjects that was assigned to the intervention arm in the study that started in 1972–3 (Oslo Diet and Antismoking Trial) did not differ according to the quartiles of fruit and berry consumption. These percentages were 49·6, 49·3, 48·6 and 49·6 % in the lowest to highest quartiles, respectively (P = 1·0). Thus, the intervention in the earlier trial did not affect the consumption of fruit and berries in the present examination. Fruit and berry consumption was related to the subject's report that he changed his diet during or after the Oslo Diet and Antismoking Trial (45·5 % of all subjects reported such change in the highest quartile of fruit and berry consumption v. 36·9, 32·5 and 25·8 % in the third, second and first quartiles, respectively; P = 0·01). Fruit and berry consumption was also related to the subject's report of paying attention to his diet (58·3 % in the highest quartile v. 51·4, 42·3 and 33·8 % in the third, second and first quartiles, respectively; P < 0·001).
The carotid IMT in the highest quartile of dietary intake of fruit and berries was 0·89 (se 0·18) v. 0·93 (se 0·23), 0·94 (se 0·22) and 0·96 (se 0·25) mm in the third, second and first quartiles (P = 0·047), giving a mean difference of 0·075 (se 0·027) mm (P = 0·033) between the highest and lowest quartile of intake of fruit and berries. Consumption of fruit and berries was associated with the consumption of cereals and bread, vegetables, fish and fish products, cakes, and tea, indicating an overall healthier diet in high consumers of fruit and berries (Table 4). Fruit and berry consumption was thus associated with a diet composed of less total and saturated fat and protein, more dietary cholesterol and carbohydrates and a higher energy intake (Table 4).
Table 4 Dietary intake according to quartiles of consumption of fruit and berries (n 558)*
(Mean values and standard deviations)

* An ANOVA test with ln-transformed variables was used for continuous variables. The χ2 test was used for categorical variables.
In a partial correlation analysis between fruit and berry consumption and IMT controlled for the baseline potential confounders (age, smoking and total energy intake) the correlation coefficient was − 0·159 (P < 0·001). Further controlling for the intermediate exposure variables (consumption of milk, cream and ice cream, dietary cholesterol and saturated fat) the partial correlation coefficient was − 0·142 (P = 0·001), giving a reduction of 11 % in correlation coefficient. This rather small reduction indicates that most of the effect of fruit and berries on IMT is mediated independently of the intermediate exposure variables. The multivariate analysis showed that the inverse relationship between fruit and berry consumption and IMT remained after adjustment (Table 5). The difference in IMT for each increase in one portion of fruit and berries (150 g) was estimated. The IMT was 2·4 % lower for each portion increase in the consumption of fruit and berries (calculation not shown).
Table 5 Results of the multivariate linear regression analysis of intima-media thickness (IMT; dependent variable) and consumption of fruit and berries (n 547)*
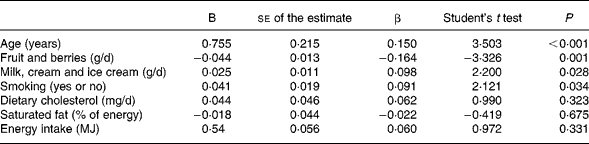
B, regression coefficient; β, standardised coefficient; R, multivariate regression coefficient; R 2, percentage of the variability of IMT explained by all variables in the test.
* R for the total model 0·257; R 20·066; se 0·209; P < 0·001.
Discussion
In these elderly men, who had taken part in a dietary intervention and smoking cessation study more than 25 years previously (the Oslo Diet and Antismoking Trial), we found that an increased consumption of fruit and berries was associated with a reduced thickness of the carotid IMT. This association persisted after adjustment for a number of prognostic and potentially confounding factors. Fruit and berry consumption was associated with a positive response to the question of whether the subject had changed his diet during or after the previous trial.
Though carotid IMT is not a hard CVD endpoint, a preponderance of data has shown significant correlations between the carotid arterial wall IMT and the risk of coronary events and stroke. A strength of the present study is that we found expected relationships between IMT and risk factors for atherosclerosis including systolic blood pressure, HDL-cholesterol and smokingReference Chambless, Folsom, Davis, Sharrett, Heiss, Sorlie, Szklo, Howard and Evans29, Reference Trøseid, Hjerkinn, Seljeflot, Klemsdal, Bergengen, Brievik and Arnesen30. While we are unaware of previous data linking fruit intake with carotid IMT, the results of a previous investigation of change in carotid IMT and dietary change are in line with our findingsReference Bemelmans, Lefrandt, Feskens, Broer, Tervaert, May and Smit14. The authors reported primarily on the association of a decrease in saturated fat intake and less progression of carotid IMT; however, a close perusal of their work reveals that an increase in fruit intake in the course of 2 years tended to be associated with less adverse change in carotid IMT.
Our findings are compatible with previous observational data showing that a high fruit intake may reduce mortality among elderly Swedish menReference Strandhagen, Hansson, Bosaeus, Isaksson and Eriksson31. Other evidence has linked carotenoid consumption with decreased cardiovascular mortality in the elderly in Massachusetts, USAReference Gaziano, Manson, Branch, Colditz, Willett and Buring32. Furthermore, higher levels of plasma oxygenated carotenoids and α-carotene were inversely related to the increase of carotid IMT in the course of 18 monthsReference Dwyer, Paul-Labrador, Fan, Shircore, Merz and Dwyer18. Rather than focusing on carotenoids or other antioxidants that are found in fruit and berries we studied the intake of the foods themselves, as fruit and berries contain a wide range of micronutrients, antioxidants and phytochemicals that may protect against CVDReference Blomhoff21. Moreover, dietary recommendations involve the intake of whole foods, rather than nutrients. Indeed, supplementation of the diet with antioxidants has not generally shown favourable effects on CVD morbidity or mortalityReference Woodside, McCall, McGartland and Young33 and antioxidant vitamin levels are only weakly related to a reduced risk of CHDReference Knekt, Ritz and Pereira34.
The explanation of a lack of an association between intake of vegetables and carotid IMT is not clear. In recent analyses from the Nurses' Health Study and the Health Professionals' follow-up study fruit was associated with a greater reduction in risk of CVD than vegetablesReference Hung, Joshipura, Jiang, Hu, Hunter, Smith-Warner, Colditz, Rosner, Spiegelman and Willett8. Among specific groups of fruit and vegetables, green leafy vegetables were most strongly associated with a reduction in the risk of CVDReference Hung, Joshipura, Jiang, Hu, Hunter, Smith-Warner, Colditz, Rosner, Spiegelman and Willett8. A recently published meta-analysis also found that fruit was associated with a greater reduction in CVD than fruit and vegetable consumptionReference Dauchet, Amouyel, Hercberg and Dallongeville35. The intake of green leafy and other fresh vegetables was low in our cohort (data not shown), while carrots were the main vegetable consumed by our sample of men. Carrot consumption tended to be weakly associated with carotid IMT (β − 0·081; P = 0·06).
Our categorisation of fruit included fresh, frozen and canned fruit and fruit juices while the category of berries included fresh and frozen berries but not berry jams. The relationship between berry consumption alone and carotid IMT did not reach statistical significance (β − 0·055; P = 0·2). However, the amount of berries consumed was low (1·7, 2·6, 5·0 and 9·8 g/d in the first, second, third and fourth quartiles, respectively, of the total intake of fruit and berries). The consumption of berries is generally seasonal in Norway; however, the FFQ were evenly completed during all months of the year. We identified one other study that examined the relationship of the intake of berries to CVDReference Rissanen, Voutilainen, Virtanen, Venho, Vanharanta, Mursu and Salonen36; in that study the effect of separate categories of fruit, vegetables or berries was not reported.
In a previous meta-analysis van't Veer et al. Reference van't Veer, Jansen, Klerk and Kok37 showed that an increase in fruit and vegetable intake of 150 g/d was associated with a 16 % lower mortality from CVD. Dauchet et al. Reference Dauchet, Amouyel, Hercberg and Dallongeville35 found that the risk of CHD was decreased by 4 % for each additional portion per d of fruit and vegetable intake and by 7 % by each additional portion of fruit intake. In the present study the mean intake of fruit and berries ranged from one-half portion per d in the lowest quartile of intake to less than three portions per d in the highest quartile. This difference of 348 g/d was associated with an approximately 5·5 % adjusted difference in mean IMT between the lowest and highest quartiles. This difference is less than that reported in studies that examined morbidity and mortality endpoints. The present study sample was homogeneous in regard to age and sex, a factor that may limit the likelihood of finding large differences between subgroups.
The amount of fruit and berry consumption was based on an FFQ covering the previous 6 months. A major disadvantage of the FFQ is that it requires subjects to remember and estimate the amount and frequency of consumption of a large number of foods. However, its advantage is that the questions were framed as a dietary history starting with the breakfast meal and the frequency questions were followed by a question regarding portion size. Because the FFQ was first filled out by the subjects at home and then checked by a nutritionist, errors in estimating portion sizes seem less plausible.
The subjects in the study carried a high risk of CVD because of high cholesterol levels or a high coronary risk score identified before their participation in an intervention study. About half of the subjects had received dietary intervention in the Oslo Diet and Antismoking Study about 27 years earlier, while the other half were in the control group of the present study. This dietary intervention focused on the reduction of dietary fat and cholesterol and did not specifically involve recommendations to increase intakes of fruit, vegetables and berries. Despite this, report of having changed the diet during or after the Oslo Diet and Antismoking Study was associated with a higher consumption of fruit and berries, regardless of the subject's assignment to the intervention or the control group.
Study limitations
The major limitation of the study is that dietary intake of fruit and berries may merely reflect a healthy diet and favourable lifestyle habits associated with the diet, like physical activity, moderate alcohol consumption and socio-economic level. Imprecision in the measurement of these confounders results in incomplete adjustment for their effects, and in potential bias in the estimate of the effect of fruit and berry consumption. The association observed between age and fruit and berry consumption may be a survival effect of a healthier diet.
In this cross-sectional study we cannot completely adjust against potential sources of bias. However, adjustment for BMI, which may be determined by physical activity, alcohol and educational level, did not change the results (data not shown). Because of the cross-sectional design of the study we cannot ascribe causal effects to the intake of fruit and berries on IMT and our observation may be due to chance. Fruit and berry intake was associated with a greater probability of prevalent CVD, probably reflecting dietary changes after the onset of disease. Use of statins was related to fruit and berry consumption but not to IMT or the presence of CVD and did not change the observed association when added to the multivariate analysis (data not shown). The effect of educational level on IMT was not evident in contrast to earlier studies where this was shown to be a significant determinantReference Rosvall, Östergren, Hedblad, Isacsson, Janson and Berglund38. Our measure of education may have been too crude to differentiate clearly between levels.
Conclusions
The present study provides evidence that a diet that is rich in fruit and berries is associated with less atherosclerosis in the carotid artery in elderly men with a high risk of CVD.
Acknowledgements
We thank Lise Bergengen for performing the carotid ultrasound measurements, Liv Breivik for assistance with coordinating the study and Ingar Holme for statistical advice. The present study was financed in part by the Norwegian Cardiovascular Council and the Norwegian Retail Company RIMI.








