According to the estimates from the International Obesity Taskforce, at least 155 million school-aged children worldwide are overweight or obese. Within that figure, approximately 30–45 million are classified as obese, accounting for 2–3 % of the children aged 5–17 years in the world(1). The European Association for the Study of Obesity estimates that 16–22 % of European adolescents aged between 14 and 17 years are overweight or obese, with an annual increase of the prevalence of approximately 2 % in the 1990s and early 2000s(Reference Baker, Farpour-Lambert and Nowicka2). Childhood obesity is usually accompanied by associated comorbidities such as type 2 diabetes mellitus, insulin resistance or the metabolic syndrome(Reference Cali and Caprio3, Reference Ochoa, Santos and Azcona4).
In relation to the genetics of obesity, SNP of the fat mass and obesity-associated (FTO) gene have recently been strongly associated with excessive body weight for height and adiposity. Thus, FTO has been identified as a candidate gene contributing to childhood obesity in several European cohorts(Reference Dina, Meyre and Gallina5–Reference Haworth, Carnell and Meaburn7). This gene, located in human chromosome 16, has been proposed to have a nucleic acid demethylation activity that might regulate the expression of genes involved in metabolism, leading to obesity(Reference Fawcett and Barroso8).
Carriers of the minor frequency A allele of rs9939609, one of the most prevalent FTO genetic variants, have previously been identified with greater BMI in several studies(Reference Cecil, Tavendale and Watt9–Reference Rendo, Moleres and Marti Del Moral11). Moreover, this polymorphism has been reported to interact with energy intake patterns in children, with carriers of the minor A allele consuming more fat and total energy than non-carriers(Reference Cecil, Tavendale and Watt9, Reference Timpson, Emmett and Frayling12, Reference Wardle, Llewellyn and Sanderson13).
With regard to specific macronutrient dietary composition, Sonestedt et al. (Reference Sonestedt, Roos and Gullberg14) have shown that fat and carbohydrate intake modifies the association between the rs9939609 genetic variation in the FTO gene and obesity in a Swedish population.
Therefore, the aim of the present study was to study whether dietary fat composition modifies the association between obesity risk and the FTO genetic variant (rs9939609) in a Spanish case–control study of children and adolescents.
Subjects and methods
The study population included 354 Spanish children and adolescents (49 % males) aged 6–18 years who were enrolled in a case–control study (Grupo Navarro de Estudio de la Obesidad Infantil; GENOI). The subjects were recruited from the Paediatric Departments at the Virgen del Camino Hospital, Clínica Universidad de Navarra and other Primary Care Centres in Navarra (Spain). Cases were subjects with a BMI above the ninety-seventh percentile of the Spanish BMI reference data for age and sex(Reference Sobradillo15). Exclusion criteria were exposure to hormonal treatment or development of secondary obesity due to endocrinopathy or serious intercurrent illness. Controls were healthy subjects with a BMI below the ninety-seventh percentile of the same reference.
Written consent to participate was requested from both parents and adolescents aged above 12 years. The study protocol was performed in accordance with the ethical standards of the Declaration of Helsinki (as revised in Hong Kong in 1989, in Edinburgh in 2000 and in South Korea in 2008), and was approved by the ethics committee of the University of Navarra.
Procedures
Trained researchers conducted face-to-face interviews with participants and their parents based on standardised procedures. A semi-quantitative FFQ, previously validated in Spain(Reference Martin-Moreno, Boyle and Gorgojo16), containing 132 food items was filled in, in order to evaluate dietary patterns. Complete data were available for 288 children and adolescents (53 % males). Familial medical history was collected using a specific questionnaire. Weight and height were measured with an electronic scale (type SECA 861; SECA, Birmingham, UK) and a telescopic height-measuring instrument (type SECA 225; SECA), respectively, to establish BMI-standard deviation score (SDS) according to the criteria of Cole et al. (Reference Cole, Bellizzi and Flegal17). Skinfolds were measured with a Holtain skinfold calliper (Holtain, Crosswell, Crymych, Pembs, UK) and waist and hip circumferences with a flexible non-stretchable measuring tape (type SECA 200; SECA) using validated protocols. Percentage of body fat was determined by bioelectrical impedance (TBF-300A Body Composition Analyzer/Scale; TANITA, Tokyo, Japan). Venous blood samples were collected to obtain DNA samples.
Genotyping
DNA was extracted from the buffy coat fraction using a commercial kit (MasterPure™; Epicentre, Madison, WI, USA). All the subjects were genotyped for the rs9939609 polymorphism of the FTO gene using Taqman SNP allelic discrimination (ABI PRISM 7900; Applied Biosystems, Madrid, Spain). The probes and the primers for these assays were designed by Applied Biosystems. Replicate quality control samples were included in every genotyping plate with more than 99 % concordance.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences software 15.0 (SPSS Inc., Chicago, IL, USA). A χ2 test was used to evaluate the Hardy–Weinberg equilibrium. The Kolmogorov–Smirnov test was used to determine variable distribution.
The differences in anthropometric, biochemical and energy intake variables between the polymorphism genotypes were tested with ANCOVA adjusted for age and sex (for normally distributed variables) or the Mann–Whitney U test. Multivariate logistic regression models were fitted to assess the association between the genotypes and obesity risk after adjusting for confounder factors. For the evaluation of the association between the rs9939609 SNP of the FTO gene and obesity risk, fat intake values were dichotomised by the median. The level of probability was set at P < 0·05 as statistically significant.
Result
The distribution of genotypes for the rs9939609 polymorphism of the FTO gene variant was in Hardy–Weinberg equilibrium in the study population. The minor A allele frequency was 0·46. The prevalence of the polymorphism was higher in cases compared to controls, both in the codominant and in the dominant model, with a marginal statistical significance (P = 0·060 and 0·098, respectively, χ2 test; Table 1).
Table 1 Baseline characteristics and prevalence of the rs9939609 polymorphism of the fat mass and obesity-associated gene in children and adolescent obese and control subjects
(Mean values with their standard error of mean, after adjusting for age and sex and number of subjects and percentage values)

SDS, standard deviation score; TE, total energy.
As expected, anthropometric measurements such as weight, BMI-SDS, waist and hip circumferences or fat mass were significantly different (P < 0·001) between cases (n 208) and controls (n 146). With regard to energy intake, consumption of PUFA was significantly higher in the control group (P = 0·002), whereas obese subjects had a higher intake of MUFA (P < 0·001; Table 1).
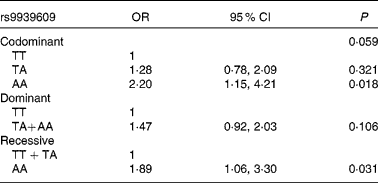
Logistic regression models were performed to study the association between this SNP of the FTO gene and obesity risk, including a recessive (T carriers v. AA), a dominant (TT v. A carriers) and a codominant (TT v. TA v. AA) model adjusted for age, sex, total energy (kJ) and total fat (g) intake. The OR for obesity increased for each additional A allele in the genotype (Table 2). Children and adolescents homozygous for the mutation (AA) had higher risk of becoming obese than those without the mutation (OR 1·89; 95 % CI 1·06, 3·30).
Table 2 Obesity associated with the rs9939609 polymorphism of the fat mass and obesity-associated gene in codominant, dominant and recessive models
(Odds ratios and 95 % confidence intervals adjusted for age and sex)
Next, we analysed the effect of the rs9939609 mutation of the FTO gene on obesity risk, depending on the fatty acid consumption. A relationship between SFA intake and obesity was found (Fig. 1(a)). After dichotomising for the median, we observed that a higher SFA intake ( ≥ 12·6 %) contributes to a greater obesity risk. A statistically significant interaction term (FTO SNP × SFA consumption) on BMI-SDS was also identified (P for interaction = 0·035). In subjects with a lower intake of SFA ( < 12·6 % of total energy), the mutation did not seem to affect BMI-SDS, whereas A allele carriers with a higher intake of SFA had a significantly higher BMI-SDS (P = 0·009) than TT subjects (Fig. 2).

Fig. 1 OR for obesity risk in children and adolescents depending on the consumption of (a) SFA (percentage of total energy, dichotomised by the median) and (b) PUFA:SFA ratio (dichotomised by the median) and the rs9939609 SNP of the fat mass and obesity associated (FTO) gene (dominant model). OR are adjusted for age and sex. TT subjects are considered as the reference group (OR = 1).

Fig. 2 BMI-standard deviation score (SDS) of children and adolescents according to SFA consumption (percentage of total energy, dichotomised by the median) and the presence of the fat mass and obesity associated (FTO) rs9939609 polymorphism in a dominant model. Values are means, with their standard errors represented by vertical bars. ![]() , TT; ■, A carriers. * Mean values were significantly different (P < 0·05).
, TT; ■, A carriers. * Mean values were significantly different (P < 0·05).
Furthermore, as seen in Fig. 1(b), having a PUFA:SFA intake ratio higher than 0·43 (median) seems to protect against obesity risk. We observed an interaction between the rs9939609 SNP of the FTO gene (dominant model) and PUFA:SFA intake ratio (P for interaction = 0·034), and a borderline statistical interaction between the rs9939609 SNP of FTO and SFA consumption (P for interaction = 0·080).
Discussion
In the present study, we confirm the previously reported association between the rs9939609 polymorphism of the FTO gene with obesity in a case–control population of children and adolescents. Moreover, we characterise the role of dietary fat intake distribution and its interaction with this genetic variant as a risk factor for obesity.
The frequency of the minor A allele was 0·46, similar to those described in other Spanish populations(Reference Razquin, Martinez and Martinez-Gonzalez18) and other European cohorts of children and adolescents(Reference Muller, Hinney and Scherag19, Reference Tanofsky-Kraff, Han and Anandalingam20).
Case–control studies have been demonstrated to be a suitable tool to screen the effects that a genetic polymorphism may have on obesity development. It has been previously reported that the accuracy of the phenotype measurement is crucial in the ability to detect gene–environment interactions for traits such as BMI(Reference Andreasen, Stender-Petersen and Mogensen21). On this point, the subjects of the present study were a homogeneous sample of cases and controls of similar age and belonging to the same social environment. As expected, control subjects showed statistically significant differences compared with obese children and adolescents in all measured anthropometric traits.
The rs9939609 polymorphism of the FTO gene has been largely related to greater adiposity. With regard to this observation, the data from the present study showed a significant increase in obesity risk per A allele present in the genotype. Homozygous carriers for the mutation are at a greater risk of developing obesity than non-carriers (OR 1·89; 95 % CI 1·06, 3·30). This increase in obesity risk was similar to that observed by Wardle et al. (Reference Wardle, Carnell and Haworth22) in a population of British children and slightly higher than those observed by other authors(Reference Jacobsson, Danielsson and Svensson10, Reference Loos and Bouchard23, Reference Price, Li and Zhao24), both in children and adult cohorts.
The present results confirm that the effect of the mutation is influenced by dietary fatty acid composition. The percentage PUFA, SFA and PUFA:SFA ratio over total energy intake modulates obesity risk associated with the rs9939609 polymorphism of the FTO gene. Specifically, children and adolescents carrying the A allele of the mutation and consuming more than 12·6 % SFA (as percentage of total energy) had an increased risk of being obese. In a similar way, we observed an interaction between the FTO polymorphism and the PUFA:SFA ratio. Indeed, subjects with the A allele of the FTO gene, with a PUFA:SFA ratio lower than 0·43, had a 2·3 times higher risk of becoming obese than the non-carriers of the risk allele consuming a higher PUFA:SFA ratio.
Genetic associations and fatty acid–gene interactions are widely explored in obesity development and onset(Reference Smith and Ordovas25). In this sense, Luan et al. showed an interaction between PUFA:SFA dietary intake ratio and the Pro12Ala polymorphism of the PPARG gene. In this study population, Ala carriers showed a decrease in their BMI as the PUFA:SFA ratio increased, whereas wild-type homozygotes were not affected by fatty acid intake(Reference Luan, Browne and Harding26). Other gene variants have also showed effects of interactions with dietary fatty acid consumption on obesity risk. In 2007, Corella et al. (Reference Corella, Lai and Demissie27) found that a APOA5 gene variation modulates the effects of dietary fat intake on BMI and obesity risk in an adult American cohort. With regard to SFA, a recent study carried out in Mediterranean and Asian populations has also shown the effect of a gene–saturated fat intake interaction on the association between the APOA2 promoter polymorphism and body weight(Reference Corella, Tai and Sorli28). All these studies demonstrate that fatty acid intake could affect obesity risk in a different way according to the individual genotype of the subject, and therefore, the study of these nutrient–gene interactions would be an important tool in obesity prevention and personalised nutrition.
With regard to the rs9939609 polymorphism of the FTO gene, there are some studies in children that link the SNP with a higher total energy and fat intake(Reference Cecil, Tavendale and Watt9, Reference Timpson, Emmett and Frayling12, Reference Wardle, Llewellyn and Sanderson13, Reference Tanofsky-Kraff, Han and Anandalingam20) and diminished satiety sensation(Reference Wardle, Carnell and Haworth22). A recent study has showed that fat and carbohydrate intake modifies the association between the rs9939609 genetic variation of the FTO and obesity(Reference Sonestedt, Roos and Gullberg14), but specific dietary fatty acid composition and its interaction with this SNP and obesity risk has not been studied so far. A study carried out in our group by Razquin et al. (Reference Razquin, Martinez and Martinez-Gonzalez18) in high cardiovascular risk Spanish subjects aged 55–80 years showed that subjects carrying the A allele of the mutation gained significantly less weight compared to wild-type subjects (TT) after 3 years of intervention with the Mediterranean diet. This diet is characterised by a high intake of PUFA and a low intake of SFA, and confirms the implication of dietary fatty acid distribution in the relationship between FTO gene variant and body weight.
To our knowledge, this is the first study linking fatty acid intake with the FTO rs9939609 polymorphism on obesity risk. One limitation of the present study is sample size, and therefore, studies in larger populations, leading to a better understanding of how dietary macronutrients and the rs9939609 SNP of the FTO gene could interact and modify obesity risk, are needed to support the findings of the present study.
In summary, the present study confirms that the rs9939609 variation of the FTO gene is associated with a higher obesity risk in this case–control study of children and adolescents. Moreover, we report for the first time the influence of dietary fatty acid distribution on the effect of this polymorphism on obesity risk.
Acknowledgements
Research relating to the present study was funded by grants from the Navarra Government (Health and Education Department) and Línea Especial, Nutrición y Obesidad (University of Navarra). The scholarships to A. M. from the Navarra Government and to T. R.-U. from the University of Navarra's Asociación de Amigos are fully acknowledged. A. M., J. A. M. and M. A. M.-G. were involved in the design, funding and writing of the manuscript. M. C. A.-S. was involved in the design, conduct and writing of the manuscript. A. M., T. R.-U. and M. C. O. were involved in the conduct, analysis and writing of the manuscript. The authors have no competing interests.






