Non-alcoholic fatty liver disease (NAFLD) presents as a serious global health issue estimated to affect approximately 30 % of adults worldwide(Reference Younossi, Golabi and Paik1,Reference Katsiki, Perez-Martinez and Anagnostis2) . This chronic liver disease is closely associated with unhealthy lifestyle behaviours including poor dietary habits, sedentary behaviours and physical inactivity(Reference Huang, Behary and Zekry3). Current clinical practice guidelines advise weight loss, primarily achieved through diet and exercise, for the management of NAFLD(Reference Rinella, Neuschwander-Tetri and Siddiqui4), but adherence to such recommendations remains a challenge(Reference Arredouani5). Telehealth has emerged as an encouraging approach to deliver and monitor personalised lifestyle programs that can significantly increase reach and accessibility for people with chronic diseases by overcoming common barriers including access to evidence-based programs, cost, time constraints and geographical location(Reference Kwon, Choi and Jang6–Reference Jaglal, Haroun and Salbach8). However, the limited digital health, lifestyle-based interventions conducted to date in those with NAFLD have reported mixed findings when examining the influence of text-message support, web-based platforms or apps designed to target weight loss, increasing physical activity or acceptability as an outcome(Reference Lim, Johal and Ong9–Reference Pfirrmann, Huber and Schattenberg12).
Although weight loss is at the forefront of NAFLD lifestyle recommendations, a common consequence of dietary-induced weight loss is a concurrent loss of muscle (lean) mass(Reference Batsis, Gill and Masutani13), which has been shown to be a risk factor for both onset and progression of NAFLD(Reference Cai, Song and Chen14). Studies conducted in healthy adults and those with chronic conditions including NAFLD have shown that progressive resistance training (PRT) can increase muscle mass with some evidence that higher protein intakes can preserve or attenuate muscle loss during weight loss(Reference Wycherley, Noakes and Clifton15–Reference Deibert, Lazaro and Schaffner17). In healthy young and older adults, there is also evidence that the combination of PRT with a higher protein intake may provide additional benefits to muscle mass (and strength) compared with PRT alone, especially in those with low habitual intakes(Reference Morton, Murphy and McKellar18). While most previous research has focused on animal protein and protein supplements such as whey protein, there is some evidence that plant-based sources of protein do not differentially impact PRT-related gains in muscle mass if the total dose of protein (∼1·6 g/kg/d) is comparable(Reference Lim, Pan and Toh19,Reference Hevia-Larraín, Gualano and Longobardi20) .
For other health outcomes, there is growing evidence that the source of protein may be important(Reference Song, Fung and Hu21). For instance, foods of animal origin are a source of animal protein and saturated fatty acids which may increase insulin resistance and CVD risk while foods of plant origin are a source of plant proteins, fibre and bioactive compounds, which are associated with cardiometabolic benefits(Reference Alnouri, Amar and Atanasov22,Reference Kahleova, Levin and Barnard23) . Data from population-based studies suggest a high consumption of animal protein (especially red and/or processed meat) may increase the risk of NAFLD(Reference Alferink, Kiefte-de Jong and Erler24,Reference Peng, Xie and Pan25) , while greater intake of plant proteins and fish may be protective(Reference Rietman, Sluik and Feskens26,Reference Khazaei, Dehghanseresht and Mousavi27) . A diet that emphasises plant sources of protein is also in line with the Mediterranean diet composition that is recommended in NAFLD clinical guidelines(Reference Rinella, Neuschwander-Tetri and Siddiqui4). Therefore, it is likely that lifestyle strategies that encompass PRT combined with a higher total protein intake may be beneficial for optimising body composition, while a higher protein intake from predominately plant-based sources may provide other potential cardiometabolic health benefits in those with NAFLD. However, to our knowledge no study has assessed the feasibility and safety of a lifestyle intervention delivered and monitored by healthcare professionals via telehealth incorporating a home-based, muscle strengthening program combined with a higher protein intake from predominately plant-based sources in adults with NAFLD.
The aim of this randomised controlled trial was to evaluate the feasibility (retention and adherence) and safety of a 12-week telehealth, home-based exercise and nutritional support program incorporating a personalised muscle strengthening exercise program and healthy eating recommendations focusing on a higher protein intake from predominately plant-based sources in middle and older aged adults with NAFLD. Secondary exploratory aims were to evaluate changes in macronutrient intake including protein intake from different protein sources (plant and animal), anthropometric measures, habitual physical activity and physical function.
Methods
Study design
This was a 12-week randomised controlled study investigating the feasibility and safety of delivering an exercise, dietary and behavioural change support intervention via telehealth to middle-aged and older adults with NAFLD. Following baseline assessments, participants were randomised (in blocks of 2), stratified by age (< 65 years, ≥ 65 years), by an independent researcher not directly involved in the study, to receive either the telehealth exercise and protein (Pro-Ex) intervention or usual care (controls). Study recruitment occurred over an 8-month period (May 2022 to November 2022) with the intervention delivered in two cohorts: cohort 1 October 2022 to December 2022; cohort 2 November 2022 to February 2023. Research staff and participants were not blinded to the group allocation given the nature of the intervention to educate and adopt diet and exercise changes. Participants were assessed at baseline, 6 and 12 weeks, with exception of protein intake that was also assessed at weeks 2 and 9. All assessments and training were performed remotely at the participant’s home. The study was conducted according to the guidelines laid down in the declaration of Helsinki, and all procedures involving human subjects were approved by the Deakin University Human Research Ethics Committee (2021-359) and prospectively registered with the Australian and New Zealand Clinical Trial Registry https://www.anzctr.org.au (ACTRN12621001706864). Written informed consent was provided (electronically) by all participants prior to participation.
Participants
A total of twenty-eight men and women aged ≥ 45 years with self-reported NAFLD, as diagnosed by a doctor, determined via routine ultrasound or biopsy and/or at least one elevated serum alanine aminotransferase level (> 20 U/L female, > 30 U/L male), were recruited from social media (Facebook), general practice and liver clinics throughout Australia. We acknowledge the recent guidelines and associated change in nomenclature definition of metabolic dysfunction-associated steatotic liver disease (MASLD); however, registration of the study protocol (13/12/2021) and participant recruitment preceded consensus and publication of the new definition. Participants were excluded based on the following criteria: current/prior involvement in PRT (> 1/week) or moderate-intensity physical activity (> 150 min/week) in the last 3 months; self-reported weight loss/gain of > 5 kg in the last 3 months; renal disease or impairment, orthopaedic, cardiovascular or respiratory disease preventing participation in the intervention or those with absolute contraindications to exercise based on the American College of Sports Medicine guidelines(Reference Shephard28); no evidence of another form of liver disease; currently following a vegetarian or vegan diet; intake of ≥ 2 serves of protein rich (fortified) supplements per week; an average intake of ≥ 1 (female) or ≥ 2 (male) alcoholic drinks per day and currently undertaking a dietary intervention from a dietitian/nutritionist. Participants were also required to obtain consent from their consulting physician (if they answered yes to any of the contradictions to exercise)(Reference Shephard28), and they needed to possess a mobile phone, tablet or computer with text message functioning, home internet connection and own or have access to scales suitable for measuring body weight. All participants were initially screened via an online questionnaire (n 408), with participants meeting the initial eligibility criteria (n 92) undertaking further screening via telephone. A total of thirty-six participants were deemed eligible, with twenty-eight participants agreeing to participate in the study and who were randomised to either group (Fig. 1).
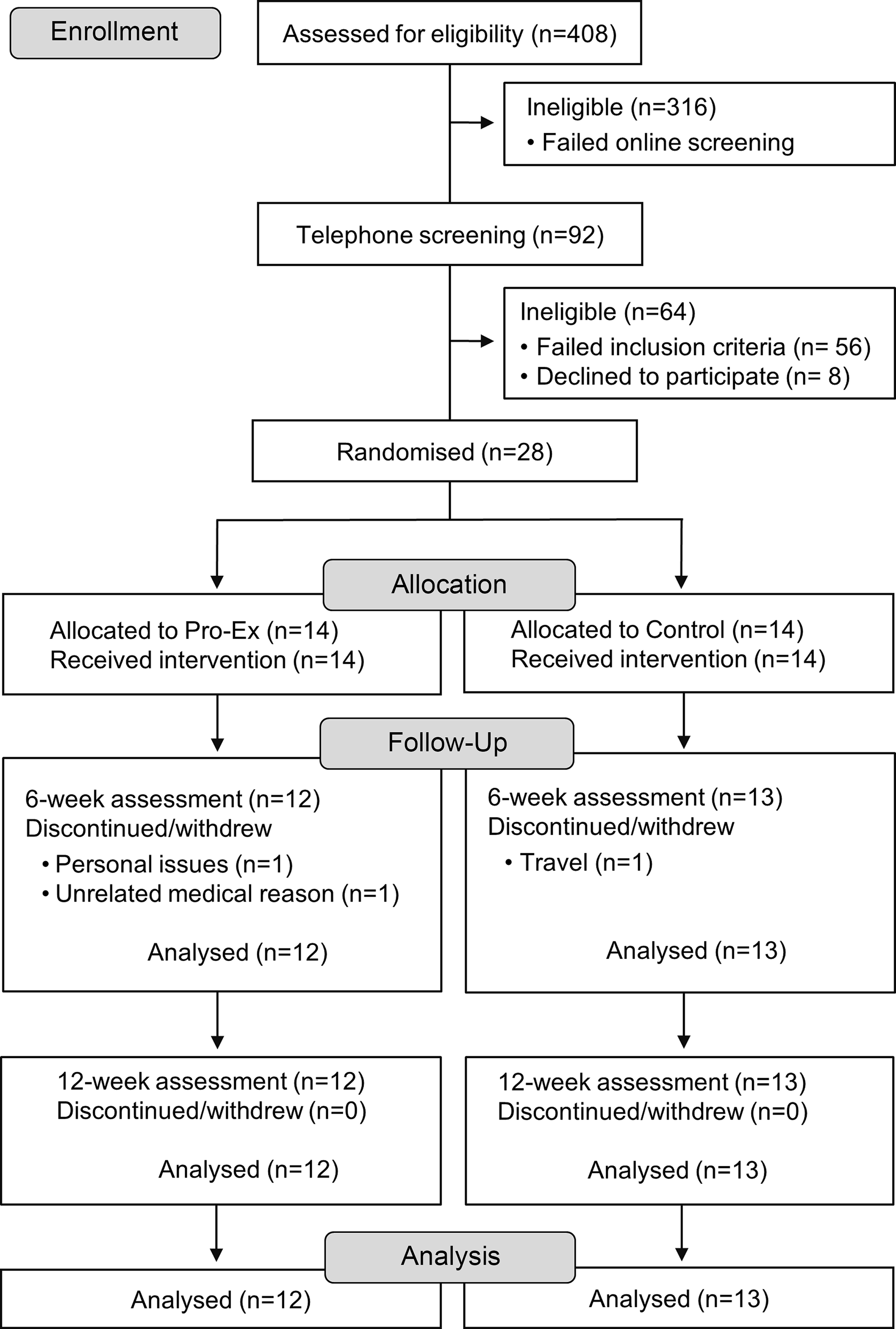
Fig. 1. Study flow chart. Pro-Ex, protein plus exercise.
Exercise intervention
Participants allocated to the Pro-Ex group were prescribed a 12-week muscle strengthening program by a qualified accredited exercise physiologist (EP) using a commercially available web-based exercise prescription platform and participant app (TeleHab, VALD Health) accessible via their phone or tablet. The TeleHab app provided participants with narrated videos of all exercises to demonstrate correct technique, and participants were asked to rate their perceived exertion (RPE) and any pain (both on a scale of 0–10) upon completion of each exercise and provide any written comments regarding their prescribed exercises, all of which were monitored remotely by the EP thrice weekly to check on participants progress and promote safety. All exercise sessions were saved in the TeleHab platform to monitor adherence and progress. Exercise prescription was personalised by the EP based on each participant’s baseline assessment and medical and physical activity history. Participants were prescribed an ∼25-minute whole body muscle strengthening program consisting of 6–8 exercises utilising major muscle groups (two sets of ten repetitions for each exercise) at a moderate intensity (5 ‘hard’ to 8 ‘very hard’ RPE scale) to be completed thrice weekly (on non-consecutive days). In week 6, the EP prescribed (via TeleHab) a new program (set of exercises) for the remaining 6 weeks of the intervention. Some examples of the prescribed exercises include sit-to-stands, squats, lunges, wall/inclined push-ups, upright row (resistance band) and triceps extension (resistance band). Prior to commencing the programme, participants attended an online (Zoom) consultation with the EP to receive training on using the TeleHab app, tips for exercising safely at home and to ensure participants were performing the prescribed exercises correctly. In weeks 2 and 6 participants attended small group (∼2–3 participants) consultations (via Zoom) with the EP who was able to answer any questions and promote motivation and a sense of community for participants. In addition, all participants were instructed and encouraged to engage in aerobic activity (e.g. brisk walking) twice weekly (total ≥ 60 min per week) throughout the 12-week program, which they recorded in a daily activity calendar.
Dietary intervention
For the 12-week intervention, an isoenergetic healthy eating plan was devised by a nutritionist which emphasised a protein intake of 1·2–1·5 g/kg/d from predominately plant-based foods (legumes, tofu, nuts, peanuts and seeds), as well as fish and white meat sources. This was translated into recommendations of at least three serves/day of protein from all sources consisting of two serves from plant sources and 1–1 ½ serves from animal-based protein sources, which were based on each participant’s age and sex. Serving guidelines were then provided regarding daily protein from plant sources (total 2 serves/d from nuts and seeds and/or tofu/legumes and/or beans) and animal sources (total 7–10 ½ serves/week from recommendations for seafood (≥ 2 serves/week), poultry (≤ 2 serves/week) eggs (2–4 serves/week), red meat (< 2 serves/week) and processed meat (≤ 1 serve/week)). Protein intakes (plant and animal sources) were recorded via an online protein checklist at baseline to guide participant recommendations about their healthy eating plan. Further checklists were completed in weeks 2, 6, 9 and 12 to monitor adherence. Dietary recommendations were based on a heart healthy, modified Mediterranean style dietary pattern informed by current clinical guidelines for NAFLD(Reference Rinella, Neuschwander-Tetri and Siddiqui4,Reference Anania, Perla and Olivero29) . In week 1, participants received written resources providing information on protein serving sizes, recommended servings from different protein sources, sample meal plans and plant-based recipes. Further education about the healthy eating plan and how to complete the 24-h record were also delivered in week 1 via an online (Zoom) consultation with the study nutritionist. At week 2 and 6, participants attended small group (∼2–3 participants) online (Zoom) consultations with the nutritionist to receive information and tips on increasing plant-based protein intake and adhering to protein serving recommendations, feedback on protein checklists and motivational tips to adopt the healthy eating plan.
Behavioural change support
In addition to receiving regular support from the EP and nutritionist, participants in the intervention group received regular text messages (3–4 messages/week) with health focused and motivational messages to support behavioural change. This included reminders and tips for increasing daily serves of protein (predominately plant-based) and adhering to other dietary recommendations, physical activity/movement and more generic messages providing motivational support.
Control group (usual care)
Participants assigned to the control group received usual care from their general practitioner or liver clinic specialist. They also received generic handouts at the start of the study providing information on the importance of healthy eating (increasing fruits (≥ 2 serves) and vegetables (≥ 5 serves), choosing wholegrains, lean proteins and reduced fat versions of dairy foods and healthy mono and polyunsaturated fats and limiting processed foods) based on recommendations from the Australian Heart Foundation(30) and being physically active as per the Australian Physical Activity Guidelines(31).
Measurements
Feasibility
Feasibility was assessed via retention and adherence as outlined below.
Retention
Retention was calculated as the proportion of participants who completed the 12-week intervention. Acceptable retention for this study was defined as < 10 % attrition.
Adherence
Adherence was recorded for both the exercise and dietary recommendations as follows; (1) the total number of exercise sessions completed relative to the total number prescribed (expressed as a percentage) and (2) the number of serves of protein-rich foods consumed relative to the number recommended from plant-based protein sources (total 2 serves per day from nuts and seeds and/or tofu and legumes and/or beans) and animal protein sources (7–10 ½ serves per week from seafood and/or eggs and/or poultry and with less than 2 serves from red meat and less than or equal to 1 serve from processed meat) and total protein (those participants meeting both the above daily plant and weekly animal serving recommendations). Acceptable adherence for this study was defined as at least 66 % (two out of three sessions/week) to the muscle strengthening exercise program and ≥ 80 % adherence (to ensure participants protein intake reached at least 1·0–1·2 g/kg/d) to the recommended serves (average over 12-weeks) of both plant and animal protein sources assessed via protein checklists in weeks 2, 6, 9 and 12. For example, if the recommendation was ≥ 2 serves/d, adherence would require an average intake of at least 1·6 serves/d. For comparison, adherence to the above dietary recommendations was also reported for controls.
Adverse events (safety)
An adverse event was defined as any intervention-related event that results in withdrawal or modification to the exercise or healthy eating program. Participants in the Pro-Ex group were asked to record any adverse events directly into TeleHab so that they could be reviewed by the research investigators. Information on any potential adverse events were also collected by the EP and nutritionist during the video consults in weeks 2 and 6.
Additional measures
Dietary assessment
Changes in macro and micronutrient intakes and food groups were assessed via three separate 24-h food records completed at baseline, 6 and 12 weeks. Food records were completed using the commercially available web-based dietary app (Research Food Diary) accessible via the participant’s phone or tablet. Dietary information was analysed using Foodworks nutrient analysis software (Xyris).
Anthropometry
Due to the telehealth nature of this study, anthropometry measures (height, body weight and waist circumference) were self-reported at baseline, 6 and 12 weeks. Participants were sent tape measures and provided written instructions on how to measure their waist circumference (in cm). Body mass index (BMI) was calculated as weight (kg) divided by height (m2).
Physical activity
The validated short International Physical Activity Questionnaire was used to assess physical activity(Reference Craig, Marshall and Sjöström32). Questions covered the frequency, intensity and duration of walking, moderate and vigorous physical activity and total time spent sitting per day (sedentary behaviour) over the past 7 d. Total time (min/week) spent walking and in moderate-to-vigorous activity and sedentary time were calculated at baseline, 6 and 12 weeks.
Physical function
Physical function was assessed using the 30 s sit-to-stand (STS) test. Participants were asked to use a firm chair, approximately 46 cm high, with no wheels or arm rests, with the same chair used for each assessment. Participants were shown a demonstration video of the 30 s STS test, then instructed to place their chair against a wall and position their device (e.g. smart device or laptop) ∼2·0 m away from the chair at an angle of ∼45° and adjust their device so the researcher could see their entire body and chair. Participants started the test from a seated position with hands across their chest and on the command ‘go’ were instructed to stand fully upright, then return to a seated position repeatedly for 30 s, with the final score being the number of completed STS in 30 s counted by the researcher. The 30 s STS has been shown to be a valid indication of lower limb strength(Reference Cruz-Jentoft, Bahat and Bauer33) and is feasible and safe when delivered and evaluated via telehealth as a remote assessment of physical function(Reference Rees-Punia, Rittase and Patel34).
Medical and health history
History of chronic diseases (hypertension, CVD, lung disease, diabetes and kidney disease), smoking (never, previous and current smoker), current medications (number and type) and education (high school, trade/technical, university/tertiary) were evaluated by a lifestyle questionnaire competed at baseline.
Statistical analysis
As this was a feasibility study, no formal sample size calculations were required as per current recommendations,(Reference Lancaster, Dodd and Williamson35) and significance testing was not conducted. Descriptive variables were calculated using Stata/se software version 17.0 (StataCorp), and all data were reported as mean with sd, median with interquartile range or number with proportions, unless stated. For the exploratory outcomes of physical activity, 30 s STS performance and anthropometric measures and effect sizes (Cohen’s d) were calculated to explore trends for between-group differences for the changes over 12 weeks. Within-group changes were calculated as the mean change (absolute or percentage) from 6 and 12 weeks compared with baseline. Between-group differences for the change were calculated as the mean difference between groups for the change from 6 or 12 weeks compared with baseline. Effect sizes for between-group differences for the changes from baseline were calculated according to the mean change in Pro-Ex minus the mean change in controls divided by the baseline pooled sd of both groups. Cohen’s d values of < 0·2, 0·2 to < 0·5, 0·5 to < 0·8 and ≥ 0·8 were used to demonstrate trivial, small, moderate and large effect sizes, respectively(Reference Cohen36).
Results
The baseline characteristics of participants are shown in Table 1, with groups being similar with the exception that participants in Pro-Ex had a higher weight and BMI than controls. The mean ± sd age of all participants was 63·5 ± 7·3 years (range 47–77 years) with 93 % being female and 36 % (n 10) classified as overweight (BMI 25–29·9 kg/m2) and 54 % (n 15) as obese (BMI ≥ 30 kg/m2). A total of twenty-two (79 %) participants reported taking a prescribed medication, with the mean number for those prescribed medication being 2·1 ± 1·9. Overall, 46 % (n 13) of participants reported the presence of one or more chronic disease, which included CVD (n 1), hypertension (n 10) and diabetes (n 2).
Table 1. Participant characteristics
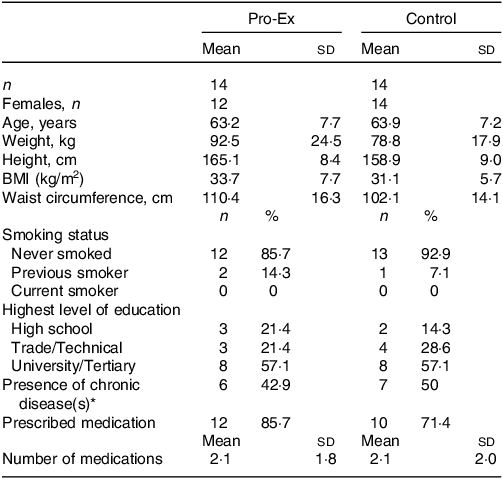
Pro-Ex, protein plus exercise. All values are expressed as mean and sd or number (%).
* Presence of chronic diseases include self-reported hypertension, CVD and/or diabetes.
Adverse events
Over the 12-week program, there was one minor exercise-related adverse event (musculoskeletal complaint of knee pain related to a pre-existing knee injury) reported from a total of 241 completed exercise sessions. The participant continued to exercise with a modified program for 2 weeks before returning to the full program.
Feasibility
Retention
Study retention was 89 % (25 of 28 participants completed the study) which was similar for Pro-Ex (86 %) and Con (93 %). Three participants (Pro-Ex group n 2, control n 1) withdrew during the first 6 weeks due to personal issues, an unrelated medical reason and overseas travel (Fig. 1).
Attendance at consultations
All participants in Pro-Ex (n 14) attended the initial one-on-one sessions with their EP and study nutritionist at the start of the study. Attendance at the small group sessions in week 2 with both the EP and nutritionist was 86 % (n 12), while in week 6, attendance was 64 % (n 9) and 71 % (n 10), with the EP and nutritionist, respectively, for the eleven participants in Pro-Ex that remained in the study.
Exercise adherence
The mean ± sd adherence to the thrice weekly muscle strengthening program (Pro-Ex) was 52 ± 36 % (median 55 %, interquartile range 15–69 %). This included two participants who withdrew at weeks 3 and 5, three participants who stopped training within the first three weeks and two participants who completed more sessions than they were prescribed (108 % and 121 % adherence). A further two participants reported knee (n 1) and back (n 1) pain unrelated to the exercise program, which resulted in one participant stopping the exercise program in week 3, and the second participant exercising at a reduced capacity in weeks 9–11. Overall, 36 % (n 5) of participants completed, on average, < 1 session per week, 29 % (n 4) completed 1–< 2 sessions per week and 36 % (n 5) completed ≥ 2 sessions per week. When assessing only those participants who completed the 12-week exercise program (nine of fourteen participants), the mean ± sd adherence to the muscle strengthening program was 73 ± 26 % (median 64·7 %, interquartile range 62–74 %). The mean ± sd adherence to the twice weekly (at least 60 min in total) aerobic exercise recommendations was 52 ± 41 % (median 63 %, interquartile range 0–100 %).
Dietary adherence
Overall, the mean proportion of participants in the Pro-Ex group who met (at least 80 %) of the plant protein (serves/day) and animal protein (serves/week) recommendations over the 12-week intervention was 32 % and 42 %, respectively, with only 14 % (n 2) of participants meeting the total protein recommendations for both animal and plant sources (Table 2). Adherence to the plant protein serves/day was highest in week 6 (50 %), before decreasing slightly at weeks 9 and 12 (33·3 %), while adherence to the recommended serves/week of animal protein increased from 14·3 % at baseline to 57·1 % in week 2 and then varied between 16·7 and 58·3 % throughout the rest of the intervention. The proportion of control participants whose habitual plant protein intake met recommended serves/day throughout the study period was 0 % while the proportion who met the recommended serves/week for animal protein varied from 15·4 to 38·5 % throughout the 12-weeks. There were no control participants who met both the recommended plant and animal protein serve at any time.
Table 2. The number and proportion (%) of participants who adhered to at least 80 % of the recommended serves and the mean daily serves of plant, animal and total protein at baseline and throughout the 12-week intervention in the Pro-Ex and control group
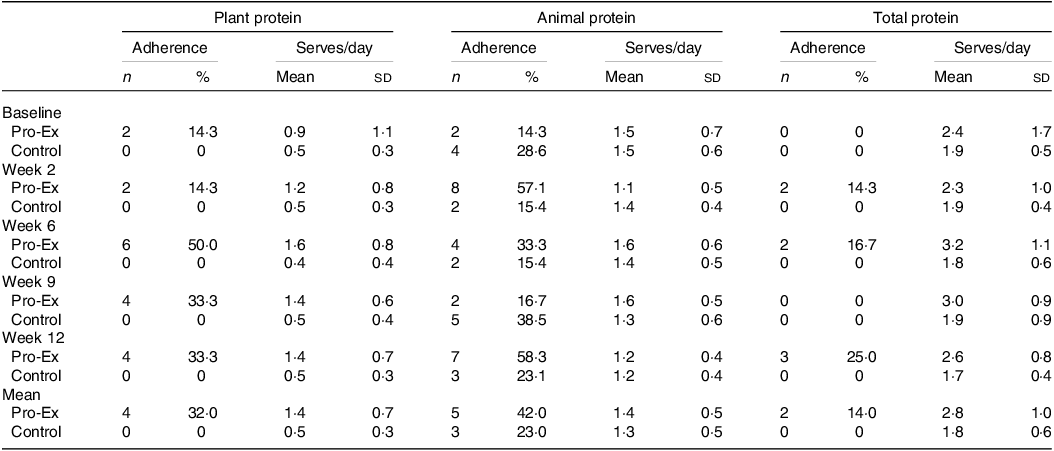
Pro-Ex, protein plus exercise group. All values are expressed as number and proportions (%) or mean and sd. The number (and proportion) of participants classified as adhering to the plant protein recommendations was based on meeting ≥ 80 % of the 2 serves/d. For adherence to the animal protein recommendations this was based on meeting ≥ 80 % of the 7–10 ½ serves/week which included from seafood and/or eggs and/or poultry and with less than 2 serves from red meat and less than or equal to 1 serve from processed meat per week. For total protein adherence this included meeting both plant and animal protein recommendations. Pro-Ex - baseline and week 2 (n 14) and weeks 6, 9 and 12 (n 12); Controls – baseline (n 14) and weeks 2, 6, 9 and 12 (n 13). Mean represents average from weeks 2 to 12.
Daily serves of total, animal and plant protein
Despite modest adherence to the specific protein (plant and animal) recommendations, participants in Pro-Ex did display some improvements in their daily serves of total and plant-based protein (Table 2). Overall, the mean ± sd serves/day of total protein for Pro-Ex increased from 2·4 ± 1·7 at baseline to 3·2 ± 1·1 at week 6 before decreasing to 2·6 ± 0·8 in week 12. In controls, mean serves/day of total protein remained relatively stable throughout the study (range 1·7 ± 0·4 to 1·9 ± 0·5 serves/d). Pro-Ex also increased their mean serves/day of plant protein from 0·9 ± 1·1 at baseline to 1·6 ± 0·8 at week 6 and 1·4 ± 0·7 at week 12, while intakes for controls remained stable (∼0·5 serves/d). Regarding serves/day of animal protein, mean intakes were comparable between Pro-Ex and controls at baseline (1·5 ± 0·7 v. 1·5 ± 0·6) and decreased slightly in both groups at week 12 (both 1·2 ± 0·4 serves/d).
Serves of protein from different plant and animal sources
The number of mean weekly serves of protein from different sources and proportion of participants meeting ≥ 80 % adherence to the recommended serves throughout the study for Pro-Ex and controls is shown in Table 3. Overall, throughout the intervention, Pro-Ex increased their intake of seafood (mean change 1·3 serves/week), nuts and seeds (0·2 serves/d) and legumes (0·3 serves/d) and reduced their intake of red meat (0·6 serves/week) and processed meat (1·2 serves/week). For controls, intakes from both plant (serves/day) and animal-based sources (serves/week) of protein remained relatively consistent over the 12-week intervention. In terms of the proportion of participants achieving ≥ 80 % adherence to our recommendations for specific protein sources, there was little or no change for participants in Pro-Ex with regards to nuts and seeds, eggs and poultry but a higher proportion of participants achieved the recommendations for legumes and tofu (7–32 %), seafood (50–78 %), red meat (71–86 %) and processed meat (36–66 %) (Table 3). For controls, there were no discernible changes in the proportion of participants meeting the recommendations.
Table 3. Mean daily plant-based and weekly animal-based serves of protein from different protein sources and the proportion of participants meeting ≥ 80 % adherence to the recommended intakes in Pro-Ex and controls
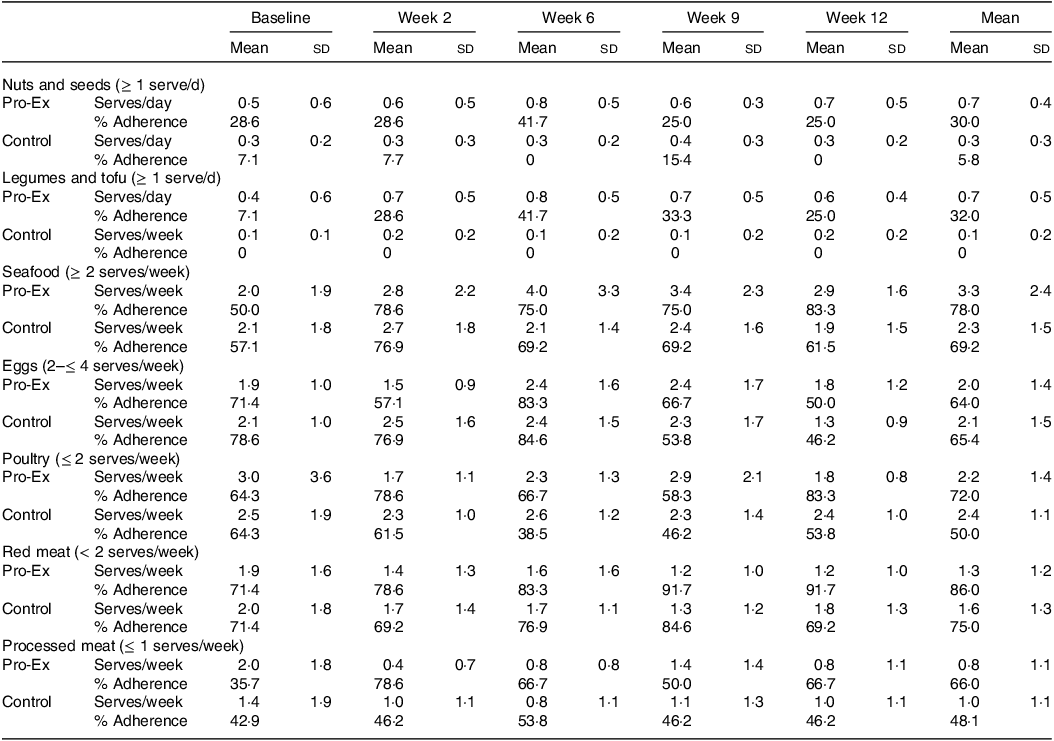
Pro-Ex, protein plus exercise group. All values are expressed as mean and sd or proportions (%). Adherence was calculated as the percentage of participants meeting ≥ 80 % of recommendations for each protein source as indicated. Number of participants with protein data: Pro-Ex – baseline and week 2 (n 14) and weeks 6, 9 and 12 (n 12); Controls – baseline (n 14) and weeks 2, 6, 9 and 12 (n 13). Mean represents average from weeks 2 to 12.
Dietary intake
As shown in Table 4, both the Pro-Ex and control group had similar reductions in total energy, carbohydrate and total fat intake from baseline to 12-weeks. However, the mean daily protein intake in the Pro-Ex group increased by 13·1 g (95 % CI: −3·8, 29·9 g) after 12-weeks but decreased by 22·1 g (95 % CI: −41·4, −2·7 g) in controls, which represented a large effect (d = 1·23) for the between-group difference. While both groups reduced their total and saturated fat intake over time, this tended to be greater in Pro-Ex compared with controls (total fat: small effect d = –0·31; saturated fat: moderate effect d = –0·57).
Table 4. Baseline and within-group changes after 6 and 12 weeks in total energy and macronutrient intake, physical activity, 30 s sit-to-stand performance and body composition measures in Pro-Ex and controls and between-group differences for changes with effect size (Cohen’s d)
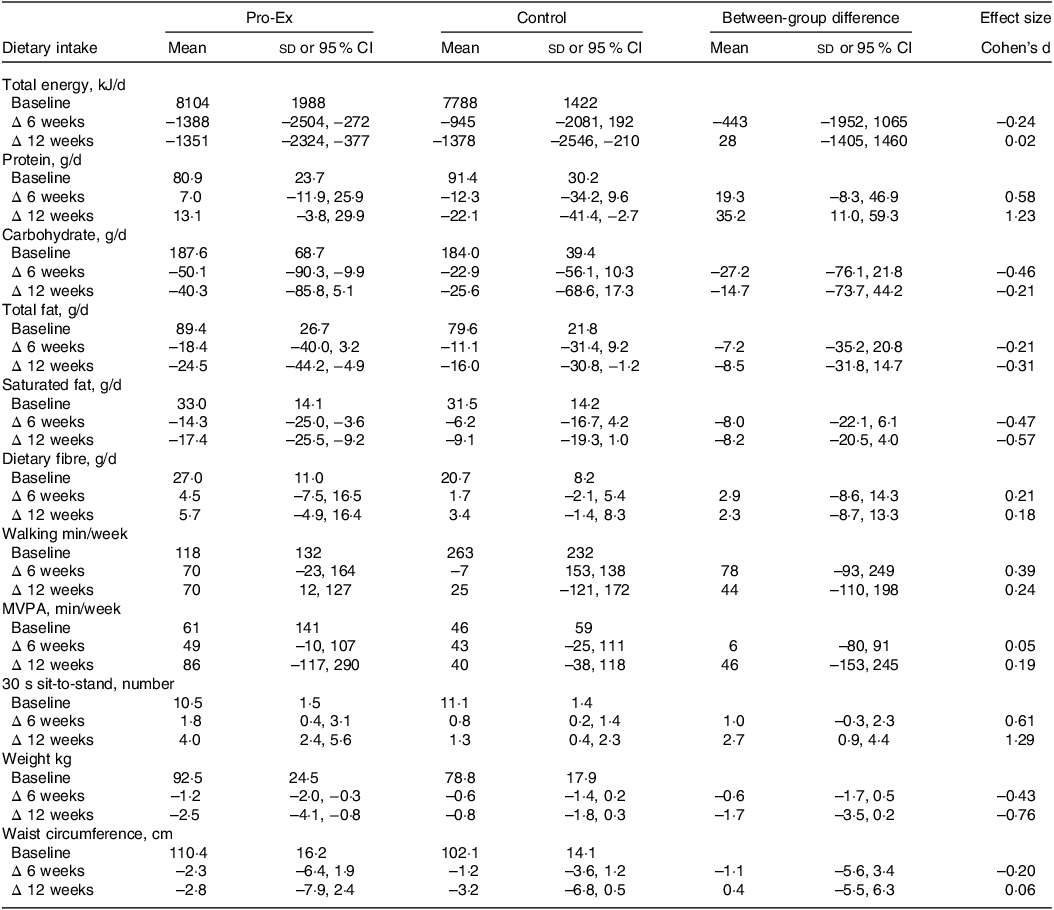
Pro-Ex, protein plus exercise group; MVPA, moderate-vigorous physical activity. All baseline values are unadjusted means and sds, while within-group changes from baseline at 6 and 12 weeks and between-group differences are reported as mean (95 % CI). Effect sizes (Cohen’s d) represent the net difference for the change between Pro-Ex minus the change for controls, divided by the baseline pooled sd. All change values are unadjusted means (95 % CI) and expressed as absolute changes from baseline.
Physical activity
Total weekly walking time in the Pro-Ex group increased on average by ∼70 min throughout the study compared with an average −7 (week 6) to +25-minute (week 12) change in controls, which represented a small effect (d = 0·24) for the between-group difference at week 12 (Table 4). Weekly moderate-to-vigorous activity increased on average by ∼45 min in both group at 6 weeks and 86 min (95 % CI: −117, 290) in Pro-Ex and 40 min (95 % CI: −38, 118) in controls at week 12, with no sizeable effect for between-group differences (d = 0·05–0·19).
Anthropometry and physical function
After 12 weeks, there was a mean 2·5 kg (95 % CI: −4·1, −0·8 kg) decrease in self-reported weight within Pro-Ex compared with a mean −0·8 kg (95 % CI: −1·8, 0·3 kg) change in controls, which represented a moderate effect (d = –0·76) for the between-group difference (Table 4). Both groups experienced similar mean reductions in self-reported waist circumference (Pro-Ex −2·8 cm (95 % CI: −7·9, 2·4 cm) v. control −3·2 cm (95 % CI: −6·8, 0·5 cm), d = 0·06) after 12 weeks. For physical function, Pro-Ex demonstrated a mean 4·0 (95 % CI: 2·4, 5·6) increase in 30-second STS number at 12 weeks compared with a mean change of 1·3 (95 % CI: 0·4, 2·3) in controls, which represented a large (d = 1·29) effect.
Discussion
The key findings from this feasibility intervention trial in adults with NAFLD were that remote delivery and monitoring of a 12-week, home-based lifestyle program via telehealth incorporating a thrice weekly muscle strengthening program with dietary advice to increase plant-based protein intake was safe as demonstrated by the single minor adverse event (exercise related) and with high retention (89 %), but adherence in terms of meeting our predefined criteria of ≥ 66 % (exercise program) and ≥ 80 % (protein recommendations) was modest. Mean adherence to the 12-week muscle strengthening program was 52 %, and while mean daily serves of plant-based protein increased (0·9 to 1·4 per day) and animal protein decreased (1·5 to 1·2 per day) after 12 weeks, the proportion of participants meeting our recommendations for plant protein (2 serves/d) was 32 % and animal protein (1–1 ½ serves/d) was 42 %. For secondary exploratory outcomes, there was evidence for greater weight loss (moderate ES), improvements in 30 s STS performance (large ES) and changes in macronutrient intake (increased total protein intake, large ES; decrease saturated fat, moderate ES) in Pro-Ex v. controls after 12 weeks.
The finding that our home-based exercise program which was delivered and monitored remotely by EPs via telehealth using a smart-device exercise app was safe, is consistent with prior telehealth-based exercise studies conducted in older adults and those with chronic diseases(Reference Chiang, Shen and Chen37) including people with NAFLD(Reference Pfirrmann, Huber and Schattenberg12,Reference Motz, Faust and Dahmus38) . However, mean adherence to the thrice weekly muscle strengthening program in our study was modest (mean 52 %) when considering all fourteen participants initially randomised to the Pro-Ex group. It is difficult to explain these findings given participants were prescribed an individually tailored exercise program and received regular monitoring and support throughout the intervention via the TeleHab app and video conferencing with the study EP, both of which have been reported to enhance adherence in previous telehealth exercise interventions in older adults including those with NAFLD(Reference Pfirrmann, Huber and Schattenberg12,Reference Daly, Gianoudis and Hall39) . Furthermore, participants received weekly behavioural support messages and exercise reminders via text and were followed up via phone and/or email by the EP if two or more consecutive sessions were missed. It is possible that the modest adherence may relate to the fact that our participants were an older (mean age 63 years) high-risk cohort in which 93 % were overweight/obese and 43 % reported having further comorbidities (e.g. type 2 diabetes, CVD). Indeed, there is evidence that adherence to home-based exercise programs in people with chronic diseases is lower compared with those with fewer health conditions and who are non-obese(Reference Picorelli, Pereira and Pereira40). However, other telehealth-based exercise interventions conducted over 8–36 weeks in those with chronic diseases including NAFLD have reported significant heterogeneity in terms of adherence (range 1–84 %)(Reference Pfirrmann, Huber and Schattenberg12,Reference Motz, Faust and Dahmus38,Reference Tabak, Brusse-Keizer and van der Valk41) . This has been attributed to factors including the level of participant monitoring/ supervision and feedback provided, the mode of delivery (i.e. online web portal) not being adequately motivating for at home exercising and the length of intervention. It is worth highlighting that when we considered only participants who completed the 12-week exercise program (n 9), mean adherence to the muscle strengthening program was 73 %. This provides some evidence to support the feasibility of our home-based, exercise program delivered and monitored via telehealth for participants that were able to complete the study.
Another key outcome from our study was that overall adherence to the specific plant, animal and total protein recommendations was relatively modest with 14–42 % meeting our recommended targets. While this might be due in part to our relatively stringent (and high) predefined protein target levels (e.g. at least 80 % adherence), there are several other likely factors that may explain these findings. Although all participants did receive a one-on-one consultation with the study nutritionist upon study commencement, and mean attendance to group nutrition counselling sessions was 79 %, and participants were regularly monitored via protein checklists and food records, all elements which have been recognised to improve adherence during dietary interventions(Reference Tinker, Rosal and Young42,Reference Carels, Darby and Rydin43) , it would seem that this high risk cohort may have required more frequent and individualised support, especially during the second half of the intervention when adherence plateaued. In part support of this notion, prior interventions assessing Mediterranean style diets over 12-weeks, including in those with NAFLD, have reported significant dietary change with more frequent (weekly or fortnightly) dietary counselling sessions and/or regular contact via phone with the study nutritionist(Reference Properzi, O’Sullivan and Sherriff44,Reference Opie, O’Neil and Jacka45) . Despite the modest adherence to the daily/weekly protein serving targets in our study, we did observe some improvement in mean serves of plant and total plant protein, which is further reflected by Pro-Ex experiencing a net 35 g increase in protein intake and 8·2 g reduction in saturated fat relative to controls. While these findings provide some evidence that our dietary approach did elicit some changes in the type of protein consumed, it would appear participants found it difficult to transition from animal to predominately plant-based protein sources. At baseline, the mean intake of plant protein in Pro-Ex was relatively low (0·9 ± 1·1 serves/d) with only 14 % (n 2) of participants reporting consuming tofu/tempeh and 29 % (n 4) consuming > 1 serve of legumes per week, whereas the average intake of animal protein was 1·5 serves per day. There is some evidence from previous studies assessing adherence to Mediterranean style dietary patterns that dietary adherence is impacted by how different an intervention is from a participant’s usual intake(Reference Downer, Gea and Stampfer46,Reference Beunza, Toledo and Hu47) . This suggests that a more gradual transition may be needed when transitioning from an animal to a predominantly plant-based protein diet.
An interesting observation from our study was that although participants in Pro-Ex were not prescribed a hypocaloric diet, they did experience a mean 1·7 kg (95 % CI: −3·5, 0·2; Cohen’s d = –0·76) reduction in body weight after 12-weeks compared with controls. While these findings must be interpreted with some caution since weight was self-reported, this may relate to the finding that participants in Pro-Ex reduced their daily total energy intake by on average 1351 kJ/d, which was associated with a relative 21 % (40 g) reduction in total carbohydrate intake and a 16 % (13 g) increase in protein intake. High-protein (including plant protein) diets are known to increase satiety and the thermic effect of food(Reference Westerterp-Plantenga, Lemmens and Westerterp48,Reference Nielsen, Kristensen and Klingenberg49) and have a strong association with weight loss(Reference Hansen, Astrup and Sjödin50). While evidence regarding the effect of different protein sources for weight loss are inconclusive(Reference Basciani, Camajani and Contini51,Reference Sucher, Markova and Hornemann52) , a longitudinal study conducted in 1730 healthy adult men reported vegetable protein intake was inversely associated with overweight/obesity, while animal protein was positively related, suggesting protein sources may be important for weight regulation, independent of total energy intake(Reference Bujnowski, Xun and Daviglus53). Another potential factor that may have contributed to the weight loss in our study was that participants in Pro-Ex increased their weekly walking and moderate vigorous activity by 44 and 46 min, respectively, at 12 weeks relative to controls.
The finding that our 12-week intervention was also associated with an improvement in physical function (30 s STS performance) is likely due to the nature of the muscle strengthening program in which participants were prescribed exercises that targeted lower leg strength (e.g. squats, lunges). Our finding is consistent with previous research demonstrating resistance training is beneficial for improving physical function in community dwelling older adults(Reference Zhang, Zhang and Du54). More specifically, previous exercise interventions of 12 weeks have reported significant net improvements ranging from 2·5 to 3·0 relative to controls in the number of sit-to-stands completed in 30 s(Reference Pedersen, Vorup and Nistrup55–Reference Liao, Tsauo and Huang57), which is consistent with our finding (mean net 2·7 increase in the number completed in 30 s relative to controls, large effect size of Cohen’s d = 1·29). This likely represents a clinically meaningful change as there is some evidence that an improvement of 2·0–2·5 stands in an individual’s score represents a minimal detectable change to exceed measurement error(Reference Gill, Hely and Page58,Reference Zanini, Crisafulli and D’Andria59) .
Several limitations need to be considered when interpreting the study findings. First, dietary adherence was self-reported using protein checklists which are subject to recall error. Second, anthropometric measures (e.g. weight and waist circumference) were self-measured as all assessments were completed at the participant’s home. Third, this study was not designed nor powered to detect between group differences in weight, physical activity and physical function and thus these represent exploratory findings; however, data from this study can inform sample size calculations for future trials. Finally, the two male participants (93 % were female) limit the generalisability of our findings. Future research should incorporate a stronger focus on participant support and monitoring and further individualisation of dietary recommendations, to enhance adherence to such a dietary intervention.
Conclusion
This feasibility study indicates that in adults with NAFLD a lifestyle intervention encompassing a home-based muscle strengthening exercise program combined with recommendations for a higher protein intake from predominately plant-based sources when delivered via telehealth was safe, but adherence to the prescribed program was modest. This suggests that more intensive support from healthcare professionals may be required to elicit long-term changes related to exercise and diet for people with NAFLD. However, exploratory analysis provide some evidence that this telehealth-based approach may represent a viable model of healthcare service delivery to support weight management and improve dietary quality and physical function in adults with NAFLD. However, future large-scale, adequately powered and longer-term intervention trials including reinforcement sessions/consultations at regular intervals to enhance adherence (and a follow-up to evaluate any residual benefits) are needed to address these and related clinical outcomes to determine if it represents an effective approach for eliciting clinically meaningful improvement for this population.
Acknowledgements
C. L. F. was supported by a Deakin University Higher Degree by Research Scholarship (DUPR 0000018830). Deakin University had no role in the design, analysis or writing of the article.
C. L. F., E. S. G., S.-Y. T. and R. M. D. study design and question. C. L. F. conducted the research. C. L. F., G. A. and R. M. D. analysed the data. C. L. F. wrote the manuscript. C. L. F., E. S. G., S.-Y. T., G. A. and R. M. D. reviewed and edited the manuscript. R. M. D. is the guarantor of this work and takes responsibility for the integrity of the data and the accuracy of data analysis.
R. M. D. reports honoraria for educational presentations from Abbott Nutrition and Fresenius Kabi. S.-Y. T. is currently serving as First Editor of British Journal of Nutrition. All other authors declare no conflict of interest.







