Acrylamide (AA) is a human neurotoxin and is classified as a probable human carcinogen (class 2A) by the International Agency for Research on Cancer based on results from animal studies(Reference Hagmar, Tornqvist and Nordander1–3). Earlier, AA exposure was thought to occur solely through tobacco smoke and occupational exposure(Reference Bergmark4, 5). In 2002, however, Swedish researchers detected AA in carbohydrate-rich foods (especially potato products) formed by Maillard reactions(Reference Mottram, Wedzicha and Dodson6–Reference Zyzak, Sanders and Stojanovic8), when these foods were prepared by frying, roasting or baking at temperatures above 120°C(Reference Tareke, Rydberg and Karlsson9). Since then, epidemiological studies have been conducted in the attempt to assess cancer risk according to dietary AA intake in human subjects(Reference Larsson, Hakansson and Akesson10–Reference Schouten, Hogervorst and Konings16). The use of self-reported dietary intake from FFQ combined with databases including information regarding AA content measured in foods has been the chosen method in these studies. Overall, most of the studies found no association between estimated dietary AA exposure and cancer risk for different sites(Reference Larsson, Hakansson and Akesson10–Reference Schouten, Hogervorst and Konings16).
Recently, attempts to clarify whether the apparent null associations between dietary AA intake and cancer risk reported in most studies are evident, or whether they merely are the consequences of inappropriate measurements of dietary AA exposure by FFQ, have been made. A way to overcome the problem of inappropriate measurement of dietary AA exposure by FFQ could be the use of an objective biomarker of AA exposure. Hb-AA adducts and the epoxy metabolite of AA, Hb-glycidamide (GA) adducts, have been used. These have been shown to reliably reflect the internal dose of the adducts during 120 d before blood sampling in accordance with the lifetime of erythrocytes(Reference Tornqvist, Fred and Haglund17). Hb-AA has been found to reflect the general exposure to AA(Reference Bergmark4), whereas Hb-GA is a biomarker of the internal dose of GA(Reference Paulsson, Athanassiadis and Rydberg18). These metabolites have been used for the validation of FFQ-estimated dietary AA intake, but published studies have shown varying results(Reference Bjellaas, Olesen and Frandsen19–Reference Hagmar, Wirfalt and Paulsson24).
So far, only two studies have related AA exposure to cancer risk by the use of biomarkers reflecting AA exposure(Reference Wilson, Balter and Adami23, Reference Olesen, Olsen and Frandsen25), of which one found a significant positive association between Hb-AA and the risk for oestrogen receptor-positive breast cancer(Reference Olesen, Olsen and Frandsen25).
As AA is a probable carcinogen to human subjects, it is important to identify the food sources that predict Hb-adduct levels. The direct relationship of diet measured by FFQ and Hb-adduct concentrations has only been reported in two previous studies, both limited by few participants(Reference Bjellaas, Olesen and Frandsen19, Reference Wilson, Vesper and Tocco21). The aim of the present study was to investigate to what extent dietary variables estimated from an FFQ were able to predict the Hb-AA and Hb-GA adduct concentrations in a large population of non-smoking women attending the Danish Diet, Cancer and Health cohort.
Materials and methods
Study population
A population of 868 postmenopausal women from a nested case–control study in the prospective Diet, Cancer and Health cohort were selected for the present study(Reference Olesen, Olsen and Frandsen25). The Diet, Cancer and Health cohort was established with the primary aim of investigating the aetiological role of diet on cancer previously described in detail(Reference Tjonneland, Olsen and Boll26). Briefly, between December 1993 and May 1997, a total of 79 729 Danish women living in the greater Copenhagen and Aarhus areas were invited to participate in the cohort. The criteria for invitation were age between 50 and 64 years, born in Denmark and no previous diagnosis of cancer registered in the Danish Cancer Registry. The invited women were identified by the unique ten-digit identification number that is allocated to every Danish citizen by the Central Population Registry. Among the invited women, a total of 29 875 agreed to participate, corresponding to a participation of 37 %(Reference Tjonneland, Olsen and Boll26). The Diet, Cancer and Health study and the present study were conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Regional Ethical Committees on human studies in Copenhagen and Aarhus, and by the Danish Data Protection Agency. Written informed consent was obtained from all subjects.
Of the 868 women included in the present study, nineteen women were excluded due to a lack of blood sample or sample loss during the Hb-adduct analyses, eight were excluded due to missing information on selected variables (smoking or diet) and four were excluded due to outlying values for the selected variables. Finally, 300 women were excluded due to being a current smoker at baseline, leaving a total of 537 non-smoking women for the analyses.
Data collection
All participants attended one of two established study centres in Copenhagen or Aarhus. The responders received by mail a 192-item semi-quantitative FFQ and completed this before attending the study centres. Development and validation of the FFQ have been described previously(Reference Haraldsdottir, Tjonneland and Overvad27–Reference Tjonneland, Haraldsdottir and Overvad30). Women were asked to report their average intake of foods and beverages during the last 12 months within twelve possible categories ranging from never to ≥ 8 times/d. The calculations of the daily intake of specific foods and nutrients for each participant were conducted using the software program FoodCalc(Reference Lauritsen31), using specifically developed standardised recipes and portion sizes.
Dietary variables selected for the statistical analyses in the present study were only food items that were known to contain an appreciable amount of AA, and were available from the FFQ. The following dietary variables were chosen and quantified into the following quantities: 200 g/d for coffee; 20 g/d for cereals (oatmeal, rye bread, refined bread, whole-grain bread and crispbread); 5 g/d for potatoes (baked, fried and chips); 10 g/d for cakes and chocolate (pound (sand) cake, Danish pastry, biscuits/crackers and chocolate); 5 g/d for snacks (peanuts, crisps and cracklings). The quantities reflected the observed variation in intake in this population (considered from the interquartile range). The population had a quite low intake of the included food items (Table 1), and therefore, these increments in daily intake were chosen.
Table 1 Baseline characteristics of the 537 non-smoking women
(Number of subjects, medians and percentiles)
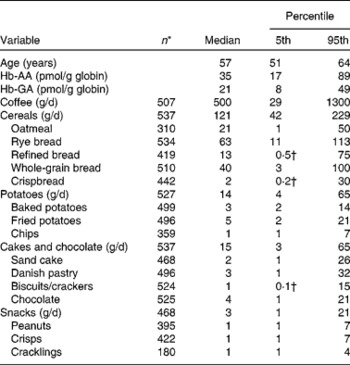
AA, acrylamide, GA, glycidamide.
* n indicates the number of the non-smoking women having an intake of the given variable.
† The value is zero when rounded off to the nearest whole number.
At the study centres, each women had a non-fasting blood sample taken and were asked to fill in a questionnaire dealing with questions about lifestyle habits, including alcohol and smoking behaviours, reproductive factors, health status and social factors. Anthropometrical measurements such as weight and height were carried out by professional staff members for every cohort participant; BMI was calculated as weight (kg) divided by height squared (m2).
Analytical method
The blood samples were analysed for Hb-adducts at the National Food Institute, Denmark, in accordance with the method previously described by detail by Bjellaas et al. (Reference Bjellaas, Olesen and Frandsen19). Briefly, from the thawed erythrocyte fractions, globin was purified and stored at − 20°C until further analysis. Globin was subjected to a modified Edman reaction, where phenyl isothiocyanate reacts with N-terminal amino acid (valine) in Hb, undergoes cyclisation and decouples from the Hb molecule releasing a phenylthiohydantoin derivative of N-alkylated valine adducts. The released phenylthiohydantoins were purified by solid-phase extraction and analysed in triplicate on a liquid chromatography ion-trap MS using multiple reaction monitoring. In total, forty-two batches were run.
The limit of quantification (LOQ) was 2·4 pmol/g for Hb-AA and 7·3 pmol/g for Hb-GA.
Statistical methods
The study population in the present study included women from a nested case–control study, and therefore, a test for heterogeneity between cases and controls was performed using a linear regression. No statistically significant differences between case and control status for Hb-AA (P heterogeneity = 0·30) or for Hb-GA (P heterogeneity = 0·73) were found.
Women who smoked at blood sampling were excluded, as smoking is a major source of AA exposure(Reference Bergmark4), and we were concerned about not being able to completely adjust for this potentially strong confounder. Smoking exposure, however, is expected to be of short term(Reference Schettgen, Rossbach and Kutting32, Reference Smith, Perfetti and Rumple33), and it was therefore considered to retain ex-smokers in the present study. The combined group of never- and ex-smokers did not differ from either never- or ex-smokers in Hb-adduct concentrations. Furthermore, when excluding ex-smokers, who discontinued smoking within the year before blood sampling, no appreciable difference between recent ex-smokers and never-smokers was observed. Therefore, ex-smokers and never-smokers were included in the analyses.
Multiple linear regression analysis was applied to model the associations between the intake of AA-rich foods and Hb-adduct concentrations. The Hb-adduct concentrations were log-transformed to improve normality of the distributions. Linearity was tested for all continuous variables by linear splines with three or nine boundaries placed at the quartiles and by an F test(Reference Greenland34). The smoking variables (time since cessation and duration) were log-transformed in order to be linear. For all other variables, we found no departure from linearity.
The association between dietary sources of AA and Hb-adduct concentrations was analysed using a one-way ANOVA and was adjusted for age as well as time since smoking cessation (among ex-smokers) and duration of smoking (among ex-smokers) to correct for a potential small residual effect of previous smoking. The estimates were back-transformed to describe the percentage difference in Hb-adduct concentrations for a defined difference in the intake of a given food item. The estimates were presented as the percentage change in Hb-adduct concentrations and the related 95 % CI and P values. Furthermore, the explained variation in the Hb-adduct concentrations from the mutually adjusted model was assessed by R 2 values.
All Hb-AA concentrations were above the LOQ, and linear regression procedures (general linear model; GLM) were used. For Hb-GA, nineteen samples were below the LOQ value, and therefore, a parametric model with left censoring was used (LIFEREG). Levels of Hb-GA below the LOQ were afterwards handled as left-censored. The model is also known as the Tobit model(Reference Greenland35).
The R 2 value could not be calculated for the mutually adjusted model for Hb-GA since the LIFEREG procedures were used. To calculate an R 2 value, all values below the LOQ were allocated one value representing all of the values below the LOQ. To evaluate the impact of choosing one representing value, different values were allocated (LOQ = 4, LOQ = 5, LOQ = 6 and LOQ = 7) and analysed in different models using the general linear model procedure. The estimates were visually considered in each model and compared with the estimates from LIFEREG. No marked difference was observed, and the R 2 value from the model with LOQ = 5 was chosen.
The procedures general linear model (GLM) and LIFEREG in the Statistical Analysis Systems statistical software package release 9.1 (SAS Institute, Cary, NC, USA) were used for the analyses on a TextPad platform.
Results
Baseline characteristics of the 537 non-smoking women included in the study are shown in Table 1. The median age at entrance to the study was 57 years (5th percentile 51, 95th percentile 64). The median Hb-AA concentration was 35 pmol/g globin (5th percentile 17, 95th percentile 89), and the median Hb-GA concentration was 21 pmol/g globin (5th percentile 8, 95th percentile 49).
Table 2 shows the estimates for the association between dietary variables and the Hb-adduct concentrations and the corresponding 95 % CI and P values in a minimally and a mutually adjusted model. In the minimally adjusted model, a higher intake of coffee was statistically significantly associated with a 5 % per 200 g/d (95 % CI 3, 7; P < 0·0001) higher Hb-AA concentration, whereas a higher intake of chips was statistically significantly associated with a 20 % per 5 g/d (95 % CI 9, 33; P = 0·0004) higher Hb-AA concentration. When mutual adjustments were performed, these associations persisted, as higher intakes of both coffee and chips were statistically significantly associated with a 4 % per 200 g/d (95 % CI 2, 7; P < 0·0001) and an 18 % per 5 g/d (95 % CI 6, 31; P = 0·002) higher Hb-AA concentration in the mutually adjusted model (Table 2). This model explained 17 % of the variation in Hb-AA concentration.
Table 2 Estimates indicating the associations between dietary variables and the Hb-adduct concentrations among the 537 non-smoking women in the Diet, Cancer and Health Cohort
(Δ Estimates and 95 % confidence intervals)
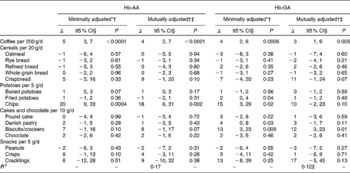
AA, acrylamide; GA, glycidamide; LOQ, limit of quantification; GLM, general linear model.
* Univariate models.
† Adjusted for smoking variables (duration (years, continuous), time since cessation (years, continuous) and smoking status (never/ex)) and age (years, continuous).
‡ Each food item mutually adjusted.
§ Δ Estimates reported as the percentage difference in Hb-adduct concentration per defined unit of difference in dietary variables.
∥ The R 2 value could not be calculated for the mutually adjusted model for Hb-GA since the LIFEREG procedures were used. To calculate an R 2 value, all values below the LOQ were allocated one value representing all of the values below the LOQ. To evaluate the impact of choosing one representing value, different values were allocated (LOQ = 4, LOQ = 5, LOQ = 6 and LOQ = 7) and analysed in different models using the GLM procedure. The estimates were visually considered in each model and compared with the estimates from LIFEREG. No marked difference was observed. The presented R 2 value is from the model with LOQ = 5.
In the minimally adjusted analyses for Hb-GA, intakes of the following dietary variables were statistically significantly associated with a higher Hb-GA concentration: coffee (percentage difference 4 % per 200 g/d; 95 % CI 2, 6; P = 0·0006), fried potatoes (percentage difference 2 % per 5 g/d; 95 % CI 0, 4; P = 0·04), chips (percentage difference 15 % per 5 g/d; 95 % CI 3, 29; P = 0·02), Danish pastry (percentage difference 4 % per 10 g/d; 95 % CI 0, 8; P = 0·03) and biscuits/crackers (percentage difference 13 % per 10 g/d; 95 % CI 3, 23; P = 0·009). When mutual adjustments were performed, only higher intakes of coffee and biscuits/crackers were statistically significantly associated with a 3 % per 200 g/d (95 % CI 1, 6; P = 0·005) and a 12 % per 10 g/d (95 % CI 3, 23; P = 0·01) higher concentration, respectively. This model explained 12 % of the variation in Hb-GA concentration.
Discussion
In a sample of 537 non-smoking Danish postmenopausal women, only a few dietary determinants of Hb-adduct concentrations in the blood were identified. The most important determinants of the Hb-AA concentration were coffee and chips. The most important determinants of the Hb-GA concentration were coffee and biscuits/crackers. Overall, the FFQ only explained 17 % of the variation in the Hb-AA concentration and 12 % of the variation in the Hb-GA concentration. The strengths of the present study are the use of both Hb-AA and Hb-GA as objective biomarkers of AA exposure, and a considerably higher statistical power compared with previous studies. Further strengths include the use of a validated FFQ to obtain information on dietary intake and the availability of detailed information on several lifestyle factors including our ability to exclude smokers. The most important limitations concern the FFQ used, which was not specifically designed to obtain information on AA-containing food items. The AA content of food items has been shown to vary considerably(36), and the standard foods in the FFQ used might therefore be a problem. The use of a standard FFQ probably limits the chance of identifying associations between the dietary determinants and the Hb-adduct concentrations. For example, in other studies, crisps have been shown to contain a high amount of AA(Reference Tareke, Rydberg and Karlsson9, Reference Svensson, Abramsson and Becker37), but in the present study, crisps were not found to be a strong determinant of the Hb-adduct concentrations.
Two previous studies have examined dietary determinants in relation to Hb-adduct concentrations among non-smokers(Reference Bjellaas, Olesen and Frandsen19, Reference Wilson, Vesper and Tocco21). A Norwegian study (n 44) found, unlike the present study, that intake of coffee was not a statistically significant determinant of Hb-AA(Reference Bjellaas, Olesen and Frandsen19). Additionally, the Norwegian study found that intakes of potato products and crisp bread were positive, statistically significant determinants of Hb-AA(Reference Bjellaas, Olesen and Frandsen19). An American study (n 296) also showed processed potato products as a positive, statistically significant determinant of Hb-AA(Reference Wilson, Vesper and Tocco21). The American study also evaluated dietary determinants of Hb-GA; particularly, intakes of coffee and potato products were positive, statistically significant determinants of Hb-GA(Reference Wilson, Vesper and Tocco21).
In the present study, only 17 % of the Hb-AA concentration and 12 % of the Hb-GA concentration were explained by the dietary factors studied. In one of the previous studies, potential AA-rich foods were found to explain 48 % of the Hb-AA variation(Reference Bjellaas, Olesen and Frandsen19). The FFQ in the previous Norwegian study was composed after the findings of AA in foods, and detection of AA-rich foods may therefore have been considered in the design of this FFQ. The American study did not present the percentage of explained variation. Several factors may be related to the observed poor ability of FFQ to explain the Hb-adduct variation, but the most important is probably the pronounced variation of AA in foods; food properties (e.g. concentration of reducing sugars)(Reference Seal, de Mul and Eisenbrand38), processes during the production (e.g. process temperature)(Reference Amrein, Andres and Escher39) and the presence of specific dietary components (e.g. rosemary)(Reference Becalski, Lau and Lewis40, Reference Hedegaard, Granby and Frandsen41) are all strongly related to AA content or formation, and are impossible to assess by FFQ.
In addition to problems related to FFQ, other factors possibly affecting the results exist. Large inter-individual variations in the Hb-adduct concentrations have been observed between populations(Reference Vesper, Slimani and Hallmans42), which might be due to variations in genes important in AA metabolism(Reference Doroshyenko, Fuhr and Kunz43–Reference Paulsson, Rannug and Henderson45). This possible genetic variation and gene–environment interactions may have affected AA metabolism in the present study population, which might result in inter-individual variations in Hb-adduct levels. Such variation will increase the random error, and the ability of the dietary factors to explain the variation in Hb-adduct levels might be masked.
In human subjects, a high reproducibility of Hb-adduct concentrations over time based on two blood samples has been shown(Reference Wilson, Vesper and Tocco21), indicating that one blood sample as collected in the present study is sufficient, when using Hb-adducts as a marker for AA exposure. However, more blood samples drawn during the year could correct for potential seasonal variation in Hb-adduct concentrations.
In the present study, three dietary factors were found to be statistically significantly associated with a higher Hb-adduct concentration, namely coffee (Hb-AA and Hb-GA), chips (Hb-AA) and biscuits/crackers (Hb-GA). The intake of these food items in the studied population varied considerably; the population had high intakes of coffee but rather low intakes of chips and biscuits/crackers (Table 1). It may seem surprising that chips and biscuits/crackers despite the lower amounts eaten still were found to be significantly related to Hb-adduct levels. They are, however, very well-known AA sources and were apparently able to distinguish AA intake even in this low intake population.
In previous studies, the sum of Hb-AA and Hb-GA has been included(Reference Bjellaas, Olesen and Frandsen19, Reference Wilson, Vesper and Tocco21), as the sum has been assumed to reflect the overall measure of AA exposure. Hb-AA levels have, however, been shown to be strongly correlated with the total exposure of AA(Reference Bergmark4, Reference Fennell, Sumner and Snyder46–Reference Urban, Kavvadias and Riedel48), and may therefore be considered as a good marker of AA exposure. In the present study, it was therefore considered reasonable to include the Hb-adducts separately.
In the present study, negative associations between several dietary factors and Hb-adduct levels were shown. No factors were found to be statistically significantly associated with a lower Hb-adduct level. But several tendencies were found (for Hb-AA: rye bread, fried potatoes, pound cake and peanuts; for Hb-GA: oatmeal, rye bread, whole-grain bread and peanuts), though all associations were of a very limited magnitude. Also, the study by Bjellaas et al. (Reference Bjellaas, Olesen and Frandsen19) discovered a negative association, though for jam/preservatives. It seems biologically unlikely that any of the studied food items should be negatively associated with Hb-adduct concentrations, as the dietary factors studied are known to have considerable AA contents. An explanation for the negative associations observed could be unknown confounding factors.
In conclusion, dietary factors measured by an FFQ could only to a limited extent predict Hb-adduct levels. Our finding supports the assumption that FFQ are inadequate measuring instruments for dietary AA exposure, especially when the FFQ is not specifically designed to measure AA intake. The null findings obtained between estimated dietary AA intakes in relation to cancer risk in most of the previous studies may therefore be caused by misclassification of dietary AA exposure as a consequence of an inaccuracy of FFQ in the estimation of dietary AA exposure. To investigate AA exposure in relation to cancer risk in future studies, the use of biomarkers reflecting the AA exposure is possibly a better measure for AA exposure.
Acknowledgements
We acknowledge Katja Boll (data manager) and Jytte Fogh Larsen (project coordinator) for their assistance with the collection and handling of data. We also acknowledge Joan E. Frandsen and Helle E. Gluver (laboratory technicians, National Food Institute, Technical University of Denmark) for their technical assistance with the determination of Hb-AA and Hb-GA. The present study was supported by funds from NordForsk (the Nordic Center of Excellence HELGA). The funding agency had no influence on the design and conduct of the study; collection, management, analysis and interpretation of the data; or preparation, review or approval of the manuscript. A. T. and K. O. contributed to the original design and data collection for the Diet, Cancer and Health project. A. O. contributed to the development of the hypothesis and study design. P. T. O. and H. F. performed the Hb-AA and Hb-GA analyses. M. O. performed the statistical analysis under the supervision of J. C. and wrote the manuscript. R. E., L. D. and A. O. contributed to the interpretation of results and made critical comments during the preparation of the manuscript. All authors contributed to the writing of the manuscript and approved the final version. There are no conflicts of interest to report.




