Polycystic ovary syndrome (PCOS) is a common endocrine disease that is characterised by hyperandrogenism, ovulation disorder and ovarian polycystic change(Reference Goodarzi, Dumesic and Chazenbalk1). The global incidence rate of PCOS is approximately 5%–10%(Reference Belenkaia, Lazareva and Walker2), and this rate is even higher in China (5·61%)(Reference Jia, Gao and Yang3). Moreover, PCOS is known as the main cause of infertility and approximately 83·3% of PCOS patients suffers low fertility or infertility(Reference Goodarzi, Dumesic and Chazenbalk1). In addition, PCOS also affected patients’ long-term health, such as increasing the risk of obesity, CVD, depression and anxiety(Reference Macut, Bjekic-Macut and Rahelic4). At present, the aetiology of PCOS is not clear, but genetic, environmental and dietary factors may play a certain role in the pathogenesis of PCOS. Studies have reported associations between increasing PCOS risk and dietary factors, which might a cost-effective method for PCOS prevention(Reference Moran, Ko and Misso5,Reference Barrea, Arnone and Annunziata6) .
n-3 PUFA may exert beneficial effects on PCOS, including enhancement of endothelial function, anti-obesity effects, glycaemic and hormonal homoeostasis and anti-inflammatory effects(Reference Salek, Clark and Taghizadeh7). Previous studies have demonstrated that a specific diet containing n-3 PUFA, especially long-chain PUFA, significantly improved the clinical manifestations of dyslipidaemia, vascular endothelial dysfunction and insulin resistance in patients with PCOS(Reference Faghfoori, Fazelian and Shadnoush8,Reference Yang, Zeng and Bao9) . Komal et al.(Reference Komal, Khan and Imran10) reported that fish oil, as a source of EPA and DHA, reduced body weight and metabolic anomalies in an animal model. One meta-analysis involving 591 PCOS patients from nine randomised controlled trials indicated that supplementation with n-3 PUFA may improve the insulin resistance index and adiponectin level as well as decrease total cholesterol and total TGA levels(Reference Yang, Zeng and Bao9). Furthermore, another family of lipid mediators produced from EPA and DHA, including D- and E-series resolvins, protectins and maresins, had potent anti-inflammatory and inflammation-resolving properties(Reference Miles and Calder11), thus affecting the expression level of transcription factors related to inflammatory reactions in cells(Reference Szczuko, Kikut and Komorniak12).
There are several potential mechanisms of these healthy effects. First, n-3 PUFA enhance endothelial functions by decreasing arterial plaque build-up, increasing anti-inflammatory properties and enhancing endothelial-dependent vasodilation(Reference Salek, Clark and Taghizadeh7). Second, n-3 PUFA, especially DHA, have been shown to upregulate the mRNA levels of adipocyte protein 2, CCAAT/enhancer-binding proteins and PPAR γ, and these two different families of transcription factors could regulate adipose biosynthesis(Reference Faghfoori, Fazelian and Shadnoush8). Third, n-3 PUFA may exert beneficial effects on insulin sensitivity by attenuating endoplasmic reticulum stress, increasing β-oxidation of mitochondrial fatty acids and mitochondrial uncoupling, reducing lipid deposits and reactive oxygen species production and improving insulin sensitivity(Reference Salek, Clark and Taghizadeh7). Finally, EPA and DHA influence the process of inflammation, including reducing the formation of TNF-α, IL-6 and IL-1β in response to lipopolysaccharide exposure as well as downregulating the expression of adhesion molecules on the surface of monocytes and endothelial cells(Reference Salek, Clark and Taghizadeh7).
The metabolic effects of PUFA in PCOS pathogenesis are controversial. A previous randomised controlled trial has shown that PUFA-modulated hormonal and lipid profiles and supplementation with long-chain n-3 PUFA further improved androgenic profiles among patients with PCOS(Reference Phelan, O‘Connor and Kyaw Tun13), supporting the importance of n-3 PUFA supplementation among PCOS patients. In contrast, Roche et al.(Reference Roche and Gibney14) found null associations between the supplementation of long-chain n-3 PUFA and fasting or postprandial TAG concentrations in PCOS patients. They also found that a similar dose of long-chain n-3 PUFA reduced the concentration of TAG in non-PCOS women, indicating that some benefits of n-3 PUFA against PCOS need to be further confirmed(Reference Roche and Gibney14).
The amounts of n-3 PUFA in the diet might be significantly lower than those in supplements; however, no cohort or case–control study has directly assessed the associations between dietary and serum n-3 PUFA and PCOS. Therefore, the purpose of this study was to explore the relationship between n-3 PUFA from a normal diet and from serum phospholipids and PCOS prevalence among Chinese women to provide evidence for the strategic relevance of nutritional assessment in the management of patients with PCOS. We hypothesised that high dietary or serum n-3 PUFA may be inversely associated with PCOS prevalence.
Materials and Methods
Study participants
The present study is a 1:1 matched case–control study exploring potential lifestyle factors associated with the prevalence of PCOS. Patients who suffered from PCOS were consecutively recruited from the Department of Gynaecology, the First Affiliated Hospital of Chengdu Medical College, China, from 15 July 2016 to 28 July 2019. The diagnosis of PCOS was based on the diagnostic criteria from the Rotterdam conference in 2003 and required two out of three following parameters to be met: (i) oligo- and/or chronic anovulation, which is defined as less than eight cycles per year or a prolonged cycle of more than 36 days; (ii) clinical and/or biochemical hyperandrogenism, which is characterised by the elevation of testosterone level, the presence of acne and/or hirsutism with a modified Ferriman–Gallway score (≥ 8) and (iii) ultrasound appearance of polycystic ovaries with presence of ≥ 12 follicles per ovary with 2–9 mm diameter and increased ovarian volume > 10 ml. Of note, potential eligible cases should be diagnosed with the exclusion of other aetiologies (Cushing’s syndrome, congenital adrenal hyperplasia and androgen-secreting tumours). Cases were incident PCOS patients diagnosed within the past 3 months to minimise the effects of recall bias and lifestyle changes secondary to PCOS. The exclusion criteria for the patients were as follows: (i) suffered diseases, such as congenital adrenal hyperplasia, androgen-secreting tumour, Cushing‘s syndrome, thyroid dysfunction, gonadotropin regression, premature ovarian failure or hyperprolactinaemia; (ii) had significant changes in dietary habits in the past year or (iii) had mental or other diseases that might affect the accuracy of the answers to the questionnaire. In addition, women with a daily energy intake <600 or >3500 kcal/d were also excluded(Reference Nimptsch, Zhang and Cassidy15). Finally, 351 eligible cases were identified and 325 patients (response rate: 92·6%) were successfully interviewed for dietary habits and biological sample collection.
Healthy controls with regular menstruation and no clinical symptoms or biochemical hyperandrogenism were mainly recruited from the Department of Physical Examination in the same hospital during the same periods. Each control was individually matched to a case by 3-year age groups. The same selection criteria for cases were applied as for controls, except suffering PCOS. In total, 367 controls were identified and 325 participants were successfully interviewed, yielding a participation rate of 88·6%.
For both cases and controls, no significant difference was found between response and nonresponse participants for demographic characteristics and parameters, including age, BMI, waist:hip ratio, education, blood pressure, fasting glucose and phenotypes (all P > 0·05, data not shown).
All participants signed informed written consent forms before the interview. Ethical approval was obtained from the Ethical Committee of the First Affiliated Hospital of Chengdu Medical College (2016CYFYHEC025).
Data collection
All participants completed a computer-assisted, face-to-face interview with experienced nurses using a structured questionnaire at the first visit to the hospital. The following information was collected: (i) socio-demographic characteristics, such as age and education; (ii) lifestyle habits, such as smoking status, alcohol drinking status, physical activities and dietary intakes; (iii) history of chronic diseases and (iv) use of supplements. In addition, 10 ml of overnight fasting peripheral blood was collected from all subjects. All samples were centrifuged at 3000 rpm at 4°C for 10 min and then stored at −80°C. For each case, height, weight, waist circumference, hip circumference, blood pressure, fasting glucose, fasting insulin, total testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), sex hormone-binding globulin (SHBG), high-sensitivity C-reactive protein (hs-CRP) levels and PCOS phenotypes were extracted from the medical records. For controls, if there was missing information on their biomarkers on total testosterone, FSH, LH, SHBG and hs-CRP levels, we added these items to the physical examination programme. The related indexes were calculated using the following equations: BMI = body mass (kg) / height 2 (m2); WHR = waist circumference (cm) / hip circumference (cm).
Assessment of dietary intake
The quantitative FFQ used in this study was adapted from the 2002 China National Nutrition and Health Survey, which was designed to capture the normal intake of nutrients and major foods from Chinese individuals(Reference Li, Rao and Kong16). A total of 102 items were included in the FFQ based on the dietary habits of the Sichuan population. The main foods included cereals, cereal products, beans, vegetables, fruits, fungi, algae, nuts, livestock meat, poultry, dairy products, eggs, fish, seafood and condiments. The participants were asked to report the frequency (none, daily, weekly, monthly and yearly) and portion size of consumption for each food item. The participants were interviewed to recall the food consumed over the past year before diagnosis (cases) or interview (controls). The daily energy, fatty acids and other nutrient intakes were estimated using data from the China Food Composition Table 2009(Reference Yang, Wang and Pan17). In this study, the intake of individual n-3 PUFA included ALA (18:3 n-3), EPA (20:5 n-3), DPA (22:5 n-3) and DHA (22:6 n-3).
A validation study of the FFQ used for the 2002 China National Nutrition and Health Survey has indicated that the correlation coefficients of food intakes between the weighed food method and the FFQ method range from 0·08 to 0·76(Reference Li, He and Zhai18). Moreover, the validity and reproducibility of the revised 102-item FFQ were tested using a 24-d diet record as a reference method. The Spearman correlation coefficients between the FFQ and 3-d dietary records among twenty-four local participants for different fatty acid intakes were moderate to good (r = 0·28 - 0·59).
Measurement of fatty acids in serum phospholipids
GC was used to obtain the concentrations of fatty acids in serum phospholipids according to a previously reported method(Reference Yaemsiri, Sen and Tinker19,Reference Mannisto, Pietinen and Virtanen20) . Serum individual fatty acid levels were determined by lipid extraction and subsequent GC analysis. The extraction and purification of serum total lipids were conducted by using the method from Bligh et al.(Reference Bligh and Dyer21). Fatty acid methyl esters were separated on an Agilent 6890A gas chromatograph (Agilent) and a capillary column (SP2380, 0·25 mm × 30 m, 0·25 μm film, Supelco). 1,2-Dinonadecanoyl-sn-glycero-3-phosphocholine (C19:0) was used as an internal standard. A total of twenty-eight fatty acids were quantified, and the percentage composition of fatty acid methyl esters normalised to 100% was calculated. The intra-assay CV were 7·8% for the total n-3 PUFA and 5·2%–9·7% for individual n-3 PUFA.
Statistical analysis
The distribution of the included continuous variables was evaluated by Q-Q plots. For continuous variables, Wilcoxon rank sum test was used to compare the differences among cases and control subjects when non-normal distribution was found; otherwise, independent t-test was used. Pearson’s χ 2 test was used to test for distribution differences in categorical variables (e.g. education, smoking and alcohol drinking status) between groups. Intakes of fatty acids and other foods or food groups were adjusted for total energy intake using the residual method(Reference Willett and Stampfer22). Categorisation of continuous variables is an acceptable method to simplify the analysis and interpretation of results, but dichotomisation has been widely criticised by statisticians due to the following drawbacks: the loss of information is small with several groups but is most severe with only two groups; the risk of misclassification due to the measurement error is high and comparison with studies using different cut-points is impossible(Reference Naggara, Raymond and Guilbert23). Therefore, three groups or more are preferred when categorisation of continuous variables is adopted(Reference Naggara, Raymond and Guilbert23). Given the potential insufficient sample size and the insufficient test power due to excessive grouping, energy-adjusted dietary and serum n-3 PUFA were categorised into tertiles based on distributions among controls with the lowest tertile as the referent group.
We estimated age-adjusted and multivariate OR and their 95% CI of PCOS with intakes or serum levels of total, long-chain and individual n-3 PUFA by using conditional logistic regression stratified on matched pairs. Long-chain n-3 PUFA referred to the sum of DPA, EPA and DHA, and total n-3 PUFA referred to the sum of ALA, DPA, EPA and DHA. Multivariate models were adjusted for age (continuous), BMI (continuous), WHR (continuous), age at menarche (continuous), education (middle school or below vs. high school vs. college or above), current smokers (yes vs. no), current alcohol drinkers (yes vs. no), use of fish oil supplements (yes vs. no), physical activity (< 1 vs. 1∼3 vs. >3 times/week), SBP (continuous), diastolic blood pressure (DBP; continuous), fasting glucose (continuous) and total energy intake (continuous), which were available for all participants included in our study. The P value for trend was calculated using the Wald χ 2 test and the median value of each dietary tertile was treated as a continuous variable. We calculated the OR and their 95% CI of PCOS prevalence per sd increase in dietary or serum levels of n-3 PUFA. We also examined possible modification of the association between dietary and serum n-3 PUFA and PCOS prevalence by conducting stratified analyses of different PCOS phenotypes. Likelihood ratio tests comparing models with multiplicative interactions were applied to assess effect modifications.
Spearman‘s rank correlation analysis was used to assess the correlations between dietary and serum n-3 PUFA and serum PCOS-related parameters.
All statistical analyses were conducted using SAS (version 9·4; SAS Institute Inc., Cary, NC, USA). The criterion for statistical significance of the associations was P ≤ 0·05 (two-tailed).
Results
The demographic characteristics and related parameters of the study subjects are presented in Table 1. Regarding the demographic characteristics, the cases tended to have higher WHR, SBP and DBP as well as higher percentages of those with higher education but less physical activity compared with the controls (all P < 0·05). The percentage of fish oil supplement users was marginally higher in the cases than in the controls (12·00% vs. 8·00%, P = 0·089), but this difference did not reach significance. No significant difference was observed between the case and control groups with regard to age, BMI, age at menarche, fasting glucose, insulin, total energy intake, percentage of current smokers and percentage of current alcohol drinkers (all P > 0·05). For PCOS-related parameters, the cases had significantly higher levels of total testosterone, LH and CRP but lower levels of FSH and SHBG (all P < 0·001). For PCOS phenotypes, 34·00%, 28·90%, 21·60% and 15·50% of PCOS patients suffered from polycystic ovarian morphology (PCOM) + hyperandrogenism + ovulatory dysfunction, PCOM + hyperandrogenism, PCOM + ovulatory dysfunction and hyperandrogenism + ovulatory dysfunction, respectively.
Table 1. Demographic characteristics and biochemical parameters in case and control groups
(Mean values and standard deviations; numbers and percentages; median and interquartile range)

Abbreviations: WHR = waist hip ratio; PCOM = polycystic ovarian morphology; HA = hyperandrogenism; OD = ovulatory dysfunction.
* P values were calculated by using independent t-test for normal distributed continuous variables.
† P values were calculated by using Wilcoxon rank sum test for non-normal distributed continuous variables.
‡ P values were calculated by using Pearson’s χ 2- test for categorical variables.
The dietary intake of total n-3 PUFA was similar between the cases (1·05 [0·53, 1·40] g/d) and the controls (1·10 [0·66, 1·69] g/d) (P = 0·221), but the intake of long-chain n-3 PUFA was significantly lower in the cases (37·50 [14·50, 65·80] mg/d) than in the controls (46·60 [21·80, 95·20] mg/d) (P < 0·001). For individual n-3 PUFA, except for a null association between dietary ALA and PCOS, the median values of other individual dietary n-3 PUFA were significantly lower in the cases than in the controls (all P < 0·05) (Table 2).
Table 2. Dietary and serum phospholipid n-3 PUFA among cases and control subjects
(Mean values and standard deviations; median values and 25th, 75th percentiles)
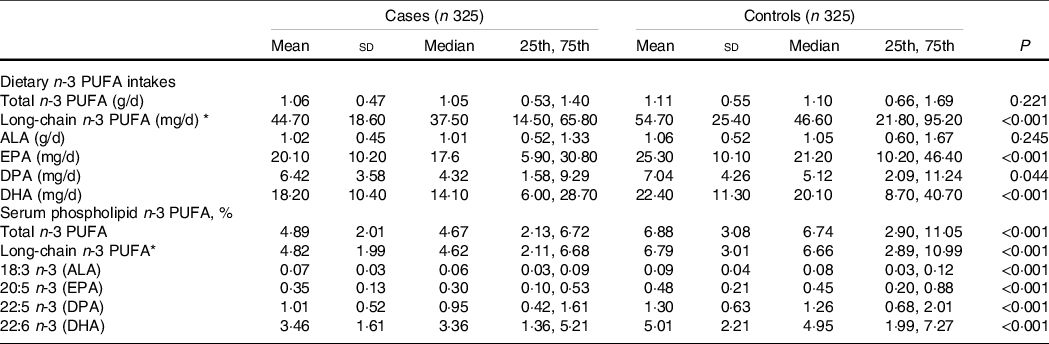
ALA, alpha-linolenic acid; DPA, docosapentaenoic acid.
Wilcoxon rank-sum test was used to compare the median levels between cases and controls.
* Long-chain n-3 PUFA = DPA+EPA+DHA.
Table 3 shows the associations between dietary n-3 PUFA and PCOS. For both age-adjusted and multivariate-adjusted models, higher dietary intakes of long-chain and individual (EPA and DHA) n-3 PUFA were all associated with a lower prevalence of PCOS. Compared with the lowest tertile (T1), the age-adjusted OR and their 95% CI of prevalence for PCOS in the highest tertile (T3) of n-3 PUFA levels were 0·62 (0·42, 0·91; P trend = 0·001) for dietary long-chain n-3 PUFA, 0·70 (0·48, 1·05; P Table 3 shows the associations between dietary n-3 PUFA and PCOS. For both age-adjusted and multivariate-adjusted models, higher dietary intakes of long-chain and individual (EPA and DHA) n-3 PUFA were all associated with a lower prevalence of PCOS. Compared with the lowest tertile (T1), the age-adjusted OR and their 95% CI of prevalence for PCOS in the highest tertile (T3) of n-3 PUFA levels were 0·62 (0·42, 0·91; P trend = 0·001) for dietary long-chain n-3 PUFA, 0·70 (0·48, 1·05; P trend = 0·049) for dietary EPA and 0·67 (0·45, 0·99; P trend = 0·035) for dietary DHA. After adjusting for potential confounders, including BMI, WHR, age at menarche, education, current smokers, current alcohol drinkers, use of fish oil supplements, physical activity, systolic blood pressure, DBP, fasting glucose and total energy intake, the OR and 95% CI were 0·61 (0·40, 0·92; P trend = 0·001), 0·68 (0·45, 1·04; P trend = 0·047) and 0·65 (0·41, 0·98; P trend = 0·030), respectively. Null associations were found between two individual n-3 PUFA (ALA and DPA) and PCOS. Similar findings were also found when using dietary n-3 PUFA per sd change.
Table 3. Association between dietary n-3 PUFA and polycystic ovary syndrome (PCOS)
(Odds ratios and 95% confidence intervals)
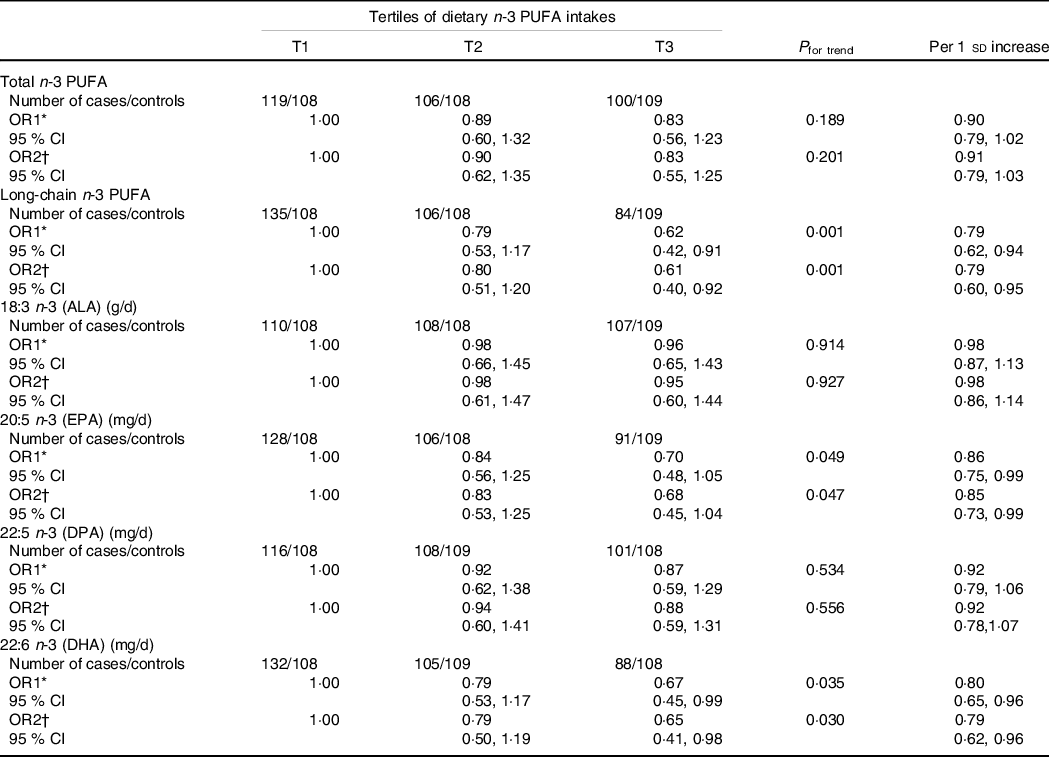
ALA, alpha-linolenic acid; DPA, docosapentaenoic acid.
With the median value of each tertile was treated as a continuous variable, the Wald χ 2 test was used to calculate P value for trend.
* OR1: adjusted for age;
† OR2: adjusted for age, BMI, WHR, age at menarche, education, current smokers, current alcohol drinkers, use of fish oil supplements, physical activity, SBP, DBP, fasting glucose and total energy intake.
The Spearman's rank correlation coefficients between dietary n-3 PUFA and serum PCOS-related parameters are shown in Table 4. Generally, dietary long-chain n-3 PUFA, EPA and DHA were negatively correlated with insulin, total testosterone and hs-CRP (Spearman correlation coefficients ranged from −0·201 to −0·115; all P < 0·05), whereas long-chain n-3 PUFA were positively correlated with FSH and SHBG (Spearman correlation coefficients = 0·116 and 0·114; both P < 0·05).
Table 4. Spearman‘s rank correlation coefficients between dietary and serum PUFA and polycystic ovary syndrome (PCOS)-related parameters among PCOS cases

FSH, follicle-stimulating hormone; LH, luteinising hormone; SHBG, hormone-binding globulin; hs-CRP, high-sensitivity C-reactive protein; ALA, alpha-linolenic acid; DPA, docosapentaenoic acid.
*P < 0·05, ** P < 0·01, *** P < 0·001.
With regard to the long-chain n-3 PUFA in serum phospholipids, both the medians of total and long-chain n-3 PUFA were significantly lower in the cases than in the controls (4·67 [2·13, 6·72] vs. 6·74 [2·90, 11·05] for total n-3 PUFA; 4·62 [2·11, 6·68] vs. 6·66 [2·89, 10·99] for long-chain n-3 PUFA; both P < 0·001). Consistently, the median values of all serum phospholipid n-3 PUFA levels were significantly lower in the cases than in the controls (all P < 0·001) (Table 2).
The associations between n-3 PUFA levels in serum phospholipids and PCOS are shown in Table 5. In the age-adjusted model, the total, long-chain and individual (EPA and DHA) n-3 PUFA in serum phospholipids were inversely associated with PCOS prevalence, whereas null associations were observed for ALA or DPA. Compared with the lowest tertile (T1), the significant age-adjusted OR and 95% CI for the highest tertile (T3) were 0·62 (0·42, 0·92; P trend = 0·003) for total n-3 PUFA, 0·58 (0·39, 0·86; P trend < 0·001) for long-chain n-3 PUFA, 0·71 (0·48, 1·05; P trend = 0·043) for EPA and 0·67 (0·45, 0·99; P trend = 0·021) for DHA, while the results for ALA and DPA were 0·93 (0·64, 1·35; P trend = 0·714) and 0·75 (0·51, 1·10; P trend = 0·127), respectively. When further adjusted for confounding factors (model 2), all of these significant associations persisted with multivariate-adjusted OR (95% CI) of 0·63 (0·40, 0·93; P trend = 0·005), 0·60 (0·38, 0·92; P trend = 0·001), 0·70 (0·45, 1·05; P trend = 0·046) and 0·68 (0·45, 1·01; P trend = 0·029), retrospectively. Notably, the inverse association for DPA became significant in model 2 (OR = 0·72 [95% CI 0·45, 1·08]; P trend = 0·048). Similar findings were observed when using serum n-3 PUFA per sd change. However, the association between serum ALA and PCOS did not reach significance (age-adjusted OR = 0·95, 95% CI 0·85, 1·09; fully adjusted OR = 0·96, 95% CI 0·85, 1·10).
Table 5. Association between serum n-3 PUFA intakes and polycystic ovary syndrome (PCOS)
(Odds ratios and 95% confidence intervals)
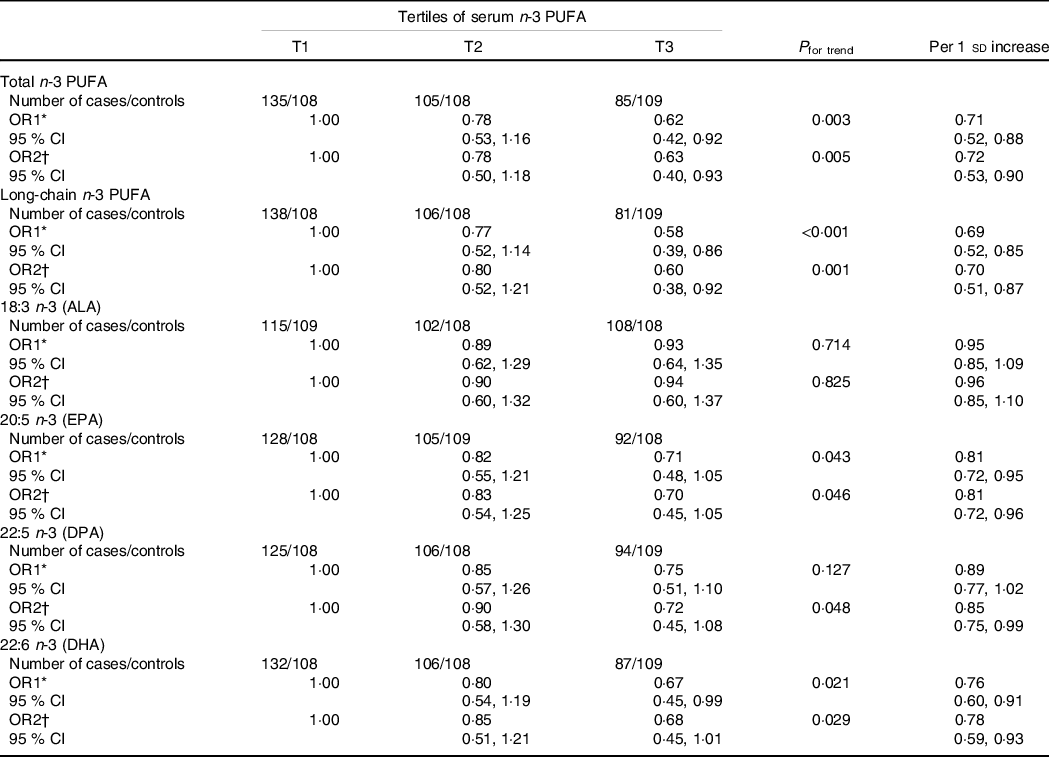
ALA, alpha-linolenic acid; DPA, docosapentaenoic acid.
With the median value of each tertile was treated as a continuous variable, the Wald χ 2 test was used to calculate P value for trend.
* OR1: adjusted for age;
† OR2: adjusted for age, BMI, WHR, age at menarche, education, current smokers, current alcohol drinkers, use of fish oil supplements, physical activity, SBP, DBP, fasting glucose and total energy intake.
With regard to the correlations between serum n-3 PUFA and PCOS-related parameters, we found that total, long-chain n-3 PUFA, EPA, DPA and DHA in serum phospholipids were negatively correlated with BMI, insulin, total testosterone, LH and hs-CRP (Spearman correlation coefficients ranged from −0·299 to −0·035) but positively correlated with FSH and SHBG (Spearman correlation coefficients ranged from 0·046 to 0·189) (Table 4).
For the correlations between dietary and serum n-3 PUFA, we noticed potentially positive correlations between energy-adjusted dietary intakes of three n-3 PUFA (EPA, DPA and DHA) and serum levels of these three PUFA (Spearman correlation coefficients ranged from 0·174 to 0·476; all P < 0·05), whereas no correlation was observed for dietary or serum ALA with other dietary or serum n-3 PUFA (Supplemental Table 1).
We further explored whether there are different associations among different PCOS phenotypes (Supplemental Table 2). The interaction effects between dietary or serum total, long-chain and individual n-3 PUFA did not reach significance among different PCOS phenotypes (P values ranged from 0·098 to 0·991).
Discussion
This 1:1 age-matched case–control study among Chinese women revealed inverse associations between n-3 PUFA from dietary and serum phospholipids, including certain long-chain n-3 PUFA with 20 or 22 carbons (EPA, DPA and DHA) and PCOS prevalence, and these associations were independent of PCOS phenotypes. Dietary and serum n-3 PUFA might have favourable effects on PCOS-related parameters (e.g. total testosterone, FSH, LH, SHBG and hs-CRP).
The present study aimed to provide population-based data on dietary and serum n-3 PUFA and their associations with PCOS. As expected, baseline potential risk factors, such as WHR, systolic blood pressure and DBP, were higher in the cases than in the controls, and the cases were more likely to have a higher percentage of those with higher education levels but less physical activity.
Data from previous studies have indicated that higher intakes or circulating levels of n-3 PUFA were associated with a lower risk of several chronic diseases, such as metabolic syndrome(Reference Zhang, Sun and Hu24), CVD(Reference Jain, Aggarwal and Zhang25) and cancers(Reference Serini and Calviello26). Our study is the first study to comprehensively show that dietary and serum n-3 PUFA, especially long-chain n-3 PUFA with 20 or 22 carbons (EPA, DPA and DHA), might have beneficial effects on PCOS.
A protective role for n-3 PUFA in PCOS aetiology is biologically plausible. At present, there are several hypotheses about the effects of n-3 PUFA on the pathogenesis of PCOS. The first hypothesis is that supplementation with n-3 PUFA, including EPA and DHA, has a certain impact on BMI in PCOS patients. Two independent clinical studies have shown that daily oral supplementation with a certain amount of n-3 PUFA for 6 months significantly reduced waist circumference and BMI among PCOS patients(Reference Oner and Muderris27,Reference Khani, Mardanian and Fesharaki28) . Obesity negatively impacts ovarian function due to its characteristics of tissue-specific chronic inflammation and oxidative stress(Reference Snider and Wood29). In the context of obesity, adipocyte hypertrophy leads to hypoxia-induced necrosis of adipocytes and infiltration of adipose tissue with circulating macrophages and T helper cells(Reference Ouchi, Parker and Lugus30). Increased levels of macrophages may induce the secretion of proinflammatory cytokines, such as TNF-α and ILs, which further produce more proinflammatory cytokines by activating the nuclear factor-κB (NF-κB) signal transduction pathway(Reference Snider and Wood29). In addition, adipokines, such as leptin and lipocalin, could also promote the release of TNF-α and IL-6, and the secretion of both cytokines and adipokines induces an inflammatory response in the ovary(Reference Snider and Wood29,Reference Nteeba, Ortinau and Perfield31,Reference Wang and Huang32) . The second hypothesis is that n-3 PUFA have beneficial effects on insulin resistance in patients with PCOS. Studies have shown that n-3 PUFA significantly alleviated insulin resistance(Reference Lepretti, Martucciello and Burgos Aceves33). n-3 PUFA could reduce the endoplasmic reticulum stress caused by metabolic abnormalities in PCOS patients, reduce lipid deposition in cells, reduce reactive oxygen species levels in cells and improve insulin sensitivity to a certain extent(Reference Kalupahana, Claycombe and Moustaid-Moussa34). IGF-I is synthesized by the ovary and the IGF-I receptor, a tyrosine kinase, shares considerable structural and functional homology with the insulin receptor(Reference Czech35). Although the affinity of the IGF-I receptor for insulin is less than that for IGF-I, the hybrid heterotetramers assembled by α and β dimers of insulin and IGF-F receptor can bind insulin and IGF-I with similar affinity and further mediate several insulin actions on the ovary(Reference LeRoith, Werner and Beitner-Johnson36). Insulin, a reproductive and metabolic hormone, could not only modulate ovarian steroidogenesis but also regulate the production of SHBG(Reference Diamanti-Kandarakis and Dunaif37). Therefore, reducing insulin resistance may be beneficial for restoring ovulatory menstrual cycles(Reference Diamanti-Kandarakis and Dunaif37). The third hypothesis is that n-3 PUFA are beneficial due to their anti-inflammatory and inflammation-resolving actions. n-3 PUFA could significantly reduce the expression of inflammatory factors. EPA and DHA decreased the expression levels of TNF-α and IL-6 induced by lipopolysaccharide(Reference Mildenberger, Johansson and Sergin38,Reference Calder39) . In addition, DHA also significantly reduced the expression levels of vascular endothelial adhesion factor-1 and vascular cell adhesion factor, which indicates that n-3 PUFA may have a potential mediation mechanism on vascular endothelial injury caused by inflammatory factors. In addition, DHA inhibited the expression of TNF-α and IL-6 by inhibiting the activity of the NF-κB signalling pathway, which was accompanied by an increase in PPAR-γ expression levels(Reference Buoite Stella, Gortan Cappellari and Barazzoni40). These results show that n-3 PUFA have a significant protective effects on the abnormal expression of inflammatory factors in patients with PCOS. Finally, n-3 PUFA may also have effects on the hormone levels of PCOS patients. n-3 PUFA inhibited the arachidonic acid-induced activation of acute regulatory protein factor and also regulate the expression of LH(Reference Szczuko, Kikut and Komorniak12). In addition, it can regulate testosterone activity in PCOS patients, inhibit testosterone production and produce intervention effects.
A previous meta-analysis involving 591 PCOS patients from nine randomised controlled trials has indicated that supplementation with n-3 PUFA improved insulin resistance and reduces total cholesterol, TAG and LDL-cholesterol, but it reported null associations between n-3 PUFA and the levels of BMI, HDL-cholesterol, total testosterone, FSH, LH and SHGB.(Reference Yang, Zeng and Bao9) The reductions in TAG might be attributed to the increased clearance, reduced synthesis and enhanced degradation of fatty acids in the liver due to the intakes of EPA and DHA(Reference Jacobson, Glickstein and Rowe41). n-3 PUFA could not only upregulate the activity of lipoprotein lipases and then facilitate chylomicron TAG clearance but also decrease VLDL synthesis and secretion, which increased plasma lipoprotein lipase activity(Reference Backes, Anzalone and Hilleman42). However, our findings showed that when the levels of certain long-chain n-3 PUFA (EPA and DHA) increased, the levels of total testosterone and hs-CRP decreased, whereas the levels of FSH and SHBG increased. hs-CRP is a biochemical marker produced in response to stimulation by IL, such as IL-6(Reference Erlinger, Platz and Rifai43), which can be downregulated by EPA and DHA(Reference Mildenberger, Johansson and Sergin38,Reference Calder39) . With regard to serum n-3 PUFA, we found that the levels of LH decreased as the levels of total, certain long-chain n-3 PUFA, EPA, DPA and DHA increased, while the levels of FSH and SHBG increased when all the serum n-3 PUFA mentioned above increased, except for ALA. PUFA may induce elevated serum FSH due to the upregulated expression of cytochrome P450 lanosterol 14a-demethylase (CYP51), a critical enzyme that converts lanosterol into cholesterol, in granulosa cells(Reference Liu, Tian and Ding44,Reference Ma, Weng and Hu45) .
The inconsistent results between our study and previous trials might be partly attributed to differences in long-chain n-3 PUFA concentrations in the dietary and serum phospholipids(Reference Yang, Zeng and Bao9), the amount of n-3 PUFA between observational studies and trials and genetic factors of different races. Our results indicated that there were differences in the associations between n-3 PUFA and PCOS in dietary and serum phospholipids. The potential reasons might be as follows: (1) the individual’s molecular response to different sources of n-3 PUFA might be greatly influenced by genetic factors; (2) epigenetic modifications due to the study types may also obscure the understanding of the clinical benefits of n-3 PUFA; (3) the bioavailability and clinical efficacy of dietary n-3 PUFA are greatly influenced by gastrointestinal digestion and the absorption process of foods and (4) there might be bias of measurement (underestimation of intake) when collecting dietary data using the FFQ, which might further lead to an underestimation of the strength of associations.
The amount of n-3 PUFA intake was 1·06 ± 0·47 g/d for the cases and 1·11 ± 0·55 g/d for the controls, and the weight percentage values of serum n-3 PUFA levels were 4·89 ± 2·01 for the cases and 6·88 ± 3·08 for the controls. The amounts were consistent with other reports conducted among Chinese individuals(Reference Zhang, Sun and Hu24,Reference Zhong, Fang and Pan46) , which showed that n-3 PUFA remain low in Chinese individuals. According to estimates from a global survey of n-3 PUFA blood levels, < 20% of the world population consumes the recommended daily n-3 PUFA doses(Reference Stark, Van Elswyk and Higgins47). Considering the potential benefits of n-3 PUFA, more efforts should be made to increase the intake of n-3 PUFA.
This study has the following strengths: (1) this was the first study to examine the associations between dietary and serum n-3 PUFA and PCOS among the general population with usual n-3 PUFA intake; (2) a wide range of potential confounders have been classified as dietary and nondietary factors and have been adjusted in different models and (3) a relatively large number of participants allowed these associations to be investigated with adequate testing power.
We should acknowledge that the present study has also some limitations. First, the major drawback of this study was that the study design was a retrospective case–control study. Thus, this study may not infer the time sequence of the impact of exposure factors on PCOS. Second, recall bias was also inevitable when participants were asked to recall their eating habits or other information over the past year because it was a case–control study. To minimise recall bias, incident cases were interviewed at the time of diagnosis. In addition, food models and pictures were used to assist participants in estimating their dietary intake. Third, selection bias was also unavoidable because this was a hospital-based case–control study. Fourth, residual confounding remained even though various dietary and nondietary confounders were adjusted in the multivariable models. Finally, there may have been measurement errors for serum fatty acids. However, the moderate-to-good correlation between dietary intakes and serum levels, as well as lower inter-assay CV, showed that the measurement of fatty acids was relatively accurate.
In conclusion, this study indicated that both higher levels of dietary and serum n-3 PUFA, especially long-chain n-3 PUFA (EPA, DPA and DHA), might be associated with lower PCOS prevalence among Chinese women independent of PCOS phenotypes. The findings suggested negative associations between n-3 PUFA and PCOS. Further cohort studies with larger sample sizes and studies conducted among various populations with different n-3 PUFA intakes should be performed to verify the present findings.
Acknowledgements
The authors acknowledge all the participants and administrators of this study.
This work was supported by General Project of Natural Science Foundation of Xinjiang Uygur Autonomous Region (No: 2018D01C256) and Talent Research Startup Fund of Guangdong Second People's Hospital.
Formulating the research question(s): L. L.; designing the study: L. L., X. Q. L., L. Lv.; carrying out the study: X. Q. L, L. Lv; analysing the data: Y. X., B. H. W., C. L. H.; interpreting the findings: Y. X., C. L. H.; writing the article: L. L., X. Q. L., B. H. W.
There are no conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114521003007







