Dyslipidaemia is an important risk factor for CVD, the leading cause of deaths in the world(1). Physiologically, genetic and lifestyle factors, especially diet, are the main determinants of plasma lipid levels, since they influence intestinal absorption, hepatic synthesis, biliary excretion and cellular use(Reference Lecerf and Lorgeril2).
Diets that are high in energy, saturated and trans-fats, and simple carbohydrates and low in unsaturated fatty acids and fibre are known to be harmful to lipid metabolism and cardiovascular health(Reference Mach, Baigent and Catapano3). All these characteristics of nutritional composition describe a dietary pattern that is already common in the world, characterised by high consumption of ultra-processed foods (UPF)(Reference Popkin4).
Sodas, sweetened artificial beverages, instant noodles, packaged salty snacks, cookies, breakfast cereals and ready-to-heat frozen foods are some examples of UPF defined by the NOVA, classification of foods based on the extent and purpose of industrial processing(Reference Monteiro, Cannon and Levy5). They result from various processes in the food industry, and their high palatability and durability as well as aggressive marketing have favoured their increasing consumption in the world(Reference Vandevijvere, Jaacks and Monteiro6).
Studies have identified UPF as risk factors for various adverse health outcomes including obesity(Reference Canhada, Luft and Giatti7), hypertension(Reference da Silva Scaranni, de Oliveira Cardoso and Chor8), cancer(Reference Fiolet, Srour and Sellem9), diabetes(Reference Srour, Fezeu and Kesse-Guyot10), CVD(Reference Srour, Fezeu and Kesse-Guyot11) and mortality(Reference Schnabel, Kesse-Guyot and Allès12). The unfavourable nutritional composition of these products is not the only factor accounting for their negative health consequences(Reference Monteiro13). Evidence now points to the role of additives (e.g. emulsifiers, preservatives and flavour enhancers), plasticisers (e.g. bisphenol A and phthalates) and neo-formed compounds (e.g. acrolein and acrylamide) in cardiometabolic health, although the magnitude of their contribution is not known for certain. Importantly, some of the evidence is still limited to in vitro or animal studies(Reference Janardhanan14–Reference Laster, Bonnes and Rocha16).
Longitudinal studies evaluating the relationship between UPF and dyslipidaemia were carried out only in children(Reference Rauber, Campagnolo and Hoffman17) or did not aim to assess the incidence(Reference Hall, Ayuketah and Brychta18). There are also some cross-sectional studies evaluating the relationship between the dietary share of UPF and metabolic syndrome and cardiovascular risk, which includes some dyslipidaemia parameters(Reference Tavares, Fonseca and Garcia Rosa19–Reference Smaira, Mazzolani and Peçanha23).
The objective of this study was thus to verify the association between consumption of UPF and incidence of dyslipidaemia in Brazilian civil servants at a 4-year follow-up.
Materials and methods
Study population and design
The current study is part of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil), a multicentre prospective cohort consisting of public employees, active or retired, from public institutions, located in six states of Brazil: the Federal Universities of Bahia (UFBA), Espírito Santo (UFES), Minas Gerais (UFMG) and Rio Grande do Sul (UFRGS); University of São Paulo (USP) and Oswaldo Cruz Foundation in Rio de Janeiro (FIOCRUZ-RJ)(Reference Schmidt, Duncan and Mill24). The objective of ELSA-Brasil is to contribute relevant information on the development and progression of chronic non-communicable diseases, particularly CVD and diabetes(Reference Aquino, Barreto and Bensenor25).
The baseline of the ELSA-Brasil study, from 2008 to 2010, enrolled 15 105 Brazilian civil servants aged 35–74 years who answered questionnaires on socio-economic conditions, lifestyle, work and health, besides undergoing laboratory and clinical tests. The exclusion criteria adopted by ELSA-Brasil were current or recent pregnancy, severe communicative or cognitive difficulty, and if retired, residence outside the metropolitan area corresponding to the study institution(Reference Schmidt, Duncan and Mill24).
Participants returned to the study sites for the first follow-up visit from 2012 to 2014, with a 94 % response rate, totalling 14 014 participants. In these two data collections, the teams were trained to guarantee the same data collection standard in all the six study sites.
In the present study, we excluded 1091 participants who did not participate in the first follow-up visit due to death (223) or failure to appear (868), diagnosis of dyslipidaemia at baseline (7595)(Reference Schmidt, Duncan and Mill24,Reference Lotufo, Santos and Figueiredo26) , report of bariatric surgery (78), implausible energy intake (89), defined as <2510 or > 25 104 kJ (<600 kcal or > 6000 kcal), or report of cholesterol-lowering diet in the last 6 months (977). The study population thus consisted of 5275 participants.
The identification of dyslipidaemia at baseline and at the first follow-up visit used the parameters from the Updated Brazilian Guidelines on Dyslipidemias and Prevention of Atherosclerosis(Reference Faludi, Izar and Saraiva27): isolated hypercholesterolaemia (LDL ≥ 4.12 mmol/L), isolated hypertriacylglycerolaemia (TAG ≥ 1.69 mmol/L), mixed hyperlipidaemia (LDL ≥ 4.12 mmol/L associated with TAG ≥ 1.69 mmol/L) and low-HDL (< 1.03 mmol/L for men and <1.29 mmol/L for women with or without association with increased LDL and TAG). In addition, the definition of dyslipidaemia considered the use of lipid-lowering drugs (statins, fibrates, nicotinic acid and ezetimibe), identified by reports, prescription and drug packages. Therefore, dyslipidaemia was defined by the presence of established biochemical parameters and/or the use of lipid-lowering drugs.
Principal variables
Consumption of ultra-processed foods
Food consumption was assessed at baseline using the semi-quantitative FFQ with 114 items based on consumption in the previous 12 months. The questionnaire’s description and validation in ELSA-Brasil have been reported in another article(Reference Molina, Benseñor and Cardoso28).
For each item, we obtained the frequency of consumption and the number of portions consumed. The amount (g/d) of each food item was calculated by multiplying the number of portions by the portion weight and the consumption frequency weight (3 for > 3 times/d, 2 for 2–3 times/d, 1 for 1 time/d, 0·8 for 5–6 times/week, 0·4 for 2–4 times/week, 0·1 for 1 time/week, 0·07 for 1–3 times/month and 0 for never/almost never).
The nutritional composition of food items was determined using the Nutrition Data System for Research (NDSR; http://www.ncc.umn.edu/products/), software of the University of Minnesota and the Brazilian Food Composition Table (TACO) of the University of Campinas (UNICAMP)(Reference Molina, Benseñor and Cardoso28). For each of the food items, we imputed the respective 99th percentile consumption for participants with consumption above this separatrix. Finally, we calculated the energy content of each food item by multiplying the daily food intake (g) by the energy in 100 g as estimated by the software (= intake grams × energy content per 100 g/100).
The UPF were identified according to the NOVA classification(Reference Monteiro, Cannon and Levy5) and have been described in other articles(Reference Simões, Barreto and Molina29). For each participant, we calculated the proportion (%) of UPF in the total weight of foods and beverages consumed (g/d). We used the proportion of weight rather than the proportion of energy to include the UPF that do not provide energy, such as artificially sweetened beverages and non-nutritional factors related to the foods’ processing, like neo-formed contaminants, additives and alterations in the structure of raw foods. This same criterion was used by Julia et al. (Reference Julia, Martinez and Allès30), Srour et al. (Reference Srour, Fezeu and Kesse-Guyot10,Reference Srour, Fezeu and Kesse-Guyot11) and Beslay et al. (Reference Beslay, Srour and Méjean31). The proportion of UPF in relation to the diet’s total weight was categorised in tertiles as the following levels of consumption: low (first tertile: 0·40–12·4 %), medium (second tertile: 12·4–20·4 %) and high (third tertile: 20·4–72·4 %) consumption.
Dyslipidaemias
LDL, TAG and HDL levels (mg/dl) were measured at baseline (2008–2010) and at the first follow-up visit (2010–2012) using the same standardised collection procedures to guarantee uniformity in all the ELSA-Brasil study sites. Participants had been fasting for 12 h. The colorimetric enzymatic method was used to obtain TAG levels. HDL and LDL levels were determined with the homogeneous colorimetric method without precipitation and Friedewald equation, respectively. All blood lipid measurements were performed with the ADVIA 1200 Siemens® equipment(Reference Fedeli, Vidigal and Leite32).
Incident cases of dyslipidaemias during the follow-up period were defined the same way as prevalent cases, that is, according to the criteria from the Updated Brazilian Guidelines on Dyslipidemias and Prevention of Atherosclerosis(Reference Faludi, Izar and Saraiva27), namely: isolated hypercholesterolaemia (LDL ≥ 4.12 mmol/L), isolated hypertriacylglycerolaemia (TAG ≥ 1.69 mmol/L), mixed hyperlipidaemia (LDL ≥ 4.12 mmol/L associated with TAG ≥ 1.69 mmol/L) and low-HDL (HDL < 1.03 mmol/L for men and <1.29 mmol/L for women with or without association with increased LDL and TAG). An additional criterion for incidence of dyslipidaemias was the use of lipid-lowering drugs as described above.
Covariables
The covariables used here were age categorically (35–44, 45–54, 55–64 and 65 years or older) in the descriptive analysis and in years in the multivariate analysis, schooling (up to complete elementary, complete secondary and university/graduate studies), smoking (never smoked/former smoker and current smoker) and consumption of alcoholic beverages (none, moderate or excessive, the latter defined as > 210 g of alcohol/week for men and 140 g of alcohol/week for women). We assessed physical activity (leisure-time domain) through the International Physical Activity Questionnaire (IPAQ)(Reference Craig, Marshall and SjöStröm33) and classified as low, moderate and high.
We defined diabetes diagnosed (no/yes) by using antidiabetic medication in the previous 2 weeks or by identifying laboratory values reaching the threshold for high fasting blood glucose (blood glucose ≥ 126 mg/dL), oral glucose tolerance test ( ≥ 200 mg/dl) or glycated Hb (HbA1C ≥ 6·5)(Reference Schmidt, Duncan and Mill24). We used this variable because diabetes is the most common co-morbidity involved in lipid metabolism in the Brazilian population. Meanwhile, diabetic individuals may present a different dietary pattern due to the food restrictions imposed by the disease.
We estimated the principal nutritional variables related to dyslipidaemias, also obtained through the FFQ and quantified by the NDSR, to describe the nutritional composition of UPF. They are total energy intake (kJ), carbohydrates (g), added sugar (g), saccharose (g), soluble fibre (g), total fibre (g), total fat (g), saturated fat (g), unsaturated fat (g), trans-fat (g) and n-3 fatty acids (g). We used the Brazilian Healthy Eating Index – Revised (BHEI-R) in its adapted form for our population (weighted for frequency of consumption of fruits and vegetables and modified considering legumes separated from other vegetables) whose calculation was described by Pires et al. (Reference Pires, Luft and Araújo34). The maximum value for the BHEI-R adapted is 100 points and high scores indicate greater adherence to consumption recommendations, that is, better quality of diet.
We assessed the nutritional status through the BMI, calculated as measured weight (kg) divided by measured height-squared (m2) and used categorically in the descriptive analysis – low/normal weight (BMI < 24·9 kg/m2), overweight (BMI from 25·0 to 29·9 kg/m²) and obesity (BMI ≥ 30·0 kg/m²) – and continuously in the multivariate analysis (kg/m²).
Statistical analysis
We described participant characteristics using absolute values and relative frequencies for categorical variables and as means and standard deviations) or medians (interquartile range) for continuous variables. Differences between tertiles of UPF consumption were assessed with the χ 2, ANOVA and Kruskal–Wallis tests, respectively.
We estimated the association between UPF consumption and dyslipidaemia using mixed-effects logistic regression models. The method was adequate for repeated measures over time, since it considers the correlation between measurements in the same individual and between individuals within clusters due to the incorporation of a correlation structure and the inclusion of a random effect(Reference Fitzmaurice, Laird and Ware35). We used uniform correlation matrix and included a random effect in the intercept of each individual. We also included a random effect for the study site, assuming that participants in the same study site are correlated with each other in a cluster, especially in relation to regional eating habits.
The added adjustment variables were age (models 1), sex and schooling (models 2), physical activity, total daily energy intake, diabetes and time since baseline (models 3). In the models 3 of the low-HDL analysis, we also included adjustment for smoking and alcoholic beverage consumption, and in the isolated hypertriacylglycerolaemia and mixed hyperlipidaemia analysis, we included adjustment for alcoholic beverage consumption. Models 4 (final models) were additionally adjusted for Brazilian Healthy Eating Index – Revised adapted and specific nutritional variables involved in plasma lipoprotein metabolism that were consumption of saturated fat, unsaturated fat, trans-fats, and soluble fibre for isolated hypercholesterolaemia, consumption of carbohydrates and n-3 fatty acids for isolated hypertriacylglycerolaemia, consumption of saturated fat, trans-fats, and soluble fibre, consumption of carbohydrates and unsaturated fat acids for mixed hyperlipidaemia, and consumption of unsaturated fat and trans-fats for low-HDL.
All the adjustment variables were considered confounders, defined a priori based on evidence from the literature, especially national(Reference Faludi, Izar and Saraiva27) and international guidelines(Reference Mach, Baigent and Catapano3) on management of dyslipidaemias and on bivariate analysis.
We also conducted a sensitivity analysis to assess the robustness of the results. Thus, we evaluated the models 4: (a) further adjusted by BMI to test the nutritional status hypothesis acting as a mediator(Reference Srour, Fezeu and Kesse-Guyot11,Reference Lopes, Araújo and Levy36) , (b) excluding 806 participants with prevalent obesity (n 4469) to further explore the role of BMI in the association and (c) using the proportion (%) of energy from UPF on total energy intake for better comparability with other studies.
All the covariables were treated as time-dependent, that is, the data both at baseline (2008–2010) and at the first follow-up visit (2012–2014) were included in the analysis, except for sex and the nutritional variables, since we only evaluated food consumption at baseline. We also conducted an interaction analysis between UPF consumption and age and physical activity regarding dyslipidaemia risk. All the tests were two-tailed, assuming 5 % significance, and the results are presented as OR and 95 % CI. All the analyses were performed in the R software, version 4.0.3.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics and Research Committees of all institutions participating in the study and also with the National Research Ethics Committee, approval reported in letter No. 976, dated 4 August 2006. Its approval in the ethics committee of the National School of Public Health/Oswaldo Cruz Foundation is registered in protocol 343/06, approved on 18 September 2006. Written informed consent was obtained from all subjects.
Results
In nearly 4 years of follow-up (mean of 3·856 years sd = 0·421, median = 3·847, range = 2·6–5·9 years), 629 individuals developed isolated hypercholesterolaemia, 857 isolated hypertriacylglycerolaemia, 458 mixed hyperlipidaemia and 842 low-HDL. Mean and standard deviation of the age was 50·6 (8·8) years, and 57·8 % of the study population were women.
Table 1 shows the participants’ principal baseline characteristics according to tertiles of proportion of UPF in the diet. Compared with participants with low consumption, those with high consumption showed significantly lower TAG and HDL mean levels and higher proportions of younger individuals, women, with more schooling, worse diet quality, with obesity, and never smokers, and fewer with diabetes and excessive drinkers. Differences in physical activity in the three groups were less pronounced. In general, the proportion of moderate physical activity was lower in the group with higher consumption of UPF than in low and medium consumers.
Table 1. Population characteristics by UPF consumption. ELSA-Brasil, Baseline (2008–2010)
(Number and percentages; mean values and standard deviations; median values and interquartile range, n 5275)
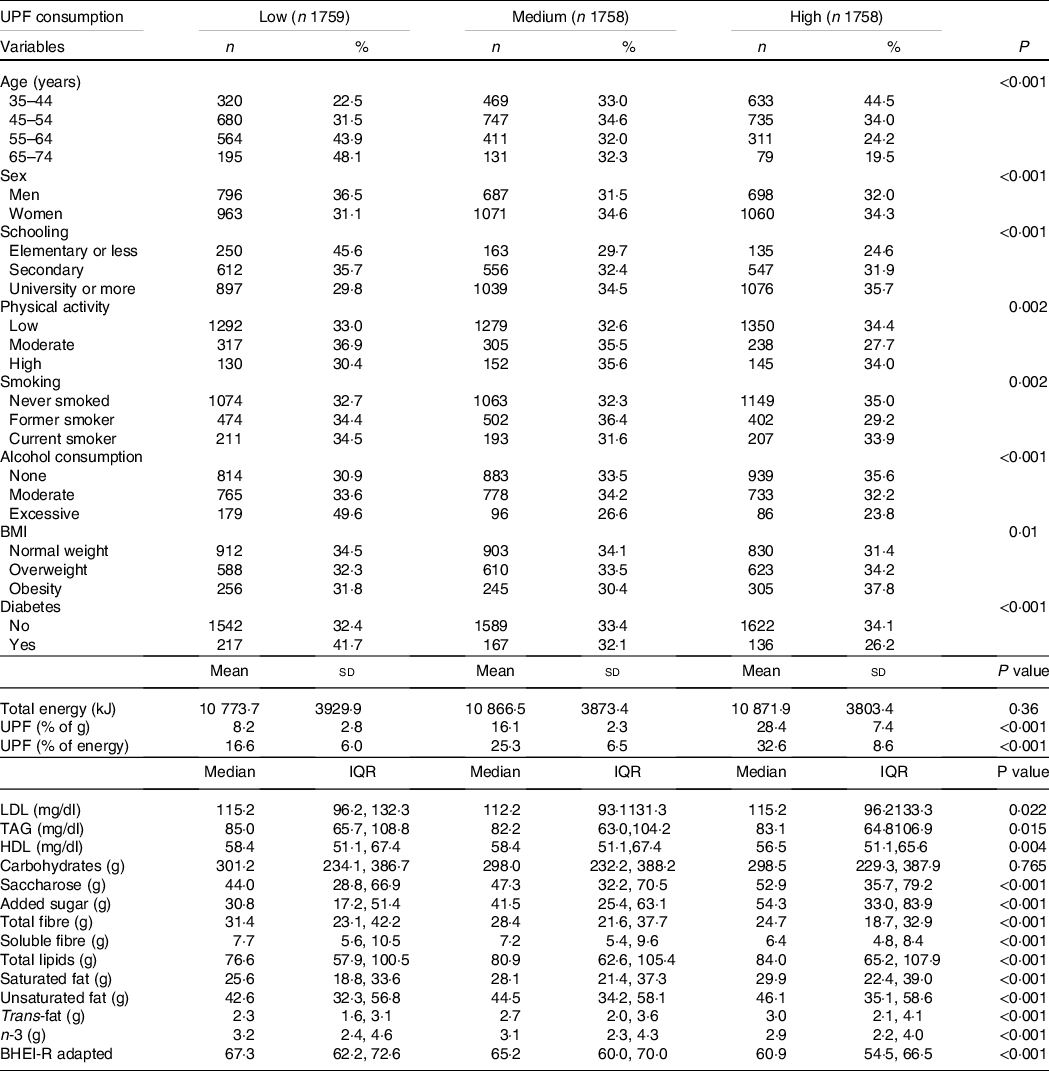
UPF, ultra-processed foods; ELSA-Brasil, Brazilian Longitudinal Study of Adult Health; BHEI-R, Brazilian Healthy Eating Index – Revised.
In relation to nutritional variables, participants with higher consumption of UPF showed significantly higher consumption of saccharose, added sugar, total fats, saturated fat, unsaturated fat and trans-fats, besides lower consumption of fibre and n-3 fatty acids. No difference was observed in total daily energy intake or carbohydrate content between tertiles of UPF consumption. The mean contribution of UPF was 17·6 % of total weight consumption (24·9 % of total energy).
Models 4 were considered as final models. Medium and high consumption of UPF were associated with incidence of dyslipidaemias, although the former was borderline for isolated hypercholesterolaemia as outcomes. Thus, individuals with medium and high consumption of UPF presented increases in the risks of development of isolated hypercholesterolaemia, by 12 % (OR = 1·12, CI 1·00, 1·27) and 28 % (OR = 1·28, CI 1·12, 1·47), of isolated hypertriacylglycerolaemia by 14 % (OR = 1·14, CI 1·03, 1·26) and 30 % (OR = 1·30, CI 1·17, 1·45), of mixed hyperlipidaemia by 21 % (OR = 1·21, CI 1·05, 1·39) and 38 % (OR = 1·38, CI 1·18, 1·62) and of low-HDL by 12 % (OR = 1·12, CI 1·00, 1·24) and 18 % (OR = 1·18, CI 1·05, 1·32), respectively, compared with participants that consumed less UPF.
Comparing the categories of consumption, all the dyslipidaemias showed a gradient in the association, such that the risk in the high-consumption group was consistently higher relative to medium consumption (Table 2).
Table 2. Association between consumption of UPF and incidence of dyslipidaemia at a 4-year follow-up. ELSA-Brasil
(Odd ratio and 95 % confidence intervals, n 5275)
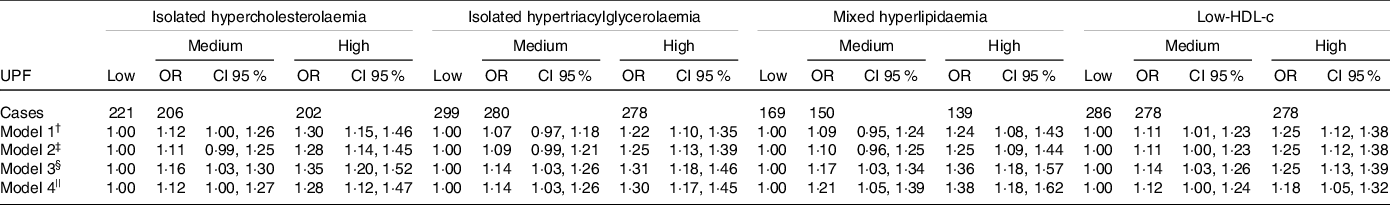
UPF, ultra-processed foods; ELSA-Brasil, Brazilian Longitudinal Study of Adult Health; BHEI-R, BHEI-R, Brazilian Healthy Eating Index – Revised.
† Model 1: adjusted for age*.
‡ Model 2: Model 1 + sex + schooling*.
§ Model 3: Model 2 + physical activity* + smoking* (only in the analysis of low-HDL) + consumption of alcoholic beverages* (only in the analysis of isolated hypertriacylglycerolaemia, mixed hyperlipidaemia and low-HDL) + total energy intake + diabetes*).
|| Model 4 (final model): Model 3 + BHEI-R adapted + specific nutritional variables (saturated fat, unsaturated fat, trans-fats, and soluble fibre for isolated hypercholesterolaemia, consumption of carbohydrates and n-3 fatty acids for isolated hypertriacylglycerolaemia, consumption of saturated fat, trans-fats, and soluble fibre, consumption of carbohydrates and unsaturated fat acids for mixed hyperlipidaemia, and consumption of unsaturated fat and trans-fats for low-HDL).
* Time-dependent variables.
In the sensitivity analysis (Table 3), the inclusion of adjustment by BMI made the measures of association smaller in relation to model 4 (final model). When we only included participants without obesity at baseline and adjusted for BMI, there were small changes in the value of estimates for all outcomes analysed and statistical significance for the medium consumption category became more borderline or was lost. There was no association between high-consumption group and low-HDL (OR = 1·00, 95 % CI 0·88, 1·14). Finally, when we used the proportion of energy from UPF, we observed an association between UPF consumption and the incidence of dyslipidaemia only for high-consumption group (in general with slightly lower estimates than the estimates found using proportion of weight of total consumption). There were no significant interactions between UPF consumption and age and physical activity in relation to the risk of dyslipidaemia (data not shown).
Table 3. Association between consumption of UPF and incidence of dyslipidaemia at a 4-year follow-up. ELSA-Brasil. Sensitivity analysis
(Odd ratio and 95 % confidence intervals)
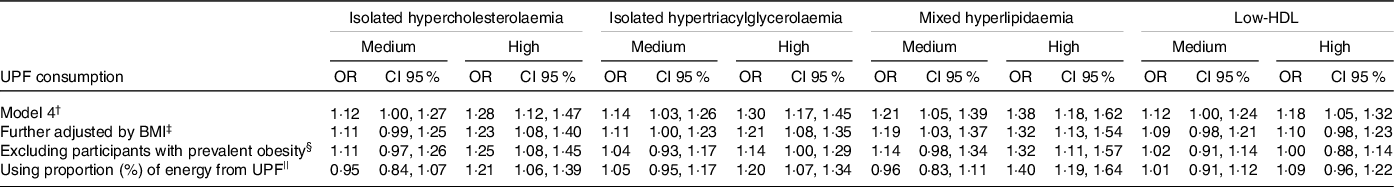
UPF, ultra-processed foods; ELSA-Brasil, Brazilian Longitudinal Study of Adult Health; BHEI-R, Brazilian Healthy Eating Index – Revised.
† Model 4 (final model, n 5275): adjusted for age* + sex + schooling* + physical activity* + smoking* (only in the analysis of low-HDL) + consumption of alcoholic beverages* (only in the analysis of isolated hypertriacylglycerolaemia, mixed hyperlipidaemia and low-HDL) + total energy intake + diabetes* + BHEI-R adapted + specific nutritional variables (saturated fat, unsaturated fat, trans-fats, and soluble fibre for isolated hypercholesterolaemia, consumption of carbohydrates and n-3 fatty acids for isolated hypertriacylglycerolaemia, consumption of saturated fat, trans-fats, and soluble fibre, consumption of carbohydrates and unsaturated fat acids for mixed hyperlipidaemia and consumption of unsaturated fat and trans-fats for low-HDL).
‡ Further adjusted by BMI: Model 4 + BMI* (n 5275).
§ Excluding participants with prevalent obesity: Model 4 + BMI* excluding participants with prevalent obesity (n 4469).
|| Using proportion (%) of energy from UPF: Model 4 using the proportion (%) of energy from UPF on total energy intake (n 5275).
* Time-dependent variables.
Discussion
Our results showed that medium and high consumption of UPF increased the risk of development of dyslipidaemias during nearly 4 years of follow-up. The findings also suggest a dose–response gradient, that is, the higher the consumption of UPF, the higher the risk of dyslipidaemias.
As far as we know, this was the first study to assess the incidence of dyslipidaemias associated with the consumption of UPF in adults. We found two studies that were longitudinal but either did not have this objective and/or did not assess the adult population. Rauber et al. (Reference Rauber, Campagnolo and Hoffman17), analysing data from 345 low-income children living in São Leopoldo, Rio Grande do Sul, Brazil, observed an increase in the levels of total cholesterol (β = 0·430; P = 0·046) and LDL (β = 0·369; P = 0·047), associated with the consumption of UPF. The second study was an American crossover type clinical trial by Hall et al. (Reference Hall, Ayuketah and Brychta18), which aimed to investigate whether the consumption of UPF affected energy intake in twenty weight-stable adults. This study, however, showed significant reductions in TAG and HDL levels compared with baseline both after the ultra-processed (experiment) and unprocessed (control) diets.
Cross-sectional evidence is also scarce. In Canada, Lavigne-Robichaud et al (Reference Lavigne-Robichaud, Moubarac and Lantagne-Lopez37) analysed UPF according to NOVA and found an association with the prevalence of low-HDL (OR = 2·05; 95 % CI 1·25, 3·38) in 811 Indigenous (Cree) adults, while no effect was observed on hypertriacylglycerolaemia. In another cross-sectional study, Smaira et al. (Reference Smaira, Mazzolani and Peçanha23) analysed fifty-six postmenopausal women with rheumatoid arthritis in São Paulo, Brazil, and did not show such an association with any plasma lipid. A recent population-based study by Ferreira et al. (Reference Ferreira, Vasconcelos and Santos22) with 655 hypertensive adults in Alagoas, Brazil, did not find a correlation between consumption of UPF and high cholesterol (P = 0·388) or TAG levels (P = 0·873).
The controversial evidence in these studies may be explained by the target populations’ particularities, which hinders extrapolations and comparisons with our findings. The reduced sample size in some studies may limit their statistical power to demonstrate effects. However, we contend that the consumption of UPF may compromise the lipid profile due to the nutritional composition and the harms from industrial processing itself. Our descriptive analysis showed higher intake of unhealthy fats and simple sugar and less fibre in the highest tertile of UPF consumption. These findings are consistent with reports in the literature(Reference da Costa Louzada, Ricardo and Steele38,Reference Rauber, da Costa Louzada and Steele39) . These food components in excess or in deficiency (in the case of fibre) are known to be harmful to lipid metabolism, especially in the presence of other unhealthy habits such as excessive alcohol consumption, smoking and physical inactivity(Reference Lecerf and Lorgeril2). Meanwhile, diets high in UPF are deficient in polyphenols, carotenoids and micronutrients that act on pathways capable of affecting oxidative stress, endothelial function and lipid homoeostasis(Reference Louzada, Martins and Canella40,Reference Korakas, Dimitriadis and Raptis41) .
In addition to nutritional composition, the plasticisers used in processing can act as endocrine disruptors and alter various physiological pathways and predispose to chronic diseases(Reference Janardhanan14). Artificial additives and neo-formed compounds involved in ultra-processing have also been associated with adverse cardiometabolic effects(Reference Zhang, Huang and Zhuang15,Reference Laster, Bonnes and Rocha16) .
As for the role of nutritional status, we found that adjustment of the models for BMI reduced the measure of association in the sensitivity analysis. This confirms that nutritional status can act as a mediator between consumption of UPF and increased dyslipidaemias and thereby increase the risk of chronic non-communicable diseases. Therefore, we chose not to use the nutritional status as a covariate in the final model (model 4). The role of BMI is to mediate, not confound and the adjustment is inappropriate for non-confounders. The inclusion of a mediator covariate in the adjustment set may produce a bias in the effect estimate(Reference Pearce and Vandenbroucke42). The hypothesis of mediation has also been raised by Lopes et al. (Reference Lopes, Araújo and Levy36) and Srour et al. (Reference Srour, Fezeu and Kesse-Guyot11).
It is well established that obesity plays an important role in the development of dyslipidaemias and chronic diseases in general. Both the high energy density of UPF and the failure to achieve satiety by consuming them favour excess weight(Reference Beslay, Srour and Méjean31,Reference Mendonça, Pimenta and Gea43) . This was also observed in studies with the ELSA-Brasil population(Reference Canhada, Luft and Giatti7,Reference Silva, Giatti and de Figueiredo44) . However, there are other still unknown mechanisms by which UPF promote weight gain(Reference Hall, Ayuketah and Brychta18). At the same time, obese individuals have unregulated production of cytokines and adipokines like TNF-α, IL-1, IL-6, leptin and resistin, which can cause endocrine disorders such as insulin resistance and lipolysis. The result is dyslipidaemia associated with obesity, characterised by elevated total cholesterol, TAG and LDL levels and lower HDL(Reference Klop, Elte and Cabezas45). In model that excluded participants with prevalent obesity of the sensitivity analysis, we observed that there is a risk of developing dyslipidaemias associated with high-consumption group even in non-obese participants at baseline, except for low-HDL, indicating that these individuals also need to avoid these products.
Consumption of UPF increased considerably in all Brazilian socio-economic strata and tended to be greater among those on lower incomes, which can be explained by the change in food production systems and the population’s increasing purchasing power(Reference Martins, Levy and Claro46). As in our results, the study by Bielemann et al. (Reference Bielemann, Motta and Minten47) showed that the consumption of UPF is higher among individuals with higher socio-economic status. Schooling, access to information and purchasing power are interconnected factors that determine food choices(Reference Bielemann, Motta and Minten47). However, nutritional knowledge and good eating habits are not always strongly correlated because knowledge about health does not translate into action when individuals are unsure how to apply it. Today, there is still unclear and conflicting information from different media sources that make food choices difficult(48). For example, claims of ‘diet’ and ‘light’ and ‘fortified’ foods can lead consumers to believe that these UPF can be healthier, encouraging their consumption, especially for higher-income strata, due to its higher cost(Reference Monteiro13).
The mean proportion of UPF in our study was 17·6 % of total weight consumption and 24·9 % of total energy. In the French NutriNet-Santé cohort(Reference Srour, Fezeu and Kesse-Guyot10), this proportion was similar, namely, 17·3 % of total weight consumption. In a recent meta-analysis conducted by Lane et al. (Reference Lane, Davis and Beattie49), twenty-eight of the forty-three observational studies included in the study showed that the mean proportion of UPF was 37 % of total energy, ranging from 17 % to 56 %, and the Brazilian mean proportion was 38 %. But caution is necessary with these comparisons, since these studies include individuals with other age groups, besides using different instruments to assess food consumption.
The literature is unanimous in showing that the consumption of UPF decreases with age(Reference Schnabel, Kesse-Guyot and Allès12,Reference Silva, Giatti and de Figueiredo44) . This is due to a generation effect, since the increasingly widespread consumption of industrial food products began in the 1980s. Since UPF are relatively recent, younger generations tend to consume more of them, and the food industry invests heavily in advertisement targeted mainly to youngsters. Meanwhile, older individuals are more prone to maintain traditional eating habits based on natural and minimally processed foods(Reference Monteiro13).
In model that used proportion (%) of energy from UPF of the sensitivity analysis, we observed an association only between high consumption of UPF and incidence of dyslipidaemia and, in general, with slightly lower estimates than the estimates found using the proportion of weight of total consumption. Therefore, the use of UPF weight as the proportion of weight of total consumption rather than energy proportion of UPF included UPF with low energy content and intrinsic processing issues such as the presence of additives and neo-formed compounds, thereby expanding the dimension of the industrial processing problem.
Our study has the strength of using a large population of both sexes with important variability in socio-economic status, age and race. In addition, the longitudinal analysis with the use of repeated measures that consider the correlation between study variables improves the estimates’ precision. Another important point was considering industrial processing with the consumption of a group of foods and not only a given item or nutrient. We conduct a sensitivity analysis to assess the robustness of the results and to allow comparability with other studies, highlighting the role of nutritional status and the variable UPF consumption as a proportion of weight of total consumption.
We also compared different magnitudes of intake and included most of the dependent variables over time rather than just the variables measured at baseline as in most of the studies.
As limitations, although the FFQ in ELSA-Brasil was validated in the Brazilian population, it was not developed to use the NOVA classification at the time it was elaborated. It is thus not possible to rule out some classification error in the dietary assessment. In addition, we did not assess food consumption in the first follow-up visit, but the FFQ can capture habitual intake in the long term (12 months), and we believe that there were no major changes in the dietary pattern that would impact our estimates in the study period.
Conclusion
In our study, consumption of UPF was associated with the incidence of dyslipidaemias, thus posing an important cardiovascular risk. This risk is proportional to the amount of consumption, so these foods should be discouraged. Since the literature with longitudinal studies is still limited, more studies are needed to corroborate these findings.
Acknowledgements
The authors thank all ELSA-Brasil participants who agreed to take part in the study.
The ELSA-Brasil study was supported by the Science and Technology Department of Brazil’s Ministry of Health and by the Ministry of Science and Technology (the Brazilian Innovation Agency-FINEP and the National Research Council-CNPq) – grant number 01 06 0010.00 RS, 01 06 0212.00 BA, 01 06 0300.00 ES, 01 06 0278.00 MG, 01 06 0115.00 SP, 01 06 0071.00 RJ). The funders had no role in the design, analysis or writing of this article.
The main author P. O. S. S. is a graduate student from Fiocruz and received a scholarship to study during her doctorate course. The co-authors are public servants from Fiocruz and besides their salary, L. O. C. has a research grant to support her research (FAPERJ – State of Rio de Janeiro Research programme), R. H. G. is research fellows of the National Council for Scientific and Technological Development – CNPq and of the Research Support Foundation of the State of Rio de Janeiro (FAPERJ), P. A. L. and M. J. M. F. have a research grant to support his research (National Council for Scientific and Technological Development – CNPq) and S. M. B. is a research fellow from National Council for Scientific and Technological Development – CNPq and is also supported by a research grant (Pesquisador Mineiro) from FAPEMIG, Brazil. The authors did not receive any additional funding to develop this paper in specific.
P. O. S. S. participated in the design, statistical analysis, data interpretation, and writing and preparation of the manuscript. M. J. M. F. and L. O. C. participated in the design, data interpretation, and writing the manuscript. R. H. G., P. A. L. and S. M. B. contributed intellectual content to the paper and helped with the final review of the paper. All of the authors contributed important intellectual content during manuscript drafting. All the authors have read and approved the final manuscript.
There are no conflicts of interest.






