Numerous studies in animals and to a lesser extent in humans have investigated the immunomodulatory effects of probiotics (PRO) (Erickson & Hubbard, Reference Erickson and Hubbard2000; Meydani & Ha, Reference Meydani and Ha2000; Gill & Cross, Reference Gill, Cross, Calder, Field and Gill2002). In humans, the oral intake of PRO increased ex vivo cytokine (interferon-α (IFN-α)) secretion of peripheral blood mononuclear cells (PBMC) (Kishi et al. Reference Kishi, Uno, Matsubara, Okuda and Kishida1996; Arunachalam et al. Reference Arunachalam, Gill and Chandra2000). Consumption of probiotics changed the relative proportions of leucocyte subsets and enhanced phagocytic activity as well as natural killer (NK) cell cytotoxicity (Gill et al. Reference Gill, Rutherfurd, Cross and Gopal2001; Olivares et al. Reference Olivares, Diaz-Ropero, Gomez, Lara-Villoslada, Sierra, Maldonado, Martin, Rodriguez and Xaus2006). The results of these studies, however, vary greatly due to the diversity of the investigated microorganisms and usage of different experimental models (Bourlioux et al. Reference Bourlioux, Koletzko, Guarner and Braesco2003; Christensen et al. Reference Christensen, Nexmann Larson, Kæstel, Buus, Sternberg, Fleischer and Frøkiær2006). The effects of prebiotics (PRE) on the human immune system are not well studied. A comprehensive overview of the experimental findings is given in two recent reviews (Schley & Field, Reference Schley and Field2002; Watzl et al. Reference Watzl, Girrbach and Roller2005). Currently, only a few human intervention studies have looked at the immunomodulatory effects of PRE alone. In one study with elderly people, supplementation with fructooligosaccharides increased the percentage of CD4+ and CD8+T cells, whereas the phagocytic activity of granulocytes and monocytes was decreased as well as expression of interleukin (IL)-6 mRNA in monocytes. However, this study did not include a placebo control (Guigoz et al. Reference Guigoz, Rochat, Perruisseau-Carrier, Rochat and Schiffrin2002). In patients with Crohn's disease, PRE stimulated the percentage of IL-10-positive dendritic cells in the intestinal mucosa (Lindsay et al. Reference Lindsay, Whelan, Stagg, Gobin, AI-Hassi, Rayment, Kamm, Knight and Forbes2006).
A synbiotic (SYN) is defined as a combination of a PRO and a PRE. Only limited data are available about the immunomodulatory potential of SYN. The SYN combination used in the present study has been investigated in a short-term and a long-term animal study. Feeding rats for 4 weeks with the SYN increased secretory immunoglobulin A production in the ileum (Roller et al. Reference Roller, Rechkemmer and Watzl2004b ). In the long-term study, azoxymethane-treated rats were fed for 33 weeks with the SYN and the animals were evaluated for the induction of colon cancer. SYN supplementation influenced primarily the immune cells of Peyer's patches, increasing their NK cell-like activity and their capacity to produce IL-10 (Roller et al. Reference Roller, Femia, Caderni, Rechkemmer and Watzl2004a ). Significantly fewer tumours occured in the SYN- and PRE-fed rats compared with controls (Femia et al. Reference Femia, Luceri, Dolara, Giannini, Biggeri, Salvadori, Clune, Collins, Paglierani and Caderni2002).
The present study examined the effects of the PRE inulin enriched with oligofructose in combination with the PRO Lactobacillus rhamnosus GG (LGG) and Bifidobacterium lactis Bb12 (Bb12) on the immune system of polypectomised human subjects and of resected colon cancer patients. We used two PRO strains because others have suggested that a combination of PRO may have a greater effect on the intestine than the individual strains (Campieri & Gionchetti, Reference Campieri and Gionchetti1999; Ouwehand et al. Reference Ouwehand, Isolauri, Kirjavainen, Tolkko and Salminen2000). Polypectomised and previously resected colon cancer patients were recruited because the overall aim of the EU-funded project was to identify new cancer risk biomarkers and to study whether SYN may reduce colon cancer risk. Immune parameters of non-specific host resistance including immune functions which are relevant for tumour cell recognition and elimination, such as the lytic activity of NK cells, were primarily analysed. Because intestinal biopsy material providing immune cells were not available, immune cells from peripheral blood were investigated.
Subjects and methods
Subjects
Subjects were recruited from the patient lists of consultant gastrointestinal surgeons in The Mercy University Hospital who fulfilled the inclusion and exclusion criteria of the study. Inclusion criteria were (1) biopsy- and histologically confirmed spontaneous but not familial adenomatous polyps within the last 5 years or (2) undergone resection for histologically confirmed colon cancer within the last 5 years. Exclusion criteria were age over 75 years, pregnancy, known lactose intolerance, clinically significant immunodeficiency, usage of antibiotics and additional gastrointestinal disorders (e.g. Crohn's disease or ulcerative colitis). Details of the inclusion and exclusion criteria are reported elsewhere (van Loo et al. Reference van Loo, Clune, Bennett and Collins2005). A total of thirty-seven colon cancer patients were recruited, of which thirty-four patients completed the trial; thirteen were females and twenty-one were males, with an age of 60·1 (sd 5·8) and 62·1 (sd 5·3) (mean and sd), respectively. In this group, fifteen subjects received placebo and nineteen subjects received SYN treatment. A total of forty-three polypectomised patients (polyp patients) were recruited, of which forty patients completed the trial; eighteen were females and twenty-two were males, with an age of 56·0 (sd 9·8) and 58·0 (sd 9·7) (mean and sd), respectively. In this group, twenty-one subjects received placebo and nineteen subjects received SYN treatment. Prior to the recruitment, the study was evaluated and approved by the Cork University Hospitals Ethics Committee.
Study design
This study was a randomised double-blind and placebo-controlled study. Subjects were randomly assigned to the SYN or placebo group. Patients in the SYN group received daily encapsulated bacteria (1 × 1010 colony-forming units of LGG and 1 × 1010 colony-forming units of Bb12) and a 10 g sachet of inulin enriched with oligofructose. An acid-resistant coating was used to prepare the capsules containing the PRO. Patients in the placebo group received daily encapsulated maltodextrin and a 10 g sachet of maltodextrin. The intervention period lasted 12 weeks. Subjects kept a 6-week diary for each phase of the intervention. Prior to the intervention (T1) and at week 6 (T2), the subjects received a numbered box containing sufficient product for 6 weeks. At T2 and after 12 weeks (T3), subjects were interviewed by the study nurse, and reactions to the product, medications taken and any adverse events that had occurred in each 6-week period were recorded. The amount of product returned was recorded to confirm compliance. Blood and faecal samples were obtained prior to intervention, and at 6 and 12 weeks after the intervention. During the study period, subjects were asked to adhere to their usual diets. Subjects were instructed to take the SYN/placebo supplement with their main meals to minimise the inhibitory effects of gastric acid on the PRO.
For logistical reasons, the isolation, stimulation and cryopreservation of PBMC took place in Cork. Phagocytic activity and oxidative burst of blood cells was measured in Cork. Preparation of faecal water was also carried out in Cork. Supernatants of stimulated cells, cryopreserved cells and faecal water samples were stored frozen in Cork until the end of the study, and then sent all at once by overnight courier on dry ice from Cork, Ireland, to Karlsruhe, Germany.
Materials
LGG was provided by Valio (Helsinki, Finland) and Bb12 was purchased from Ch. Hansen (Horsolm, Denmark). Raftilose Synergy1® was provided by Orafti (Tienen, Belgium). Raftilose Synergy1® is a 1:1 mixture of long-chain and short-chain fractions of inulin, a β(2–1)-fructan extracted from chicory (Cichorium intybus) roots.
Isolation of PBMC
Blood was drawn into tubes containing Li-heparin (Sarstedt, Nümbrecht, Germany). PBMC were isolated by density gradient centrifugation using Histopaque 1077 (Sigma, Deisenhofen, Germany) and resuspended in complete RPMI-1640 culture medium (Life Sciences, Eggenstein-Leopoldshafen, Germany), containing 10 % (v/v) heat-inactivated fetal bovine serum (FBS), l-glutamine (2 mmol/l), penicillin (100 000 U/l) and streptomycin (100 mg/l).
Cryopreservation of lymphocytes
Isolated PBMC were centrifuged at 400 g for 8 min at 20°C. Cells were resuspended in freezing medium (70 % RPMI-1640, 20 % fetal bovine serum (FBS), 10 % dimethylsulphoxide) at a concentration of 2 × 1010 cells/l. A 300 μl aliquot of the cell suspensions was placed into cooled cryogenic freezing vials and put in a cryopreservation vessel overnight at − 80°C. After 24 h, freezing vials were transferred to liquid nitrogen in which they were stored until sending them on dry ice to Karlsruhe (Germany). In Karlsruhe, cells were stored overnight at − 80°C and then transferred to liquid nitrogen until the time of analysis.
Lytic activity of NK cells
Cryopreserved PBMC were defrosted in a 37°C water bath, suspended in 10 ml of complete RPMI-1640 culture medium, centrifuged (400 g , 10 min, 20°C) and resuspended in complete medium (Fujiwara et al. Reference Fujiwara, Akiyama, Yamakido, Seyama, Kobuke, Hakoda, Kyoizumi and Jones1986). PBMC were incubated for 18 h at 37°C. After washing the cells with complete medium, they were diluted to 2·5 × 109 cells/l and used for determination of lytic activity of NK cells against K-562 cells (effector–target ratio 12·5:1) as described (Chang et al. Reference Chang, Gusewitch, Chritton, Folz, Lebeck and Nehlsen-Cannarella1993). A flow cytometer (FACSCalibur, Becton Dickinson, Heidelberg, Germany) was used to determine the percentage of killed target cells.
Phagocytic activity and intensity
Assessment of phagocytic activity (percentage of phagocytic-active cells) and phagocytic intensity (number of phagocytised Escherichia coli per phagocytic cell expressed as mean fluorescence) was based on a described flow cytometric method (O'Gorman, Reference O'Gorman, Rose, Hamilton and Detrick2002). Briefly, to measure phagocytic activity, 10 μl of opsonised BODYPY FL-labelled E. coli (Molecular Probes, Leiden, The Netherlands) and 100 μl of fresh whole blood were mixed and incubated at 37°C for 10 min. The reaction was stopped by adding 100 μl of ice-cold quenching solution (10 % trypan blue in phosphate-buffered saline (PBS)). After washing, whole blood cells were fixed with 2 ml of 1 × lysing solution (BD, Heidelberg, Germany). DNA was stained with 300 μl of propidium iodide solution (33·3 mg/l dissolved in PBS). A flow cytometer (Epics Elite, Beckman Coulter, Fullerton, CA) was used to measure the level of phagocytic activity and intensity in neutrophils and monocytes.
Respiratory burst
Fresh whole blood (100 μl) was cooled on ice to 0°C. Tubes were incubated with 20 μl of PBS (control) or 20 μl of phorbol myristate 13-acetate (8·1 μmol/l; Sigma-Aldrich) as a high stimulus for 10 min at 37°C. A 1·7 μl aliquot of dihydrorhodamine 123 (10 g/l in dimethylsulphoxide; Molecular Probes, Leiden, The Netherlands) was added and samples were incubated for 15 min at 37°C. Erythrocytes were lysed with 2 ml of 1 × lysing solution. After washing, cells were resuspended in 300 μl of propidium iodide solution (33·3 mg/l dissolved in PBS) and incubated for 10 min on ice. A flow cytometer (Epics Elite, Beckman Coulter) was used to measure the percentage of neutrophils that produced reactive oxygen species and also the intensity of the production.
Quantification of cytokine secretion
PBMC isolated from fresh whole blood were suspended at a concentration of 1 × 109 cells/l in complete medium and were stimulated with mitogens for 24 h at 37°C. PBMC were stimulated by 5 mg/l concanavalin A (Sigma-Aldrich) (IL-2/IFN-γ), by 1 μg/l lipopolysaccharide (E. coli 0111:B4, BD) (tumour necrosis factor-α (TNF-α/IL-12) or by 1 mg/l phytohaemagglutinin (lectin of Phaseolus vulgaris, Sigma Aldrich) (IL-10). Cell-free supernatants were collected and stored at − 80°C until analysis. IL-2 was measured by sandwich-enzyme-linked immunosorbent asay (ELISA) using an anti-human IL-2 monoclonal antibody (3 mg/l in PBS, R&D Systems, Wiesbaden, Germany) as capture antibody and a biotin-labelled anti-human IL-2 monoclonal antibody (50 μg/l PBS–Tween and 2 % v/v bovine serum albumin, R&D Systems) as detection antibody. Recombinant human IL-2 (R&D Systems) was used as standard. Levels of IL-10, IL-12, TNF-α and IFN-γ in the supernatants were measured with CytoSets™ commercial ELISA kits (Biosource, Solingen, Germany).
Faecal water preparation
Total faeces of one passage from the patients were collected in the early morning and stored cool until preparation. Aliquots of the total faeces were diluted with ice-cold Dulbecco's modified Eagle's medium (DMEM; 1 g of faeces +1 ml of DMEM) and homogenised in a stomacher for 2 min. The homogenates were centrifuged at 60 000 g for 2 h at 4°C. The resultant supernatants were filtered through a 0·2 μm filter with a glass pre-filter (Nalgene, Fisher Scientific, Schwerte, Germany). Aliquots of 1 ml were stored at − 80°C prior to analysis.
Quantification of prostaglandin E2 (PGE2) in faecal water
Sample purification was done with a C18 minicolumn (Amprep C18 RPN1910; 500 mg/2·8 ml; Amersham, Freiburg, Germany). Faecal water samples were centrifuged for 5 min at 2500 g (20°C). A 100 μl aliquot of the supernatants was diluted 1:10 with distilled H2O and passed through a 0·2 μm filter. A 500 μl aliquot of the filtered sample was mixed with ice-cold 500 μl water–ethanol solution (1:4) and 10 μl of glacial acetic acid, and incubated for 5 min at 20°C followed by centrifugation (2500 g , 5 min, 20°C). C18 minicolumns were primed with 5·6 ml of 10 % ethanol. Supernatants of the samples were applied to the columns which were then washed with 2·8 ml of distilled H2O and with 2·8 ml of hexane. PGE2 was eluted with 3 ml of ethyl acetate. Ethyl acetate was evaporated in a vacuum centrifuge. Pellets were stored at − 80°C prior to analysis. The levels of PGE2 were measured with a commercial EIA kit (Amersham). For measurement, pellets were resuspended with 250 μl of assay buffer.
Quantification of transforming growth factor-β (TGF-β1) in faecal water
For activation of latent TGF-β1, faecal water samples were centrifuged (2500 g , 5 min, 20°C). An 80 μl aliquot of the supernatants was mixed with 80 μl of acetic acid (2·5 mol/l)/urea (10 mol/l) and incubated (20°C, 10 min). Samples were neutralized with 80 μl of NaOH (2·7 mol/l)/HEPES (1 mol/l). Levels of TGF-β1 were immediately measured with a commercial ELISA kit (R&D Systems).
Statistical analyses
Normal distribution of the data was analysed by using the Kolmogorov–Smirnov normality test. We used repeated measures analysis of variance (ANOVA) to analyse changes of the various immune parameters. Treatment (placebo or SYN) was included as main effect, and change in immune parameters over time as dependent variables. Study group (cancer v. polyp) as a main effect was not included in the ANOVA due to significant differences in various immunological markers at baseline. When the interaction was significant, post hoc analysis of the effect of treatment group was performed by one-factor ANOVA with Dunnett's test. All statistical calculations were performed with the PROC Mixed procedure in SAS Software (version 6.12) (SAS Institute, Cary, NC, USA). Values of P < 0·05 were considered significant.
Results
Three cancer subjects withdrew; each of them was in the placebo group. One subject withdrew due to a chest infection, another subject felt unwell on the product and the third subject was diagnosed with nephrotic syndrome unassociated with the product. Two polyp subjects in the SYN group withdrew; one subject moved from Cork and the second subject withdrew due to bloating associated with the product. One polyp subject in the placebo group withdrew following diagnosis with temperal arthritis which was unrelated to the product.
In a pilot study, levels of LGG and Bb12 in faeces were measured before and after the SYN treatment. In this pilot study, up to 10 % of the lactic acid bacteria provided with the SYN survived passage through the gastrointestinal tract (van Loo et al. Reference van Loo, Clune, Bennett and Collins2005).
The percentages of phagocytic active neutrophils and monocytes and their phagocytic intensity were not modulated by the dietary intervention in either the cancer or polyp group (Table 1). The SYN treatment did not affect the percentage of neutrophils that produced reactive oxygen species and the intensity of the production in both study groups (Table 1). Lytic activity of NK cells was not significantly changed by the intake of the SYN in both groups (Table 1).
Table 1 Immune parameters measured prior to supplementation (T1), and at 6 weeks (T2) and 12 weeks (T3) after supplementation with placebo or synbiotic in two study groups (cancer and polyp group subjects) (Mean values and standard error of the mean)
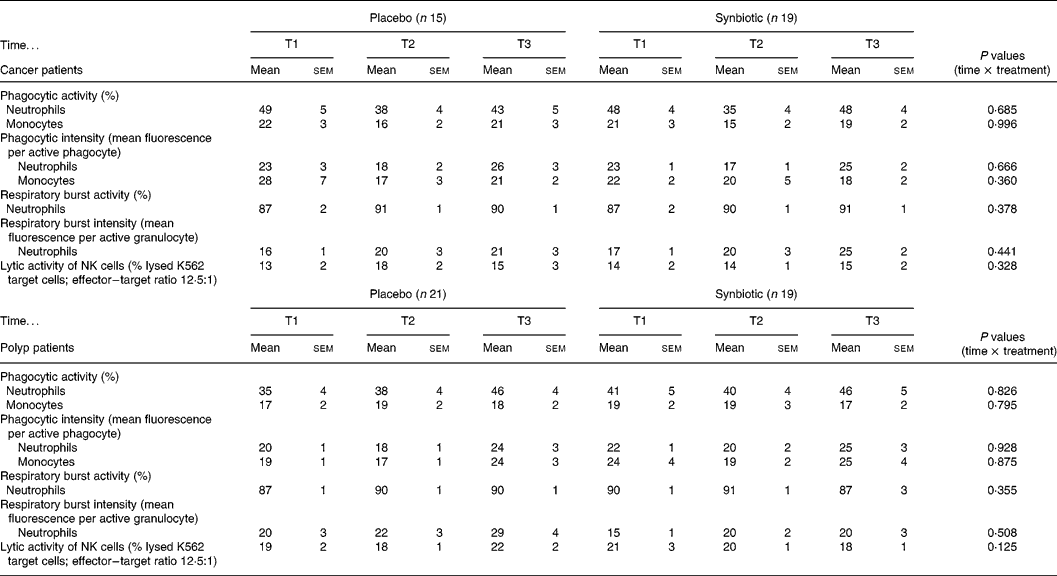
NK, natural killer.
The capacity to produce IL-2 by activated PBMC from the polyp group differed significantly between the placebo and SYN group at T3 (Table 2). While in the placebo group the IL-2 secretion increased at T3, this effect did not occur in the SYN-supplemented polyp group. In contrast, in the cancer group, subjects' IL-2 secretion was not affected by SYN treatment. There was no significant difference in production of the cytokines IL-10, IL-12 and TNF-α due to the intervention in either the cancer or polyp group (Table 2). However, in the cancer group, the treatment with SYN significantly increased the IFN-γ-producing capacity of PBMC at T3 compared with T2 (Table 2). This effect was not observed with subjects from the polyp group.
Table 2 Cytokine secretion of activated PBMC isolated from cancer or polyp group subjects prior to supplementation (T1), and at 6 weeks (T2) and 12 weeks (T3) after supplementation with placebo or synbiotic (Mean values and standard error of the mean)
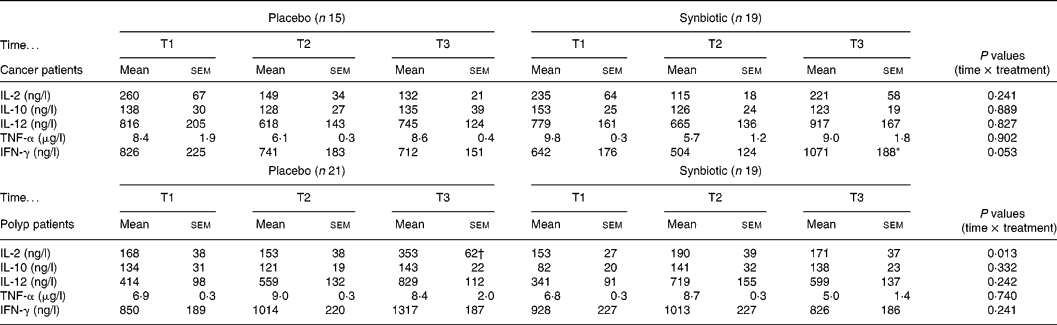
IL, interleukin; TNF, tumour necrosis factor; IFN, interferon.
* Significantly different between T2 and T3 in synbiotic-treated cancer group subjects (P < 0·05).
† Significantly different from T1 and T2 in the placebo group of polyp patients (P < 0·05).
The intake of the SYN did not affect the concentration of PGE2 and TGF-β1 in faecal water in either the cancer or the polyp group (Table 3).
Table 3 Concentration of TGF-β1 (μg/l) and PGE2 (μg/l) in faecal water of cancer or polyp group subjects prior to supplementation (T1), and at 6 weeks (T2) and 12 weeks (T3) after supplementation with placebo or synbiotic (Mean values and standard error of the mean)
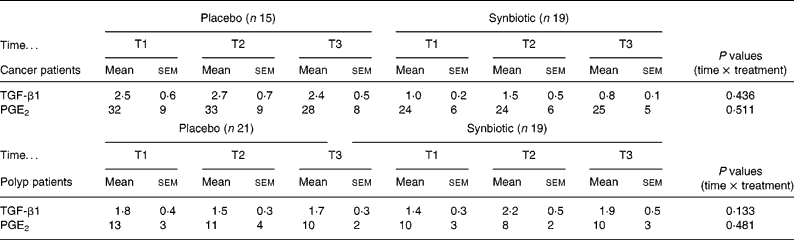
TGF, transforming growth factor; PGE2, prostaglandin E2.
Discussion
Our study was done in the context of a project funded by the Commission of the European Communities with the aim to identifiy new cancer risk biomarkers and to determine whether SYN can reduce the risk for colon cancer. The role of the present study was to investigate the impact of SYN on the immune system, which potentially mediates a protective effect of the SYN supplement on colon carcinogenesis. The immune system plays an important role in the control of tumour promotion and progression. The generation of an effective anti-tumour immune response depends on close interaction of several elements of the immune system. These include antigen-presenting cells, and different subsets of T cells, B cells and NK cells (Gabrilovich & Pisarev, Reference Gabrilovich and Pisarev2003). Considerable evidence is available from animal and in vitro studies that PRO (Rafter, Reference Rafter2003), and to a lesser extent PRE (Delzenne et al. Reference Delzenne, Cherbut and Neyrinck2003), have the potential to reduce colon cancer risk. Data from animal studies would suggest that using a combination of PRO and PRE may be the most effective strategy to maximise any anti-carcinogenic effects in the intestinal tract (Burns & Rowland, Reference Burns and Rowland2000; Femia et al. Reference Femia, Luceri, Dolara, Giannini, Biggeri, Salvadori, Clune, Collins, Paglierani and Caderni2002). Data from human studies (epidemiology and intervention), however, are rare. Until now, only one human study investigated the effect of a SYN in patients with active ulcerative colitis. The outcome of this study suggests that this SYN (containing a similar PRE to that in the present study) reduced intestinal inflammation (Furrie et al. Reference Furrie, MacFarlane, Kennedy, Cummings, Walsh, O'Neil and MacFarlane2005). Therefore, we investigated immunomodulatory effects of a daily SYN intake in resected colon cancer patients who had no intervention other than surgery, and subjects considered at increased risk for colon cancer (polypectomised patients).
In the present study, the intake of the SYN did not affect phagocytic activity, respiratory burst and the lytic activity of NK cells in either study group. Two other studies have looked at the effect of the combined application of PRO with the PRE galactooligosaccharide (Chiang et al. Reference Chiang, Sheih, Wang, Liao and Gill2000; Sheih et al. Reference Sheih, Chiang, Wang, Liao and Gill2001). In contrast to our findings, galactooligosaccharide in combination with B. lactis enhanced NK cell activity compared with the PRO alone, while galactooligosaccharide in combination with L. rhamnosus HN001 was not significantly different from the PRO alone. Intake of the PRO alone also increased phagocytic activity in both studies. Neither study included a PRE group alone or was controlled for time effects, so conclusions regarding the immunomodulatory potential of the synbiotic approach cannot be drawn. Consumption of fermented milk containing L. casei Shirota for 3 weeks resulted likewise in an enhancement of NK cell activity in subjects with low NK cell activity at baseline (Nagao et al. Reference Nagao, Nakayama, Muto and Okumura2000). In another human study with subjects with normal NK cell activity at baseline, intake of L. casei Shirota-fermented milk for 4 weeks did not influence NK cell activity, phagocytic activity or respiratory burst (Spanhaak et al. Reference Spanhaak, Havenaar and Schaafsma1998) which is in line with our results. Presumably, differences in the study design, study subjects and immune status at baseline may in part explain the different findings.
A number of lymphocyte and monocyte-specific cytokines in supernatants of ex vivo-activated PBMC were measured. In the placebo polyp group, a rise of IL-2 secretion by activated PBMC occurred at T3, whereas in the SYN polyp group IL-2 secretion did not differ between time points. Whether the SYN supplement prevented the increase in IL-2 secretion at T3 and which factors may have caused the increase in the placebo group is currently not known. Subjects were enrolled for this study over a time period of approximately 1 year. Therefore, seasonal effects can be excluded as a cause for the increase in IL-2.
While the capacity of activated PBMC to produce IFN-γ in the polyp group was not significantly affected by the SYN, in the cancer group supplementation with the SYN increased secretion of IFN-γ at T3. An increase in IFN-γ production in SYN-supplemented cancer patients could be interpreted as a positive effect due to the role of this cytokine in the immunosurveillance of cancer (Smyth et al. Reference Smyth, Godfrey and Trapani2001). A recent human intervention study measured the effect of a SYN (Bifidobacterium longum, Synergy 1) on cytokine mRNA expression in rectal biopsies from patients with ulcerative colitis. TNF-α and IL-1α mRNA expression were significantly reduced after 1 month with SYN, suggesting an anti-inflammatory effect of this SYN (Furrie et al. Reference Furrie, MacFarlane, Kennedy, Cummings, Walsh, O'Neil and MacFarlane2005). No other human SYN study measured the effect on cytokine secretion, but several studies with PRO examined IFN-γ production of PBMC. In accordance with our results, uptake of yoghurt containing live cultures during a 4-month period increased IFN-γ secretion of PBMC (Halpern et al. Reference Halpern, Vruwink, Vandewater, Keen and Gershwin1991). No information about the bacterial strains used and their dosage is available. The intake of fermented milk with L. casei Shirota for 3 or 4 weeks had no influence on IFN-γ secretion of activated PBMC and IFN-γ concentration in serum (Spanhaak et al. Reference Spanhaak, Havenaar and Schaafsma1998; Nagao et al. Reference Nagao, Nakayama, Muto and Okumura2000). After ingestion of a PRE mixture (inulin with oligofructose) in combination with a nutritional supplement for 8 weeks, the IFN-γ-secretion of activated PBMC was not influenced (Bunout et al. Reference Bunout, Hirsch, Pia de la Maza, Munoz, Haschke, Steenhout, Klassen, Barrera, Gattas and Petermann2002) which is in line with our results obtained after 6 weeks. In our study, IFN-γ secretion was only affected after an intake of 12 weeks, suggesting that only a long-term intake results in immunomodulation. Again a seasonal effect for the enhanced IFN-γ secretion can be excluded. Why the increase in IFN-γ secretion was not also seen in polyp group subjects is currently not known. However, immunological data mostly differed between the two groups, indicating that the immunological status among these groups was already different at baseline. Taken together, supplementation with the SYN did not have a strong effect on cytokine secretion by activated PBMC. Moreover, secretion of IL-10, IL-12 and TNF-α was not influenced by the SYN in either group.
Due to limitations in the availability of biopsies within the project, we were not able to isolate immune cells, such as intraepithelial lymphocytes from the intestinal epithelium or Peyer's patch cells, for measuring immune functions in the gut. As a surrogate, in faecal water we quantified concentrations of PGE2 and TGF-β1, which are produced by intestinal epithelial and immune cells, modulating immune cell functions within the gut, and which reflect intestinal production of these molecules. Levels of PGE2 have been found to be elevated in human colon cancer samples (Rigas et al. Reference Rigas, Goldman and Levine1993; Pugh & Thomas, Reference Pugh and Thomas1994). PGE2 is known to promote tumour cell survival and has diverse effects on immune cells (Harris et al. Reference Harris, Padilla, Koumas, Ray and Phipps2002), in particular NK cell activity is suppressed by PGE2 (Rhind et al. Reference Rhind, Gannon, Suzui, Shephard and Shek1999; Yakar et al. Reference Yakar, Melamed, Shakhar, Shakhar, Rosenne, Abudarham, Page and Ben-Eliyahu2003). TGF-β possesses immunomodulatory activity, acting predominantly in an immunosuppressive way which inhibits cell proliferation of many cell types (Derynck & Choy, Reference Derynck, Choy and Thomson1998). In later stages of cancer development, TGF-β1 contributes to cell growth, invasion and metastasis (Pasche, Reference Pasche2001). Supplementation with the SYN did not affect concentrations of PGE2 or TGF-β1 in faecal water in either study group.
The mechanisms by which PRO and PRE modulate the immune system are not well understood (Cross, Reference Cross2002; Perdigon & Raya, Reference Perdigon and Raya2002; Watzl et al. Reference Watzl, Girrbach and Roller2005). It is known that the gut microflora interacts with mucosal cells including epithelial cells and underlying immune cells (Marteau et al. Reference Marteau, Seksik and Jian2002). In vitro assays indicate that cell wall components of lactic acid bacteria activate immune cells such as lymphocytes and macrophages (Meydani & Ha, Reference Meydani and Ha2000). Moreover, bacterial DNA activates cells of both the innate and acquired immune systems (Hacker et al. Reference Hacker, Redecke and Hacker2002). PRE selectively stimulate the growth of beneficial bacteria in the colon (Gibson & Roberfroid, Reference Gibson and Roberfroid1995). As a result of this, PRE might modulate the immune system directly by affecting the composition of the intestinal flora. In addition, the fermentation of PRE produces short chain fatty acids in the colon (Flamm et al. Reference Flamm, Glinsmann, Kritchevsky, Prosky and Roberfroid2001) which may affect the gut-associated lymphoid tissue (Kvale & Brandtzaeg, Reference Kvale and Brandtzaeg1995; Chapman, Reference Chapman2001; Sanderson, Reference Sanderson2004).
In conclusion, no negative effects were observed in cancer and polyp patients with daily consumption of this SYN for 12 weeks, but more definitive testing is required to indicate conclusively that this product is safe. The SYN supplement had only minor effects on the immune system of the subjects in both groups. It is possible that SYN supplementation in humans preferentially affects the gut-associated lymphoid tissue rather than the systemic immune system. Therefore, future studies with humans and SYN should aim to focus on the effects at the level of the gut-associated lymphoid tissue. Further, we have only tested two strains of lactic acid bacteria; however, other strains could result in a more significant modulation of the immune system.
Acknowledgements
We gratefully acknowledge the excellent technical assistance of Susanne Merkel and Gisela Schultheiss. The authors thank Dr Herbert Thijs, Center for Statistics, Hasselt University, Diepenbeek, Belgium, and Lothar Korn, Federal Research Centre for Nutrition and Food, Karlsruhe, for their statistical consultation. The study was supported by the Commission of the European Communities, project No. QLRT-1999-00 346.





