Lupins are used in the diet of growing pigs in some countries including Australia, providing both amino acids (AA) and energy for maintenance and growth(Reference van Barneveld1, Reference Kim, Pluske and Mullan2). The type of variety(Reference van Barneveld and Cranwell3) and mechanical grinding(Reference Wigan, Batterham, Farrell, Hennessy and Cranwell4) of lupins are known to affect the digestible energy (DE) content in pigs; however, studies are lacking investigating the effects of these two factors on protein (AA) digestion. Mechanical grinding is likely to be an important factor for AA nutrition of pigs fed lupins, since lupins have a hard seed coat and kernel compared with other feed ingredients such as grains and oil seed meals(Reference Kim, Pluske and Mullan2). A decrease in particle size will most likely change the surface area available for interaction of nutrients in lupins with digestive enzymes and intestinal microbes, and cause an increase in digestibility.
AA are known to be accumulated as body protein only when they are digested and absorbed along the small intestine in pigs(Reference Nyachoti, de Lange and McBride5). AA escaping small intestinal digestion enter into the large intestine where they are mainly broken down into NH3 and volatile fatty acids (VFA) by micro-organisms(Reference Wrong, Edmonds and Chadwick6). NH3 is the main nitrogenous compound absorbed from the large intestine and most of this NH3 is converted to urea in the liver and excreted through the renal system, although some of the circulating NH3 diffuses back to the caecum and is used for growth of micro-organisms(Reference Younes, Demigne and Behr7, Reference Younes, Garleb and Behr8). Therefore, protein digestion and absorption in the large intestine of pigs makes only a small contribution to body protein deposition(Reference Nyachoti, de Lange and McBride5). Consequently, quantification of digestible AA measured at the end of the small intestine is a pivotal measure of the conversion of ingredient AA to body protein. In addition, protein fermentation produces NH3 and branched-chain fatty acids (BCFA)(Reference Pluske, Pethick and Hopwood9, Reference Kim, Mullan and Hampson10), while straight-chain VFAAPB are produced from carbohydrate fermentation that is known to provide energy to the pig (i.e. acetic and propionic acids) and colonocytes (i.e. butyric acids)(Reference Pluske, Pethick and Hopwood9). Additionally, extensive carbohydrate fermentation in the gastrointestinal tract (GIT) increases the relative weight of the GIT and decreases empty body weight(Reference King11, Reference van Nevel, Seynaeve and van de Voorde12) and dressing percentage(Reference King11) accordingly. However, relationships between particle size of lupins and digestion and fermentation characteristics in pigs are not established to date.
Two experiments were conducted to test the following hypotheses: (1) decreasing particle size of lupins will increase total-tract apparent protein digestibility irrespective of lupin variety; (2) decreasing particle size of lupins will increase apparent ileal digestible AA content linearly in growing pigs within the range of particle sizes seen commercially (600–1300 μm); (3) decreasing particle size of lupins will decrease the amount of N entering into the large intestine and in turn decrease indices of protein fermentation such as NH3 and BCFA.
Materials and methods
Animals and experimental design
In Expt 1, sixty-three individually housed entire male pigs (Large White × Landrace) weighing 63·5 (sd 7·28) kg were randomly allocated to a 2 × 4 factorial arrangement of treatments plus a wheat control, with the respective factors being (a) two lupin varieties at 350 g/kg inclusion level (Tanjil v. Mandelup); (b) four mean particle sizes of lupins (746, 888, 1099 and 1136 μm). In Expt 2, forty-two individually housed male pigs (Large White × Landrace) weighing 29·8 (sd 2·9) kg were allocated to a completely randomised experiment with six treatments (n 7). The diets were a wheat control diet plus five diets containing 350 g/kg lupins in replacement of wheat, with lupins having varying mean particle sizes (567, 995, 1198, 1250 and 1304 μm).
Diets, housing, feeding
For Expt 1, diets were manufactured using two varieties of Western Australian lupins (Mandelup and Tanjil, harvested in the 2005/2006 season) having varying particle sizes (746, 888, 1099 and 1136 μm, see Table 1). These varieties were chosen because they represent the major lupins harvested in Western Australia and therefore fed to pigs. Similarly, the particle sizes represent the particle sizes used by commercial feed manufacturers and producers with on-farm mixing facilities. Particle size distribution and mean particle size of ground lupins were measured using the American Society of Agricultural Engineers method(13) (Table 2). Wheat was separately ground using a 6 mm screen. A wheat control diet was formulated to contain wheat (947·3 g/kg), rapeseed oil (30 g/kg), limestone (15 g/kg), dicalcium phosphate (5 g/kg), salt (0·7 g/kg), vitamin and mineral mix (1 g/kg), and indigestible marker (1 g/kg) to determine the DE content of the dietary components other than test lupins. The eight test lupin diets were formulated by replacing 350 g wheat per kg diet with test lupins from the control diet. Titanium dioxide (TiO2, 1 g/kg) was added as an indigestible marker to calculate digestibility coefficients. To minimise the influence of feed intake on energy digestibility, all diets were offered at three times maintenance energy level based on the individual metabolic body weight (3 × (0·458 MJ × live weight0·75)/diet DE; about 90 % of ad libitum intake). Water was freely available at all times through a nipple drinker. Pigs were fed each diet for a total of 10 d, comprising a 7-d adaptation period followed by a 3-d ‘grab sample’ collection of faeces. Faecal samples were collected at 08.00, 10.00, 12.00, 14.00 and 16.00 hours on the day of collection. Faeces were then bulked per pig, and subsampled for analysis. The DE content of lupins was determined by comparing the DE content of the control diet and the lupin-based diets. Also, digestibilities of DM, gross energy and crude protein in test diets were determined to establish relationships with lupin particle sizes.
Table 1 Diet composition (g/kg) and analysed chemical composition (g/kg, air-dry basis) of the experimental diets and ingredients used in Expt 2
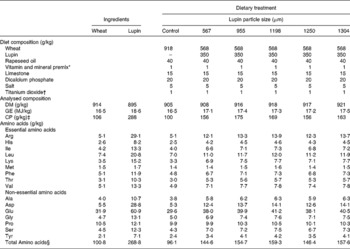
GE, gross energy; CP, crude protein.
* Provided the following nutrients per kg of air-dry diet: vitamins: A 7000 IU (2100 μg), D3 1400 IU (35 μg), E 20 mg, K 1 mg, B1 1 mg, B2 3 mg, B6 1·5 mg, B12 15 μg, calcium pantothenate 10 mg, folic acid 0·19 mg, niacin 12 mg, biotin 30 μg; minerals: Co 0·2 mg (as cobalt sulphate), Cu 10 mg (as copper sulphate), I 0·5 mg (as potassium iodine), Fe 60 mg (as ferrous sulphate), Mn 40 mg (as manganese oxide), Se 0·3 mg (as sodium selenite), Zn 100 mg (as zinc oxide), antioxidant as Endox (Kemin Industries, Inc., Des Moines, IA, USA) 20 mg (BJ Grower 1, BioJohn Pty Ltd, WA, Australia).
† Sigma-Aldrich Inc., St Louis, MO, USA.
‡ CP (N × 6·25).
§ Sum of amino acids excluding cystine and tryptophan.
Table 2 Particle size distribution (g/100 g) of ground lupins used in Expt 1 and 2*
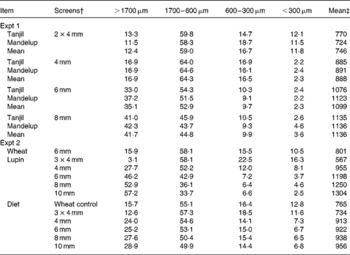
* Values are mean of duplicate determination.
† Lupins were ground through a hammermill fitted with different screen sizes, 2 × 4 mm = double grinding through a 4 mm screen, 3 × 4 mm = triple grinding through a 4 mm screen.
‡ Mean particle size (μm) was determined using a method by American Society of Agricultural Engineers(13).
For Expt 2, six experimental diets were prepared consisting of a wheat control diet plus five diets containing 350 g/kg lupins in replacement of wheat, with lupins (cv. Mandelup) having varying particle sizes (567, 995, 1198, 1250 and 1304 μm). Ingredient composition and chemical contents of the experimental diets and ingredients used in the experimental diets are presented in Table 1. Wheat was ground through a hammer mill to a mean particle size of 801 μm using a 6 mm screen and then used across all experimental diets. Lupins were ground through a hammer mill to achieve a mean particle size of 567, 955, 1198, 1250 or 1304 μm by triple grinding through a 4 mm screen, and by a single grinding through 4, 6, 8 and 10 mm screens, respectively. Particle size distribution and mean particle size of ingredients and the experimental diets are presented in Table 2. The wheat control diet was included to calculate nutrient digestibility and digestibility of dietary components of lupins by the difference method(Reference Kim, Mullan and Hampson14). No other protein sources were added and diets were offered in an unbalanced form to minimise interactive effects of other protein sources on AA digestibility. TiO2 (1 g/kg) was added as an indigestible marker to calculate nutrient digestibility. Rapeseed oil (40 g/kg) was added to prevent possible segregation between ingredients and the digestibility marker, particularly in the diets containing coarsely ground lupins.
Upon arrival at the research facilities, pigs were individually housed in a concrete-slatted pen with a space allowance of 1·62 m2. Pigs were randomly allocated to the experimental diets based on live weight and fed the respective experimental diet ad libitum for 2 weeks. In week 3, pigs were fed at three times maintenance energy to eliminate any influence of feed intake on digestibility. Daily feed allowance was halved and the pigs received an equal amount of diet at 09.00 and 16.00 hours. Fresh water was available throughout the experiment through a nipple drinker set in the pen.
Post-mortem procedure (Expt 2)
On the day before euthanasia, pigs received their daily feed allowance as one meal at 09.00 hours to ensure consumption of all experimental diets on the euthanasia day. The average body weight at the end of the experiment was 46·7 (sd 4·21) kg. On the day of sampling, pigs were fed their morning meal in a staggered fashion, such that the period of time between the commencement of feeding and euthanasia was approximately 9 h ± 20 min(Reference Donkoh, Moughan and Smith15). After 9 h from the start of feeding, pigs were administered a single intramuscular injection of 0·2 ml Zoletil (10 mg tiletamine+10 mg zolazepam, Zoletil® 100, Virbac Pty Ltd, Peakhurst, NSW, Australia) plus 0·1 ml Xylazil (10 mg xylazil, Ilium Xylazil-100, Troy Laboratories Pty Ltd, Smithfield, NSW, Australia) to induce general anaesthesia. Blood samples were collected from the anterior vena cava into heparinised vacutainers and pigs were then euthanised with a lethal dose (10 ml) of sodium pentobarbitone solution administered intravenously (Lethobarb, May and Baker Pty Ltd, Melbourne, Australia). The abdomen was immediately opened from the sternum to the pubis following cervical dislocation and the GIT was removed. Digesta samples were collected within 5 min of euthanasia from the stomach, terminal ileum (40 cm from ileo-ceacal junction), caecum, mid-colon and rectum. All the samples were immediately placed on ice and later frozen at − 20°C for subsequent analyses of AA, VFA, NH3, protein and energy. A 3–4 cm section of tissue sample from the mid-colon was removed and carefully washed with PBS and preserved in 10 % buffered formalin solution for histological examination. The pH of digesta was measured by inserting the electrode of a calibrated portable pH meter (Schindengen pH Boy-2, Schindengen Electric MFG, Tokyo, Japan) into the collected samples. Within 30 min of collection of ileal digesta samples, viscosity was determined with a Brookfield LVDV-II+cone-plate rotational viscometer (CP40, Brookfield Engineering Laboratories Inc., Stoughton, MA, USA) according to the method described by Pluske et al. (Reference Pluske, Black and Pethick16).
Chemical analyses
DM was measured using the Association of Official Analytical Chemists official method 930.15(17). The N content was determined with a LECO FP-428 Nitrogen Analyser (LECO Corp., St Joseph, MI, USA) using a combustion method (Association of Official Analytical Chemists official method 990.03)(17). The gross energy content was determined using a ballistic bomb calorimeter (SANYO Gallenkamp, Loughborough, Leics, UK). TiO2 was measured by spectrophotometry at 410 nm after acid hydrolyses (7·4 m-H2SO4)(Reference Short, Gorton and Wiseman18).
The plasma urea N content was determined using an enzymatic (urease) kinetic method (Randox, Crumlin, Co., Antrim, UK). NH3-N in fresh digesta samples was measured according to a method described by Weatherburn(Reference Weatherburn19). To determine VFA concentrations, thawed digesta samples were diluted 1:1 (w/v) with distilled water, mixed, centrifuged and the supernatant fraction was analysed chromatographically. The supernatant fraction (0·1 ml) was added to 1 ml internal standard solution containing valeric acid before processing with capillary GC. A working standard and a control (distilled water) were included in each run of the analysis, with the working standard containing acetic acid (60 mm), propionic acid (20 mm), isobutyric acid (6·67 mm), butyric acid (20 mm), isovaleric acid (10 mm), valeric acid (10 mm) and caproic acid (4 mm). The Hewlett Packard 5890A capillary GC (Agilent Technologies, Forrest Hill, Vic., Australia) was maintained at injector and flame ionisation detector settings of 260 and 265°C, respectively, and an initial and final oven temperature of 120 and 240°C, respectively. The carrier gas flow rate was 5 ml/min and the split-flow rate was 70 ml/min. The Hewlett Packard Chemstation integration system was used to calculate the VFA concentrations from the area of the peaks.
AA contents in ingredient, diets and digesta samples were measured according to a method described by Cohen(Reference Cohen, Cooper, Packer and Williams20). Briefly, a 200–300 mg sample was hydrolysed with acid (6 m-HCl) to convert protein-bound AA to free AA. The AA in the hydrolysate then underwent pre-column derivatisation with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate. The AA derivatives were then separated and quantified by reverse-phase HPLC (ACQUITY UPLC system with UV detector, Waters Corporation, Milford, MA, USA). A Waters AccQ-Tag Ultra column (BEH C18, 2·1 × 100 mm; 1·7 μm) was used for all analyses. Column temperature employed was 55°C, detection was at 260 nm and the flow rate 0·7 ml/min.
Apparent digestibility of dietary components in ileal and faecal samples was calculated as:
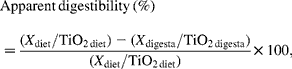
where X is dietary components such as energy, N and AA.
Standardised ileal digestibility of AA was calculated using a determined apparent ileal digestibility and pre-published(Reference Stein, Trottier and Bellaver21) basal endogenous AA flow as:

Statistical analyses
For Expt 1, the pig was considered as the experimental unit. The treatment effects were assessed by two-way ANOVA for a factorial design with the main effects being lupin variety and particle sizes. Effects were considered as fixed effects in the model. Where the effect of variety and interaction between variety and particle size on all measured variables was NS, data were pooled and analysed for effects of particle size on digestibility of diet components and DE content of lupins. Polynomial regression analysis(Reference Payne22) was conducted to examine the linear and quadratic effects of particle size on digestibility and DE content. Statistically significant difference between treatments was accepted at a level of probability < 0·05.
For Expt 2, data were subjected to ANOVA within which the linear and quadratic influence of particle size on AA digestibility was examined using polynomial regression analysis. The individual pig was considered as the experimental unit. Statistical difference between treatments was accepted at a level of probability < 0·05.
All statistical analyses were conducted using the statistical package Genstat 11 for Windows (VSN International Ltd, Hemel Hempstead, Hertfordshire, UK).
Results
Expt 1
Variety of lupins had no effect on total-tract apparent digestibility of DM, gross energy and crude protein, and hence the DE content of lupins was not significantly different in the two varieties (mean DE of 13·5 and 13·6 MJ/kg for Mandelup and Tanjil, respectively). Moreover, interactions between variety and particle size were NS (P>0·05) for all measured variables. The treatment-effect ANOVA showed that DM (P = 0·174) and energy digestibility (P = 0·106) due to decreasing particle size were not statistically significant. However, decreasing particle size of lupins significantly increased crude protein digestibility (P < 0·01). Linear regression analyses of pooled data showed that decreasing particle size of lupins from a mean particle size of 1136–746 μm linearly increased total-tract apparent digestibility of crude protein (crude protein, 70·4–76·3 %, linear effect P < 0·001) of the test diets. Also, decreasing particle size of lupins tended to linearly increase the DE content of lupins (13·2–13·9 MJ/kg, linear effect P = 0·067, Fig. 1).
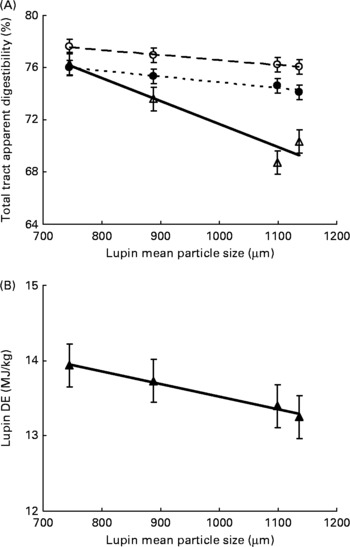
Fig. 1 Expt 1 – effect of lupin particle size on (A) total-tract apparent digestibility of DM (○), gross energy (GE; ●), crude protein (CP; △), and (B) digestible energy (DE) content of lupins (▲). The linear relationships were significant for DM (P < 0·05), GE (P < 0·05), CP (P < 0·001) and lupin DE (P < 0·1).
Expt 2 – ileal and total-tract apparent digestible energy and nitrogen content
Decreasing particle size of lupins from 1304 to 567 μm linearly increased ileal (P < 0·001) and total-tract (P < 0·01) apparent digestibility of N (Table 3). Ileal digestible N content significantly increased from 20·5 to 36·5 g/kg as the particle size of lupins decreased from 1304 to 567 μm. However, decreasing particle size had only marginal effects on ileal and total-tract energy digestibility (P < 0·10) due mainly to the large between-animal variation. Ileal and total-tract DE contents of the coarsely ground lupin (1304 μm) were 4·1 and 12·2 MJ/kg, respectively, while the corresponding figures were 8·0 and 14·1 MJ/kg, respectively, when the lupin was ground to 567 μm.
Table 3 Effect of lupin particle size on ileal and total-tract apparent digestibility of nitrogen and energy in lupin seeds determined with 45 kg pigs
(Mean values with their standard errors)
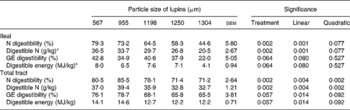
GE, gross energy.
* N and GE contents of the lupin were 46 g/kg and 18·6 MJ/kg, respectively.
Apparent and standardised ileal digestibility of amino acids
Apparent and standardised ileal digestibilities of AA in response to particle size of lupins are presented in Table 4. Decreasing particle size of lupins significantly increased apparent and standardised ileal digestibility of all AA (P < 0·05– < 0·001) excluding proline. The effect of decreasing particle size on the increased apparent and standardised ileal digestibility of AA was linear (P < 0·05– < 0·001).
Table 4 Effect of lupin particle size on apparent and standardised ileal digestibility of amino acids (AA (%)) in lupin seeds determined with 45 kg pigs
(Mean values with their standard errors)
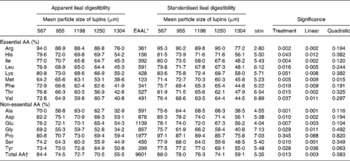
EAAL, endogenous amino acid loss.
* EAAL (mg/kg DM intake) values published by Stein et al. (Reference Stein, Trottier and Bellaver21) were used for calculation of standardised ileal digestibility of AA.
† Sum of AA excluding cystine and tryptophan.
Apparent and standardised ileal digestible amino acids content
Apparent and standardised ileal digestible (SID) AA contents of lupins in response to varying particle size of the lupins are presented in Table 5. Decreasing the mean particle size of lupins from 1304 to 567 μm increased SID lysine, methionine, threonine, isoleucine, leucine and valine from 8·8 g, 0·8 g, 4·9 g, 6·4 g, 10·0 g and 5·9 g/kg to 12·7 g, 1·2 g, 8·4 g, 10·6 g, 16·6 g and 10·2 g/kg, respectively.
Table 5 Effect of lupin particle size on apparent and standardised ileal digestible amino acids (AA) content (g/kg) in lupin seeds determined with 45 kg pigs
(Mean values with their standard errors)
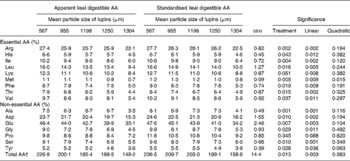
EAAL, endogenous amino acid loss.
* EAAL (mg/kg DM intake) values published by Stein et al. (Reference Stein, Trottier and Bellaver21) were used for calculation of standardised ileal digestible AA.
† Sum of AA excluding cystine and tryptophan.
Fermentation characteristics in the large intestine
Particle size of lupins had no effect on NH3-N content in all sites of the large intestine. The absolute amount of total VFA produced in the caecum was not influenced by particle size (Table 6). However, the decreasing particle size of lupins linearly increased total VFA production in the colon and rectum (linear effects P < 0·001 and P < 0·05, respectively). When the total VFA composition is expressed as a proportion of VFAAPB (sum of acetic, propionic and butyric acids) and BCFA (sum of valeric, isovaleric, isobutyric and caproic acids), decreasing particle size of lupins linearly increased VFAAPB molar proportion and linearly decreased BCFA molar proportion (P < 0·001, Fig. 2). For the molar proportion of individual acids, decreasing particle size of lupins linearly decreased acetic acid in the caecum and colon (P < 0·001), and linearly increased valeric acid in all sites of the large intestine (P < 0·01– < 0·001). Butyric acid was increased in all sites of the large intestine as the particle size of lupins decreased (P < 0·05– < 0·01). There were no differences (P>0·05) in the DM content of digesta at any of the gastrointestinal sites measured (Table 6).
Table 6 Effect of lupin particle size on ammonia nitrogen, total volatile fatty acids (VFA) concentration and the molar proportion of individual VFA in the gastrointestinal tract determined with 45 kg pigs
(Mean values with their standard errors)
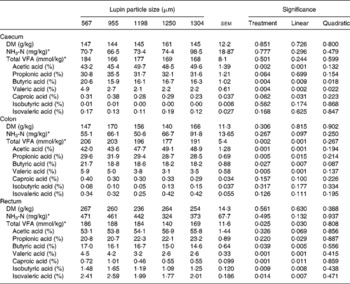
* Wet digesta basis.
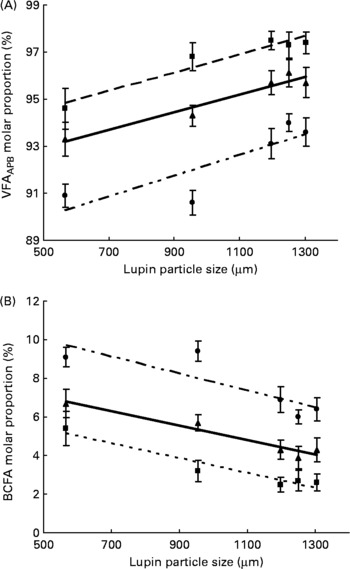
Fig. 2 Expt 2 – effect of lupin particle size on (A) the molar proportion of volatile fatty acids (sum of acetic, propionic and butyric acids, VFAAPB) and (B) branched-chain fatty acids (BCFA; sum of valeric, caproic, isobutyric and isovaleric acids) in the caecum (–■–), colon (–▲–) and rectum (–●–) of 45 kg pigs. The linear relationships were significant for VFAAPB and BCFA in the caecum, colon and rectum (at P < 0·001).
Physical properties of digesta and colon morphology
As no significant treatment effects were observed, these data are not presented. Particle size of lupins did not alter pH of digesta collected at the stomach (3·7 (se 0·44)), ileum (6·1 (se 0·22)), caecum (5·5 (se 0·10)) and colon (5·6 (se 0·10)). Also, particle size of lupins did not influence viscosity of ileal digesta (5·9 (se 2·0) mPa) and the plasma urea N content (5·9 (se 0·37) mm). Histological examination of the colonic tissue samples showed no differences in the crypt depth (195 (se 5·3) μm) and the depth of muscular layer (205 (se 14·2) μm) in response to varying particle size of lupins.
Discussion
The terminal anaesthesia technique(Reference Moughan and Ball23) was employed in the present study because it is less disruptive to the physical properties of the digesta and technically simpler compared with the various cannulation techniques(Reference Hodgkinson, Moughan, Moughan, Verstegen and Visser-Reyneveld24). Moreover, the variance in the AA digestibility data measured by the terminal anaesthesia technique has been reported as being similar to the variance in digestibility measured by other cannulation methods, such as simple T-cannulation(Reference Donkoh, Moughan and Smith15). As significant amounts of intestinal epithelium (enterocytes and mucus) can shed into the lumen when animals are killed using the captive bolt gun method(Reference Badawy, Campbell and Cuthbertson25, Reference Badawy, Campbell and Cuthbertson26), which is known to contribute more than 10 % of the total N content in the digesta samples, pigs were euthanised using sodium pentobarbitone under sedation(Reference Pluske, Black and Pethick16). To minimise variation in AA digestibility, pigs were euthanised at 9 h after the commencement of feeding(Reference Donkoh, Moughan and Smith15) and digesta samples were collected within 5 min of euthanasia. The 9 h interval was defined based on the observation that the variation in the apparent ileal N digestibility of grower pigs was narrower in ileal digesta samples collected 9 h after the commencement of feeding than samples collected at 5, 7 and 11 h (se 0·6 v. 4·3, 1·2 and 1·4, respectively)(Reference Donkoh, Moughan and Smith15). In addition, to prevent the shedding of intestinal cells and mucus into digesta samples, squeezing of the intestinal tract was avoided during collection.
Expt 1 demonstrated that decreasing particle size of lupins increased total-tract protein digestibility but not energy digestibility. Comparable and consistent results were observed in Expt 2, which confirmed that protein digestibility at the ileum and rectum was significantly affected by the particle size of lupins, while energy digestibility at the ileum and rectum was affected only marginally by variable particle size. Using starch-rich ingredients such as wheat and barley, numerous studies have shown increased total-tract energy digestibility with decreasing particle size of the grains(Reference Mavromichalis, Hancock and Senne27–Reference Kim, Mullan and Pluske29). However, as lupins contain a negligible amount of starch (6 g/kg DM) but significant amounts of NSP (443 g/kg DM(Reference Evans, Cheung and Cheetham30)), it is perhaps not surprising that energy digestibility in lupins in response to altered particle size is different to that in cereal grains. Finer grinding of lupins would have increased the substrate, particularly NSP, surface area for microbial action along the GIT. Significantly increased total amounts of VFA produced in the colon and rectum with decreased particle size (Table 6) supports this notion. Therefore, marginally increased energy digestibility in the finely ground lupins is most likely the result of increased NSP fermentation due to increased microbial fermentation, although the significantly increased N digestibility in the GIT due to decreased particle size would have contributed to the ileal energy digestibility. Nevertheless, the present results show that every 100 μm decrease in lupin particle size under 1304 μm reduces ileal digestible N and energy by 2·2 g/kg and 0·53 MJ/kg, respectively, which could have a significant effect on nutrient utilisation efficiency and environmental pollution.
The low ileal energy digestibility of finely ground lupin seeds (43 %) in the present study is identical to that reported previously by Taverner & Curic (44 %)(Reference Taverner, Curic, Robards and Packham31). Also, the ileal DE content found in the present study (8·0 MJ/kg) agrees with the value estimated with an older variety Gungurru (8·0 MJ/kg as is, 9·08 MJ/kg DM)(Reference van Barneveld1). In the present study, energy digestibility of the finely ground lupin was 76 % in the large intestine, indicating that approximately 33 % of the gross energy was digested in the large intestine. Taverner et al. (Reference Taverner, Curic and Rayner32) reported a 32 % difference between total-tract and ileal energy digestibility of lupins, which agrees with the present result. By contrast, N digestibility of the finely ground lupin at the ileum was comparably high (79 %) and was not different from the total-tract N digestibility (81 %). The present result concurs with Taverner et al. (Reference Taverner, Curic and Rayner32) who reported a 4 % unit difference between ileal and total-tract N digestibility of lupins. Collectively, the results reconcile that the major sites of digestion for energy and protein of lupins are the large intestine and the small intestine, respectively(Reference Taverner, Curic and Rayner32).
Decreasing particle size of lupins linearly increased apparent and standardised ileal AA digestibility. An early study showed that wheat ground through a 1·5 mm screen showed significantly higher ileal AA digestibility compared with a coarsely ground wheat (6 mm screen)(Reference Sauer, Stothers and Phillips33). In the present study, ileal apparent lysine digestibility was higher by 9 % units in the finely ground wheat. Decreasing particle size of soyabean meal from 979 to 185 μm significantly increased ileal digestibility of several essential AA such as methionine, isoleucine, phenylalanine and valine(Reference Fastinger and Mahan34). The present study quantified the ileal apparent digestible AA contents and estimated the standardised digestible AA contents in lupins in response to decreasing particle size. In support of the present hypothesis, every 100 μm decrease in lupin particle size under 1304 μm increased the SID lysine, methionine, threonine, isoleucine, leucine, valine and total AA contents by 0·5, 0·1, 0·5, 0·6, 0·9, 0·6 and 10·5 g/kg, respectively. Considering the purpose of lupin supplementation in diets for pigs is to supply digestible AA for lean growth, a significant loss of most AA due to coarse grinding can reduce feed conversion efficiency(Reference Healy, Hancock and Kennedy35) and therefore the financial return per kg pig meat sold. Moreover, a significant effect on reducing the flow of N into effluent can be achieved by finer grinding of lupins.
The increase in total VFA production with decreasing particle size of lupins (Table 6) concurs with a previous study by van Larr et al. (Reference van Laar, Tamminga and Williams36) who reported increased VFA production in vitro as particle size (i.e. surface area to volume ratio) of monocotyledon (maize, wheat, rye and rice) cell wall materials decreased. Carbohydrate fermentation by intestinal microbes produces straight-chain VFA (VFAAPB) such as acetic, propionic and butyric acids, while protein fermentation produces NH3 and BCFA such as valeric, isobutyric and isovaleric acids(Reference Bauer, Williams and Voigt37, Reference Macfarlane, Gibson and Beatty38). Nyachoti et al. (Reference Nyachoti, Omogbenigun and Rademacher39) and Htoo et al. (Reference Htoo, Araiza and Sauer40) demonstrated that reducing the amount of protein entering into the large intestine decreased BCFA concentration in the ileum and caecum of weaned pigs. Based on these observations, it was anticipated that decreasing particle size of lupins would decrease NH3 and BCFA production as the amount of total protein entering into the large intestine would be decreased due to increased AA digestibility at the ileum. However, the present data indicated that decreasing particle size increased the proportion of BCFA in the large intestine, while the proportion of VFAAPB derived from carbohydrate fermentation was decreased. This is probably because finer grinding increased surface area that in turn increased AA digestibility in the small intestine, but also increased the available surface area for interaction with the microbiota in the large intestine. It is generally known that the globoid and proteinaceous matrix in the legumes are located inside of the cell wall (carbohydrate) materials, which are fundamentally located in the peripheral part of the kernel's endosperm(Reference Lott and Buttrose41). Therefore, coarse grinding may less efficiently expose these proteinaceous matrixes for digestion and fermentation in the small and large intestine, respectively. Consequently, the carbohydrate fraction in the peripheral part of the endosperm cell walls would have been readily available for fermentation in the large intestine compared with the enclosed proteinaceous matrix. The increase in the amount of energy digested in the large intestine by increasing particle size of lupins could partly reconcile the present hypothesis, although part of the energy digested in the large intestine in the coarsely ground lupins could be due to protein fermentation (Table 5). Although the particle size variation in cereals and lupins might have different effects on the physico-chemical properties of digesta and microbial populations in the GIT, the decrease in the proportion of straight-chain VFA and reduction in proportion of BCFA in pigs fed coarsely ground lupins could, at least partly, explain the health-promoting effect of the pigs that received coarsely ground diets compared with the pigs received finely ground diets(Reference Mikkelsen, Hedemann and Jensen42). These changes in fermentation characteristics due to an alteration of dietary/ingredient particle sizes could possibly be associated with the reductions in pathogen-related strains in the GIT of pigs fed a coarsely ground diet compared with pigs fed a finely ground diet(Reference Mikkelsen, Hedemann and Jensen42, Reference Hedemann, Mikkelsen and Naughton43).
Hedemann et al. (Reference Hedemann, Mikkelsen and Naughton43) reported increased crypt depth in the colon of pigs fed a coarsely ground diet compared with that of pigs fed a finely ground diet. However, and although the straight-chain VFA proportion in the large intestinal digesta was increased due to increased particle size of lupins, digesta pH and colonic morphology were not different between treatments in the present study. This might have been caused by the shorter duration of the study (3 weeks), hence different responses may have occurred if pigs received the experimental diets for longer. Also, the range of dietary particle sizes employed in the present study was not enough to change digesta pH, as only lupins included at 350 g/kg had variable particle sizes, while the majority of the diet (650 g/kg) had a constant particle size. As a result, the dietary particle size ranged from 734 to 956 μm, which is only a difference of 222 μm between the coarsely prepared and the finely prepared diet.
Conclusion
Reducing particle size of lupins significantly increased ileal AA digestibility and increased total production of VFA in the large intestine. Also, reducing particle size of lupins decreased the molar proportion of the straight-chain VFA, while increasing molar proportion of BCFA in the large intestine. However, the alteration in the fermentation characteristics in the large intestine due to variable lupin particle size did not influence the morphology of the colonic mucosa. The results indicate that every 100 μm increase in lupin particle size over 567 μm reduced ileal digestible N and energy contents by 2·2 g/kg and 0·53 MJ/kg, respectively. Also, every 100 μm increase in lupin particle size over 567 μm reduced the SID lysine, methionine, threonine, isoleucine, leucine, valine and total AA contents by 0·5, 0·1, 0·5, 0·6, 0·9, 0·6 and 10·5 g/kg, respectively. The variety of lupin fed to pigs had no influence on digestibility.
Acknowledgements
The authors acknowledge the financial support from the Western Australia Agricultural Produce Commission-Pork Producers' Committee and the technical assistance by Ms Aracely Hernandez, Ms Josie Mansfield, Mr Bob Davis and Mr Roland Nicholls. Each author fully contributed to the design, experiments and interpretation of the results presented in the present manuscript. The authors have no conflict of interest to report.










