Inclusion of plant protein ingredients in commercial fish feed has rapidly increased over the last decade, with consequent changes in amino acid composition that possibly affect metabolic pathways, growth and deposition in fish(Reference Espe, Lemme and Petri1–Reference Rathore, Liaset and Hevroy4). Arginine and lysine are indispensable amino acids in fish, and antagonistic effects on uptake between the two amino acids have been reported in mammals and fish(Reference Fico, Hassan and Milner5–Reference Zhou, Shao and Xiao7). Traditional fishmeal diets have sufficient amounts of both amino acids, while plant protein-based diets often require supplementation, and optimum levels and ratios between the two amino acids, therefore, have to be investigated.
Besides the need for protein synthesis, arginine is essential for a range of metabolic pathways, such as the production of polyamines, urea, NO and creatine, and fish probably have arginine requirement beyond the maximum requirement for growth(Reference Wu, Bazer and Davis8). Arginine is the precursor for ornithine, by the enzyme arginase, which again is the only precursor for the polyamines putrescine, spermidine and spermine. Ornithine decarboxylase (ODC), the rate-limiting enzyme of polyamine synthesis, converts ornithine to putrescine, which again can be converted to spermidine and spermine, a process consuming decarboxylated S-adenosylmethionine (SAM)(Reference Pegg9), which is synthesised from methionine in an ATP-dependent process(Reference Mato, Corrales and Lu10). Polyamines are small organic molecules present in all eukaryotic cells(Reference Janne, Alhonen and Pietila11) that are positively charged at the physiological pH, allowing them to bind to the DNA and RNA, affecting transcription and translation(Reference Pegg9). Through such interactions, polyamines are capable of modulating a range of cellular mechanisms that are essential for cell growth and differentiation as well as for mitochondrial function and integrity(Reference Wu, Bazer and Davis8, Reference Flynn, Bird and Guthrie12). The enzyme spermidine/spermine-(N1)-acetyltransferase (SSAT) acetylates the polyamines, consuming acetyl-CoA, enabling them to be transported out of the cell or to be oxidised to a shorter polyamine. The turnover of polyamines then might deplete the acetyl-CoA pools in the cells and thus has the capacity to decrease malonyl-CoA concentration, releasing its inhibitory effect on carnitine palmitoyl transferase-1 (CPT-1), the rate-limiting enzyme in the oxidation of the long-chain fatty acids in the mitochondria(Reference Pirinen, Kuulasmaa and Pietila13). Transgenic mice having an increased SSAT activity were characterised by increased putrescine and decreased spermine and spermidine pools and chronic oxidative stress, as well as being significantly leaner when compared with wild-type mice(Reference Pirinen, Gylling and Itkonen14, Reference Cerrada-Gimenez, Pietila and Loimas15). Mice overexpressing ODC or decarboxylated S-adenosylmethionine decarboxylase (SAMdc), however, did not have altered polyamine metabolism(Reference Heljasvaara, Veress and Halmekyto16), highlighting the importance of SSAT regulation for polyamine metabolism.
l-Arginine supplementation is known to reduce adiposity in genetically diet-induced obese rats and obese humans with diabetes mellitus type-2(Reference Jobgen, Meininger and Jobgen17, Reference McKnight, Satterfield and Jobgen18) as well as to increase protein accretion in pig muscle(Reference Yao, Yin and Chu19, Reference Tan, Yin and Liu20). Tan et al. (Reference Tan, Yin and Liu20, Reference Tan, Yin and Liu21) showed that arginine stimulated lipogenesis in the muscle and lipolysis in the adipose tissue in pigs by differentially regulating the expression of genes in these tissues, which resulted in increased muscle mass and an improved metabolic profile. Arginine supplementation has also been shown to enhance fatty acid oxidation via the production of NO, through an increased expression of lipolytic proteins(Reference McKnight, Satterfield and Jobgen18). The mechanisms underlying the lipid-reducing effect of l-arginine supplementation are complex, but seem to involve those in favour of fat loss and/or that reduce the adipocyte mass as well as stimulate mitochondrial biogenesis and brown adipose tissue development in rodent models. Growing evidence that arginine supplementation promotes the oxidation of long-chain fatty acids and glucose, concomitantly decreasing de novo synthesis of glucose and TAG, has been presented(Reference Wu, Bazer and Davis8, Reference Jobgen, Meininger and Jobgen17). In humans, excess body fat is mainly stored as TAG in abdominal white adipose tissue (WAT), while Atlantic salmon store fat both in the viscera, liver, and in the white muscle(Reference Valente, Linares and Villanueva22, Reference Espe, Ruohonen and El-Mowafi23). Thus, arginine supplementation has the potential to improve the metabolic profile and health status of Atlantic salmon by promoting skeletal muscle growth and viscera mass reduction.
The aim of the present study was to assess whether arginine supplementation in a plant protein-based diet would affect the type of growth and polyamine metabolism and alter the energy status of juvenile Atlantic salmon.
Materials and methods
Diets
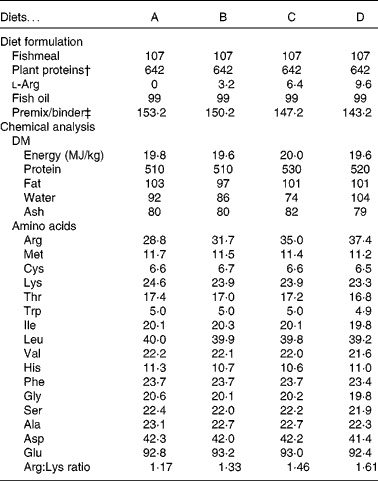
The diets contained a high inclusion of plant protein ingredients and a marine fat source. The four experimental diets were prepared to contain increasing concentrations of arginine, from diet A (low arginine) to diet D (high arginine). Lysine was supplemented at levels just below the expected requirement(Reference Espe, Lemme and Petri2) to avoid lysine interactions. Compositions of the diets are listed in Table 1. All other amino acids remained constant among the diets (Table 1). The diets were extruded and pellet size was 1·5 mm. All diets were produced by EWOS Innovation. Protein, fat and energy contents were equal in all the diets.
Table 1 Diet formulation and chemical analysis of the diets*

* All values are listed as g/kg diet, unless stated otherwise.
† Plant protein blend: soya concentrate:wheat gluten:pea protein concentrate:maize gluten:sunflower meal (0·42:0·13:0·12:0·16:0·17).
‡ Premix contained minerals and vitamins to fulfil the requirement of Atlantic salmon(39).
Feeding trial
The experiment complied with the guidelines of the Norwegian Regulation on Animal Experimentation and European Community Directive 86/609/EEC. The feeding trial was carried out in Dirdal, Norway, from December 2011 to February 2012. Each diet was randomly assigned to four replicate tanks, with each containing 140 fish with an initial weight of 5 g. A total of sixteen glass fibre tanks (0·6 m × 0·6 m) were used, with each being filled with 165 litres of fresh water. The fish were acclimatised in the experimental tanks for 2 weeks with a commercial diet before the commencement of the study. Bulk weights were measured at the commencement of the trial, every second week after switching to the experimental diets and at the end after 8 weeks with their respective diets. For the first 2 weeks, the fish were fed the experimental diets ad libitum, and later they were fed a restrictive diet, 95 % of the requirement as set by the Norwegian feed tables. Continuous light was applied, and the fish were fed continuously using automatic feeders throughout the day and night (repeatedly fed for 0·33 min and then held for 7·25 min). The mean water temperature throughout the experiment was 12·7 (sem 1·0)°C.
Sampling
From each tank, pooled samples from ten fish were bulk weighed for total body weight, liver weight and gastrointestinal weight. Liver weight and gastrointestinal weight were used to calculate the hepatosomatic and visceralsomatic indices (percentage of body weight). Pooled samples of an additional ten fish from each tank were collected and frozen on ice for later chemical analysis. Samples of liver, WAT and muscle were sampled from at least three fish from each tank, flash frozen on liquid N2 and stored at − 80°C until analysis. Heparinised blood was drawn from the caudal vein of an additional ten fish and centrifuged and pooled plasma from each tank was stored at − 80°C until analysis.
Chemical analyses
Energy, fat and protein contents of the feeds were determined as described by Espe et al. (Reference Espe, Lemme and Petri1).
Total amino acid composition in the diets and whole fish was determined after hydrolysis for 22 h at 110 °C in 6 m-HCl containing 3·125 mm-Norvaline (internal standard) and 3 mm-dithiothreitol (to protect the sulphur amino acids against oxidation) and pre-column derivatised with AccQTag™ at 55 °C as described by Waters. The amino acids were separated on an ultra-high performance liquid chromatrography (UPLC) system (Waters Aquity UPLC BEH C18 column with an internal diameter of 1·7 μm at a flow rate of 0·7 ml/min using the gradient offered by the supplier). The concentration of the amino acids was calculated using external standards supplied by Sigma. Tryptophan was determined after basic hydrolysis with Ba(OH)2 as described by Liaset et al. (Reference Liaset, Julshamn and Espe24). Total arginine gain and lysine gain were then calculated as the difference in each amino acid, in g, between the start and the end of the trial. Free amino acids were determined in the pooled samples of plasma, liver and muscle on a Biochrom 20 plus amino acid bioanalyzer (Amersham Pharmacia Biotech) using post-column derivatisation with ninhydrin as described by Espe et al. (Reference Espe, Lemme and Petri1).
Metabolites
Polyamines were analysed using HPLC and calculated with Empower (Waters). Extraction was done from 1 g of tissue in a total of 5 ml of 0·4 m-perchloric acid with an internal standard as described by Liaset & Espe(Reference Liaset and Espe25). SAM and S-adenosylhomocysteine (SAH) were determined using reverse-phase HPLC after deproteinisation in 0·4 m-perchloric acid as described by Wang et al. (Reference Wang, Kramer and Yang26). The concentrations of SAM and SAH were quantified using standards of the respective metabolites (Sigma-Aldrich). Liver samples were deproteinised in perchloric acid using a deproteinising sample preparation kit, according to the manufacturer's instructions. ATP and acetyl-CoA were measured in deproteinised liver samples using an ATP fluorimetric assay kit and a PicoProbe Acetyl-CoA Assay kit, respectively, following the manufacturer's protocol (all kits from BioVision).
Spermidine/spermine-(N1)-acetyltransferase abundance and activity
Western blotting was used to investigate the abundance of SSAT in the liver. Collection samples from each tank were homogenised in four volumes of 10 mm-Tris buffer (pH 7·6) with 1 mm-EDTA and 0·25 m-sucrose and spun at 8000 g for 40 min at 4°C, and the collected supernatant was stored at − 80°C until used. Proteins were separated on SDS gels (12 %), Western blotted on a polyvinylidene difluoride (PVDF) membrane and detected by Chemiluminescence Image Capture as described by Espe & Holen(Reference Espe and Holen27). The gels were simultaneously stained for SSAT (anti-SAT1, ab54047; Abcam) and β-actin (no. 4967; BioNordica/Cell Signaling), and the relative quantities of SSAT compared with β-actin were calculated. The values obtained in the fish fed diet A were set to 100, and those of fish fed the other diets were calculated relative to this. The enzyme activity of SSAT was determined using Ellman's reagent, measuring the amount of the product CoA-SH produced per min by SSAT as described by Lin et al. (Reference Lin, Lien and Hsu28).
Gene expression
RNA and complementary DNA were obtained from three fish from each tank, and the means obtained for each tank were used to calculate the relative values. RNA was extracted from the liver samples using the EZ1 BioRobot and the RNA Universal Tissue Kit (Qiagen), according to the manufacturer's instructions. RNA extraction from the WAT was carried out manually with TRIzol. All RNA samples were DNase treated to remove any contaminating DNA. Total RNA concentration was determined at 260 nm with the NanoDrop ND-1000 UV–Vis spectrophotometer (NanoDrop Technologies), and quality was assessed with the Agilent 2100 Bioanalyzer (Agilent Technologies). For the liver and WAT samples, the RNA integrity numbers (RIN) were 9·87 and 6·48 (sem 0·10 and 0·15), respectively. A two-step quantitative PCR was run as described by Torstensen et al. (Reference Torstensen, Espe and Stubhaug29). Briefly, 500 ng of RNA were reverse-transcribed into complementary DNA, with duplicates of each sample, using a TaqMan® Gold RT-PCR Kit (Applied Biosystems), with the following protocol: 25°C for 10 min; 60°C for 48 min; 95°C for 5 min, and stored at − 20°C. Gene expression was quantified with quantitative PCR on the Lightcycler 480 (Roche Applied Sciences), on the following programme: 95°C for 5 min; forty-five cycles of 95°C for 10 s; 60°C for 20 s; 72°C for 30 s, followed by a melt curve analysis. The quantitative PCR primers were synthesised by Invitrogen after a BLAST search, which are listed in Table 2. To confirm the specificity of the primers, Sanger DNA sequencing (Applied Biosystems 3730XL Analyzer) was performed on purified PCR products after preparation using Big Dye v.3.1, according to the manufacturer's instructions. Relative quantities were calculated against the reference genes β-actin and elongation factor 1Ab, verified in Atlantic salmon(Reference Olsvik, Lie and Jordal30).
Table 2 Primer sequences, accession numbers and sizes of the products

SSAT, spermidine/spermine-(N1)-acetyltransferase; ODC, ornithine decarboxylase; SAMdc, S-adenosylmethionine decarboxylase; CPT-1, carnitine palmitoyl transferase-1; EF1Ab, elongation factor 1Ab.
Statistical analyses
All statistical calculations were performed in Statistica 11 (StatSoft, Inc.) using a one-way ANOVA, followed by Tukey's post hoc test or, when applicable, an unequal N post hoc test. Average tank values were used as the statistical unit. Levene's test was applied to test for homogeneity in variances between the groups. P values less than 0·05 were accepted as statistically different. Regression analyses were performed in Statistica 11, determining bivariate correlations, with arginine concentration in the diet as a continuous variable.
Results
Growth
The fish fed the highest arginine diet, diet D, grew better and displayed a higher weight gain than the fish fed diet B (Table 3). Diet D also resulted in higher protein gain and fat gain compared with diet B. However, neither of these values correlated with arginine concentration in the diets, as the regression analysis gave R values of 0·23, 0·18 and 0·22 for growth, protein gain and fat gain, respectively. The hepatosomatic and visceralsomatic indices were similar between the dietary groups. Overall, all the parameters measured, including weight gain, protein and fat deposition and specific growth rate, were lower in the fish fed diet B than in those fed diet A, while the regression analysis showed a correlation between these parameters and increasing dietary arginine in the fish fed diet B to those fed diet D only.
Table 3 Growth, protein gain and lipid gain, as well as the mean total gain of the amino acids arginine (Arg) and lysine (Lys) in juvenile salmon fed the experimental diets for 8 weeks (Mean values with their standard errors, n 4)

IBW, initial body weight; EBW, end body weight; HSI, hepatosomatic index; VSI, visceralsomatic index.
a,bMean values with unlike superscript letters were significantly different (P< 0·05).
* One-way ANOVA with Tukey's post hoc test was used.
Polyamines
In the liver, putrescine concentrations increased with increasing arginine concentrations in the feed (P= 0·01; Table 4), correlating with arginine concentration in the diets (R 0·69). Spermidine and spermine concentrations in the liver remained unchanged between the dietary groups. In the WAT, there were no observed differences in either of the polyamines. The fish fed diet D, containing the highest dose of arginine, had higher concentrations of spermine in the muscle compared with the fish fed diet B, while putrescine and spermidine concentrations were unaffected. The products of SSAT, acetylated spermidine and acetylated spermine, were not detected by the method. Other polyamines, such as cadaverine, tyramine and phenylethylamine, were also measured by the method, but either they were found at levels below the detection limit or there were no differences observed.
Table 4 Polyamine concentrations in the liver, white adipose tissue (WAT) and muscle (mg/kg) of juvenile salmon (Mean values with their standard errors, n 4)

a,bMean values with unlike superscript letters were significantly different (P< 0·05).
* P values are listed as the lowest observed P value between any of the four diets. One-way ANOVA with Tukey's post hoc test was used to calculate the significance.
Gene expression and spermidine/spermine-(N1)-acetyltransferase regulation
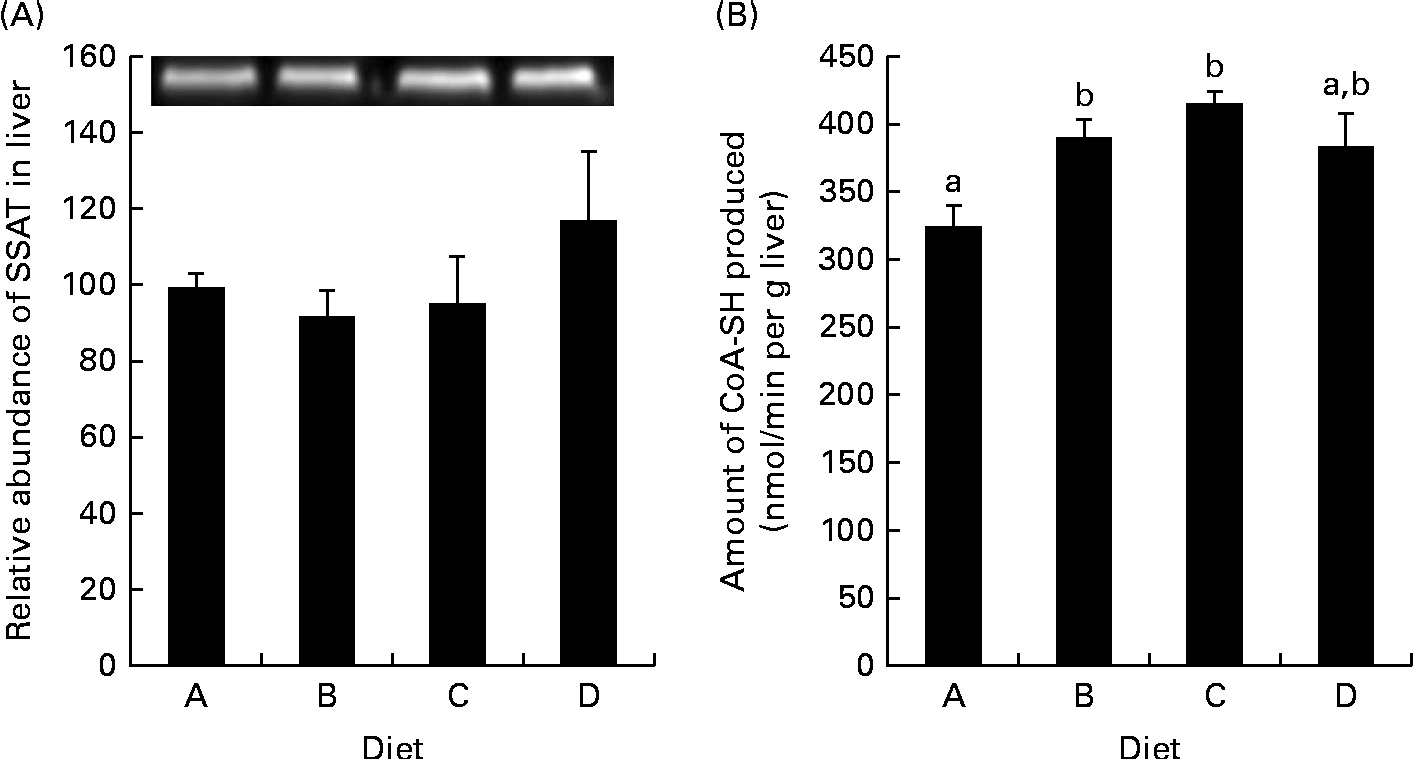
Gene expression was analysed in the fish fed diets B and D, as these two groups exhibited the greatest growth difference, and as the diets were examples of a low-arginine diet (B) and a high-arginine diet (D); the results are displayed in Fig. 1. In the WAT, ODC expression was up-regulated in the salmon fed diet D compared with those fed diet B (P= 0·01). Signs of a similar up-regulation were also found in the liver, though not significant (P= 0·13). CPT-1, however, was significantly up-regulated in the liver of fish fed diet D compared with those fed diet B, while expression in the WAT was unaffected. SAMdc expression remained unchanged in both the WAT and liver of the two dietary groups. SSAT, the enzyme acetylating spermine and spermidine by converting them back into shorter polyamines, did not appear to be regulated at the genetic level, in either the WAT or the liver, as quantitative PCR results showed no significant changes. Western blotting gave a bright clear band of SSAT at 28 kDa and a band of β-actin at 45 kDa. These results showed no differences in SSAT abundance in the liver between the dietary groups (Fig. 2(A)). The activity of SSAT, on the other hand, as measured by the amount of CoA-SH produced, was higher in the liver of fish fed diets B and C compared with those fed diet A (low arginine; Fig. 2(B)), and SSAT activity correlated with arginine concentration in the diets (R 0·63).

Fig. 1 Fold change in gene expression in the (A) liver and (B) white adipose tissue (WAT) of group D (high-arginine (Arg) diet) relative to group B (low-Arg diet). Values are means (n 4), with their standard errors represented by vertical bars. * Mean values with unlike letters were significantly different (P< 0·05; one-way ANOVA with Tukey's post hoc test). SSAT, spermidine/spermine-(N1)-acetyltransferase; ODC, ornithine decarboxylase; SAMdc, S-adenosylmethionine decarboxylase; CPT-1, carnitine palmitoyl transferase-1.

Fig. 2 Spermidine/spermine-(N1)-acetyltransferase (SSAT) abundance and activity in the liver. (A) Protein abundance of SSAT in the liver relative to the abundance of β-actin, with an inset of Western blotting bands of SSAT abundance. Diet A is set to 100, P= 0·45. (B) SSAT activity in homogenised samples collected from the liver, P= 0·004; n 4. a,bMean values with unlike letters were significantly different (P< 0·05; one-way ANOVA with Tukey's post hoc test).
Amino acids and metabolites
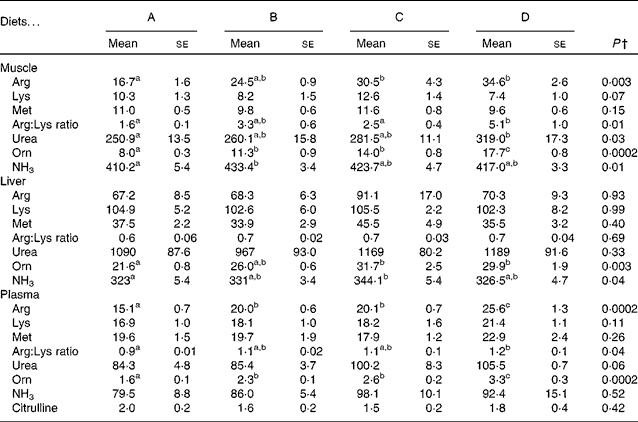
The concentrations of free arginine, lysine and methionine, as well as those of the metabolites of arginine in the muscle, liver and plasma, are listed in Table 5. In the muscle and plasma, increasing arginine in the diets resulted in increasing arginine concentrations in the tissues. In the liver, however, there was no difference in arginine between the dietary groups. Neither lysine nor methionine was altered in any of the tissues examined. The arginine:lysine ratio was affected in the muscle and plasma, with significant differences being observed between fish fed the lowest concentration of arginine (diet A) and those fed the highest concentration of arginine (diet D). In the liver, however, no changes were observed in the ratios of arginine:lysine. Ornithine concentrations in the liver, muscle and plasma increased from diet A through diet D and correlated with arginine in the feed (R 0·73, 0·94 and 0·91 and Arg = 2·0+0·05 × Orn, 2·3+0·08 × Orn and 2·3+0·004 × Orn, respectively), indicating high arginase activity. Urea, which is released when arginine is converted to ornithine, was not affected in the liver, while its concentrations increased in the muscle and plasma with increasing arginine concentrations in the diets, thus indicating that urea produced in the liver is quickly released into the blood stream. Changes in NH3 were also observed in the muscle and liver, with no differences being observed in the plasma samples. Citrulline, another metabolite of arginine, was only detected in the plasma, with no effect from the diets being observed. Overall, the liver appears to be less affected by the changing arginine inclusion in the diet, which might indicate higher metabolic capacity or need for arginine. These differences also highlight the importance of investigating more than one tissue when looking at amino acids and their metabolites. Arginine gain and lysine gain were calculated from the analysis of total amino acids in the pooled whole fish samples. No significant differences were observed in lysine gain (P= 0·07), while arginine gain was highest in the fish fed diet D, which was significantly higher than that in the fish fed diet B (P= 0·046; Table 3).
Table 5 Selected free amino acids and metabolites in the muscle, liver and plasma samples of the fish fed diets A to D* (Mean values with their standard errors, n 4)

a,b,cMean values with unlike superscript letters were significantly different (P< 0·05).
* All data are given as μmol/100 g tissue (100 ml plasma).
† P values are listed as the lowest observed P value between any of the four diets. One-way ANOVA was performed with Tukey's post hoc test or an unequal N test when applicable.
Effect on energy status
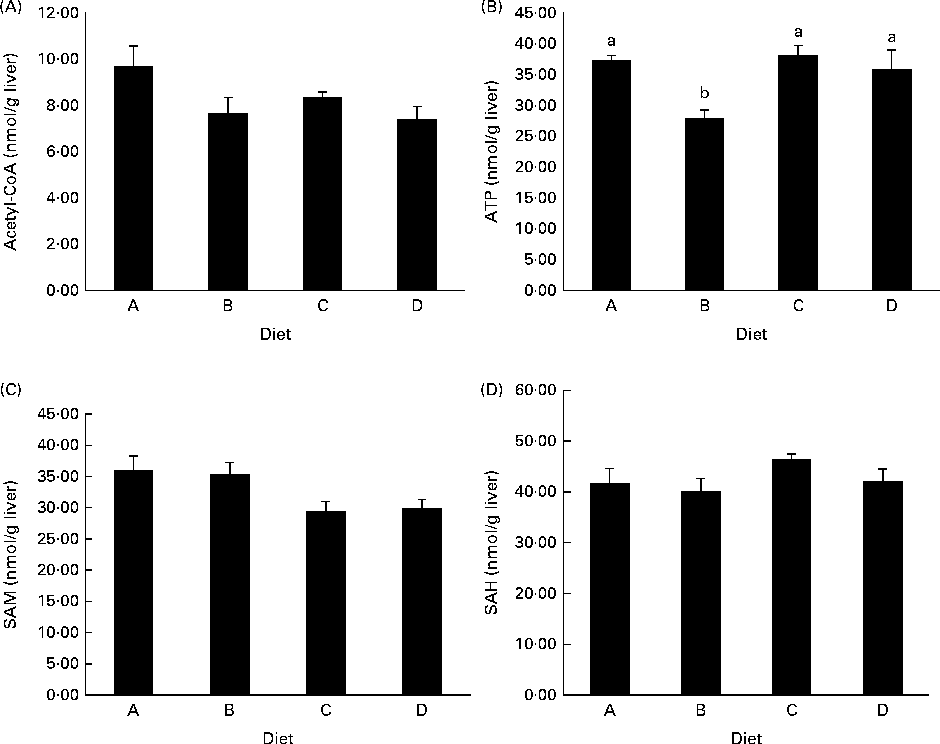
ATP concentrations were lower in the liver of fish fed diet B compared with those fed all the other diets (Fig. 3(B)), while there was no difference among diets A, C and D. Acetyl-CoA concentrations were not different between the experimental groups, though there was a trend in decreasing acetyl-CoA levels from the fish fed diet A to those fed diet D (P= 0·09; Fig. 3(A)). SAM concentrations appeared to decrease in the fish fed diets C and D compared with those fed diets A and B, while SAH levels were slightly higher in the fish fed diet C than in those fed diets A and B (Fig. 3(C) and (D)), though neither of these values reached significance.

Fig. 3 Concentrations of (A) acetyl-CoA, P= 0·09, (B) ATP, P= 0·02, (C) S-adenosylmethionine (SAM), P= 0·08, and (D) S-adenosylhomocysteine (SAH), P= 0·27, in deproteinised liver samples. P values are given as the lowest observed value between any of the four diets. Values are means (nmol/g tissue), with their standard errors represented by vertical bars (n 4). a,bMean values with unlike letters were significantly different (P< 0·05; one-way ANOVA with Tukey's post hoc test).
Discussion
Arginine supplementation has been reported to enhance muscle mass in several species(Reference Jobgen, Meininger and Jobgen17, Reference Tan, Yin and Liu20), and Yao et al. (Reference Yao, Yin and Chu19) have found that arginine activates mammalian target of rapamycin signalling in pig muscle, favouring protein synthesis and growth. This supports the present findings that protein gain was enhanced in the fish fed diet D, as this high-arginine diet resulted in the highest weight and protein gain. Diet D also resulted in the highest fat gain, which differs from that observed in studies carried out in mammals, demonstrating a loss in visceral mass after arginine supplementation(Reference Jobgen, Meininger and Jobgen17, Reference McKnight, Satterfield and Jobgen18, Reference Tan, Yin and Liu20, Reference Clemmensen, Madsen and Smajilovic31). Tan et al. (Reference Tan, Yin and Liu20) showed that arginine supplementation differentially regulated lipid metabolic genes in the muscle and adipose tissue and that intramuscular fat content, as well as the weight of the muscle and brown adipose tissue, was increased(Reference Jobgen, Meininger and Jobgen17) after arginine supplementation. However, as the concurrent loss of visceral mass exceeded the gain of muscle and brown adipose tissue mass, an overall weight loss was observed. In the present study, juvenile salmon, which are in a rapid growing life stage with minimal initial visceral mass, were used. The observed increased deposition of both fat and protein in the muscle therefore probably exceeds the possible lipolytic effect on the visceral mass by arginine, explaining the increased body weight, protein gain and fat gain observed in the highest-arginine diet. Still, weight gain, protein gain or fat gain did not correlate with arginine concentration in the diets, leading to the question whether there is a true arginine effect on growth. To the best of our knowledge, all reports on the effect of arginine on muscle and fat gain have been based on adult animals. With this in mind, it will be interesting to study arginine supplementation in growing/adult Atlantic salmon to assess whether arginine would have a whole-body lipid-reducing effect. The hepatosomatic and visceralsomatic indices remained unchanged between the dietary groups, indicating that the higher growth from diet D is equally distributed among tissues, possibly showing a whole-body growth-promoting effect of arginine supplementation. As the diets used in the present study were all low in fat and energy content compared with commercial diets fed to juvenile salmon, it is possible that arginine supplementation enabled the fish to utilise the limited energy more efficiently. However, future studies are required to investigate this. Surprisingly, while diet D (high arginine) resulted in the highest weight gain, diet A (low arginine) appeared to yield higher growth than diets B and C, though not significant. This was a pattern also observed for other parameters, such as protein gain and fat gain, some polyamine measurements and SSAT abundance in the liver. This might be due to the limited inhibition of lysine uptake by arginine in the salmon fed diet A (low arginine), thereby increasing the uptake of the already limited lysine in the diet(Reference Berge, Sveier and Lied32). Some of the lower values observed for the salmon fed diet B might be explained by the slightly lower initial body weight compared with that observed for the other experimental groups (P= 0·07). Due to this non-linear pattern in growth and deposition data, the groups fed diets B and D were chosen for gene expression analysis, as they exhibited the greatest difference in growth performance, and as the diets that they consumed represented those with adequate and surplus arginine concentrations.
Regulation of ODC is known to appear solely at the level of ODC protein abundance, and not due to its activity(Reference Pegg9), and we did indeed observe an up-regulation of mRNA expression for ODC in the WAT of the group receiving the highest arginine inclusion in the diet (Fig. 1(B)), indicating more arginine being used towards polyamine synthesis. Surprisingly, no differences in the concentrations of either of the polyamines were observed in the WAT. Ornithine was observed at increasing concentrations in the liver, muscle and plasma, correlating with increasing arginine inclusions in the diets (R 0·71, 0·94 and 0·91, respectively), again indicating more arginine being used towards polyamine synthesis. Arginine supplementation did not affect the mRNA expression of SSAT in the WAT or liver, but as the regulation of SSAT occurs at several levels including splicing, translation, activity and degradation(Reference Pegg9), it is likely that arginine affects SSAT at another level rather than during gene expression in the liver and WAT of salmon. This is supported by the present findings showing the regulation of the protein activity of SSAT in the liver (Fig. 2(B)). SSAT activity increased with the supplemented arginine, before appearing to reach a plateau. Protein abundance, as investigated with Western blotting, however, showed no difference between the dietary groups, though it displayed a tendency to increase in the fish fed the high-arginine diet. Taken together, these findings indicate that arginine regulates SSAT in the liver of salmon mainly at the post-transcriptional level.
Arginine in the diets clearly correlated with ornithine and putrescine in the liver, and taken together with the increased SSAT activity observed after arginine supplementation, this indicates increased production and turnover of polyamines. Increased synthesis of polyamines leads to higher SSAT activity and decreased degradation of the enzyme(Reference Pegg33), which increases the polyamine turnover and explains the steady pool of spermine and spermidine observed in the liver. In theory, as SSAT consumes acetyl-CoA, the cellular levels of acetyl-CoA decrease and the following decrease in malonyl-CoA levels will decrease fatty acid synthesis(Reference Pegg33). Acetyl-CoA levels did show a tendency to decrease in the liver with increasing arginine supplementation, though not reaching significance (Fig. 3(A); P= 0·09). Jell et al. (Reference Jell, Merali and Hensen34) also found no change in acetyl-CoA or malonyl-CoA levels in the liver of transgene SSAT mice overexpressing SSAT, while both molecules were clearly reduced in the WAT of the same transgene mice. They have related this observation to the fact that the liver is clearly permeable to glucose, in comparison with other tissues. Therefore, it would be of interest to analyse the concentrations of acetyl and malonyl-CoA in the WAT of arginine-supplemented salmon in the future. Malonyl-CoA is known as an inhibitor of CPT-1, the expression of which was clearly up-regulated in the liver of fish fed the high-arginine diet (Fig. 1(A)), indicating an increased oxidation of long-chain fatty acids in the liver. However, acetyl-CoA concentrations and SSAT activity were similar in the fish fed diets B and D and did not correlate with CPT-1 expression. This leads to the question whether the observed CPT-1 up-regulation is a true response to increased SSAT activity. A similar regulation of CPT-1 was not observed in the WAT. This could be due to the expression of different CPT-1 isoforms, as has been established in mammals, though the currently investigated CPT-1 isoform is the only one to be described in Atlantic salmon. Other studies have shown that arginine differentially regulates genes in the muscle and adipose tissue, up-regulating the lipolytic genes in the WAT(Reference Tan, Yin and Liu21, Reference Jobgen, Fu and Gao35). This was not observed in the present study, though future studies will further examine the regulation of fatty acid metabolism in the WAT after arginine supplementation.
The production of polyamines requires decarboxylated SAM, which is synthesised from methionine and ATP, potentially depleting cells of these compounds. Still, methionine concentrations were not affected in the tissues examined (Table 5) and ATP concentrations were not affected in the liver by the high-arginine diets (Fig. 3(B)), which is similar to what has been observed by Jobgen et al. (Reference Jobgen, Fu and Gao35). ATP concentrations were reduced in the liver of fish fed diet B, while there were no differences among the other dietary groups, indicating that the effect of arginine is not significant enough to deplete the cells of energy. SAM concentrations appeared to decrease in the liver of fish fed diets C and D, compared with those fed diets A and B (P= 0·08). The expression of SAMdc, the enzyme producing SAM, was not affected in the liver or WAT (Fig. 1(A) and (B)). As SAMdc is known to be negatively regulated by the increased spermidine and spermine levels(Reference Pegg, Xiong and Feith36), this lack of effect on SAMdc expression can be seen in relation to the stable pools of both polyamines in the liver among the experimental dietary groups. Overall, SAM does not appear to be the limiting factor for polyamine turnover after arginine supplementation.
Increased free lysine concentrations were observed with increased dietary arginine supplementation in the plasma (Table 5), as well as increasing whole-body total lysine gain, even though lysine was equal in all the diets, which is opposite to what was expected as arginine efficiently inhibits the uptake of lysine from the lumen in salmon(Reference Berge, Bakke-McKellep and Lied6). However, it is similar to what He et al. (Reference He, Kong and Wu37) have observed in the plasma of pigs, which they suggested could be due to increased NO production in the lumen from arginine, killing micro-organisms, which in turn decreases the catabolism of amino acids by intestinal bacteria, increasing the uptake of amino acids(Reference He, Kong and Wu37). The increase in whole-body total lysine content, however, may be related to the overall increased protein gain, incorporating lysine in myosin and actin, the bulking proteins of the muscle. Urea was observed at increasing concentrations in the muscle, possibly indicating arginine excess(Reference Luiking and Deutz38), though liver and plasma urea remained unchanged. In general, arginine supplementation has limited adverse effects in fish, apart from possible oxidative stress caused by increased NO production and H2O2 produced via the oxidation of acetylated polyamines(Reference Pirinen, Kuulasmaa and Pietila13, Reference Jobgen, Fu and Gao35, Reference Luiking and Deutz38). Future studies on arginine supplementation should therefore include markers for oxidative stress in salmon.
The present study demonstrated an increased turnover of polyamines in the liver, demonstrated with increased putrescine levels and SSAT activity and a declining pool of acetyl-CoA in the liver. Also, arginine supplementation affected amino acid metabolism in several tissues, increasing the concentrations of arginine metabolites. Growth results indicate arginine to be a promising supplement to enhance growth and protein deposition in Atlantic salmon, though future studies are required to further verify this, as well as to investigate how deposition is affected in different tissues in the post-smolt phase, the period when salmon deposit more lipid.
Acknowledgements
The present study was supported through the project ‘Integrated amino acid requirement’ financed by the Research Council of Norway (project no. 208352/E-40) and EWOS Innovation AS. The authors' responsibilities were as follows: A. A., M. E. and I. R. planned and designed the experiment and collected the samples. S. M. A., E. H. and M. E. collected the samples, analysed the data and interpreted the results; J. E. Z. designed the experiment and analysed the samples; S. M. A. and M. E. drafted the manuscript. All authors read and approved the final manuscript. The authors declare no conflict of interest.