Inflammation is an intrinsic part of the immune system, playing a role in the prevention of infection(Reference Medzhitov1). However, prolonged inflammation in the absence of an acute inflammatory stimulus, referred to as chronic inflammation, has been implicated in the progression of conditions such as CVD, type 2 diabetes, non-alcoholic fatty liver disease, the metabolic syndrome and depression(Reference Minihane, Vinoy and Russell2–Reference Hotamisligil4). While mechanisms by which dietary fibre may decrease disease risk are not fully understood, a higher fibre intake has been associated with lower risk of CVD, type 2 diabetes and the metabolic syndrome(Reference Meyer, Kushi and Jacobs5–Reference Chen, Chen and Wang7). Reducing or preventing chronic inflammation is a plausible mechanism, supported by observed associations between higher dietary fibre intake and lower inflammatory markers(Reference Lie, Brown and Forrester8,Reference Parikh, Pollock and Bhagatwala9) .
Some non-digestible dietary fibres are fermented in the intestine by the gut microbiota, creating by-products with the ability to inhibit and suppress inflammation and oxidative stress(Reference Cummings10,Reference Tong, Wang and Wang11) . Potential mechanisms include reducing the permeability of the intestinal membrane(Reference Macfarlane and Macfarlane12–Reference Tremaroli and Backhed14), lowering release of lipopolysaccharide from the gut and consequent production of inflammatory cytokines such as IL-6(Reference Schedlowski, Engler and Grigoleit15). In turn, IL-6 stimulates the production of C-reactive protein (CRP), an acute-phase inflammatory reactant frequently used to measure chronic inflammation(Reference Pepys and Hirschfield16,Reference Eklund17) .
A higher dietary fibre intake may alternatively impact inflammation through a reduction in adipose tissue(Reference Slavin18), as excess adiposity has been associated with elevated inflammation(Reference Cox, West and Cripps19–Reference Shelton and Miller21). Leptin is a pro-inflammatory adipokine and immune modulator produced in adipose tissue and associated with increased inflammation(Reference Scotece, Conde and Lopez22,Reference Procaccini, La Rocca and Carbone23) . In contrast, adiponectin is anti-inflammatory and high concentrations have been associated with reduced inflammation in the metabolic syndrome and other related conditions(Reference Scotece, Conde and Lopez22,Reference Nedvidkova, Smitka and Kopsky24) . Although associations between dietary fibre and inflammation have been studied in adult populations, only one study has previously investigated the concept in healthy adolescents, to our knowledge. The study of American adolescents found that a higher dietary fibre intake was linked to greater levels of adiponectin and lower levels of leptin(Reference Parikh, Pollock and Bhagatwala9). Higher dietary fibre intake was also associated with lower leptin concentrations in adults and female young adults(Reference Murakami, Sasaki and Takahashi25,Reference Kuroda, Ohta and Okufuji26) .
Inadequate dietary fibre intakes are not exclusive to adults, with adolescent fibre intakes being below recommendations in much of the Western world(Reference Edwards, Xie and Garcia27,Reference Fayet-Moore, Cassettari and Tuck28) . More research to determine the effect of low dietary fibre intake on biomarkers of health during adolescence is warranted as adolescent health may have consequences in adulthood(Reference Metcalf, Jones and Nordstrom29,Reference Biro and Wien30) . Additionally, the impact of dietary fibre sourced from different food groups (e.g. cereals and grains, fruits, and vegetables) on CRP, leptin, and adiponectin concentrations has been insufficiently studied in healthy populations. The analysis of different sources of dietary fibre is supported by two studies of adults with diabetes which found a significant association between higher cereal fibre intake and lower CRP in women(Reference Qi, van Dam and Liu31) and with higher cereal fibre intake and higher adiponectin in men(Reference Qi, Rimm and Liu32). Another study of women found associations between higher total fibre and cereal fibre intake and higher adiponectin and between higher cereal fibre and lower high-sensitivity C-reactive protein (hs-CRP)(Reference AlEssa, Ley and Rosner33). We aimed to investigate the association between dietary fibre intake, both overall and from different sources, and hs-CRP, leptin and adiponectin in adolescents participating in the 17-year follow-up of the Raine Study.
Methods
Participants
The Raine Study is a prospective study which recruited 2900 pregnant mothers (generation 1) through the major maternity hospital in Perth, Western Australia between 1989 and 1991, resulting in 2868 live births (generation 2). Multiple follow-ups have been performed from birth through to adulthood, and cross-sectional data from the 17-year follow-up are used in the present study based on availability of inflammation variables. Detailed cohort information and characteristics have been published elsewhere(Reference Straker, Mountain and Jacques34,Reference White, Eastwood and Straker35) . Briefly, the 17-year cohort included 1621 adolescents, with 748 eligible for the present study after exclusion of those without complete data for diet and inflammatory markers. Exclusion of those without anthropomorphic and lifestyle data resulted in 621 adolescents with all data (Fig. 1).

Fig. 1. Number of participants in the Raine Study 17-year follow-up and number with complete variable data. Dietary data include dietary fibre and energy intake. * Some participants are missing data from multiple variables and therefore numbers do not add up to the total number of people excluded for missing data. hs-CRP, high-sensitivity C-reactive protein.
Dietary variables
Dietary fibre and energy intake data were obtained from the 212-item Commonwealth Scientific and Industrial Research Organisation semi-quantitative FFQ, completed by the adolescent. The FFQ measures usual diet over the previous year with a series of questions about consumption frequency and serving size of various foods, including mixed dishes and beverages. Estimates of daily intake of dietary fibre and energy were provided by the Commonwealth Scientific and Industrial Research Organisation, calculated from serving size and nutrient content of each food item. The Commonwealth Scientific and Industrial Research Organisation FFQ has been validated for use in the Australian population(Reference Lassale, Guilbert and Keogh36) and within the Raine Study cohort(Reference Ambrosini, de Klerk and O’Sullivan37). Dietary fibre, sourced from cereals and grains, fruits and vegetables and nuts and legumes was determined by assigning a fibre concentration (%) to each food item (see online Supplementary Table S1, based on the Nutrient Tables for use in Australia dietary composition database) and combining it with each adolescent’s intake of those foods (g/d) to obtain a daily fibre intake from each food (g/d). These intakes from individual foods were combined to give a total fibre intake per food group. Dietary fibre intake from nuts and legumes was considered but not analysed separately due to extremely low intakes (mean intake of 0·60 g/d). Dietary misreporting was calculated from energy intake and BMR using the Goldberg method to classify participants as under-, over- or plausible reporters(Reference Goldberg, Black and Jebb38) as described elsewhere(Reference Appannah, Pot and O’Sullivan39).
Sociodemographic and lifestyle variables
Information on lifestyle factors including screen time, leisure-time physical activity, smoking status, alcohol intake and oral contraceptive use in females was obtained from self-report questionnaires completed by the adolescent at the 17-year follow-up. Screen time reported the average number of hours spent either using a computer or watching television (whichever was highest) on a weekend day. Physical activity refers to h/week spent in vigorous physical activity outside of school or work. Smoking was categorised as the average number of cigarettes smoked per d in the previous week. Alcohol intake was measured in standard drinks/week, calculated from reports of the number and variety of alcoholic beverages consumed in the previous week. Females were categorised into those currently taking oral contraceptives or not. Inflammation linked medications considered in the study included antihistamines, oral steroids, immunosuppressants, anti-cholinergics and non-steroidal anti-inflammatory drugs and was recorded as yes or no. Adolescents who were taking these medications (n 5) were excluded from analysis.
Parental ethnicity was recorded during pregnancy and used to categorise the adolescents as Caucasian (both parents Caucasian), mixed (one parent Caucasian and one parent non-Caucasian) or non-Caucasian (both parents non-Caucasian). Height (to the nearest 0·1 cm using a Holtain Stadiometer), weight (to the nearest 100 g using a Wedderburn Digital Chair Scale) and waist circumference (to the nearest 0·1 cm) were measured by trained researchers. BMI was calculated from weight (kg) divided by height squared (m2). Waist:height ratio (WHtR), a measure of central adiposity, was calculated by dividing waist circumference (cm) by height (cm). WHtR and BMI were both chosen as they measure different aspects of adiposity (general adiposity for BMI and central adiposity for WHtR) and are independently associated with fibre intake and inflammation (data not shown). Adolescents with a WHtR of 0·5 or higher were considered to have central obesity(Reference Maffeis, Banzato and Talamini40).
Inflammatory markers
Fasting blood samples (no food or beverage other than water consumed in the previous 12 h) were taken by a phlebotomist in the home of the participant. Blood samples were analysed at the PathWest Laboratory at the Royal Perth Hospital. hs-CRP was measured on an Architect c16000 Analyser with an intra-assay CV of 15·96 % and a lower detection threshold of 0·14 mg/l. Leptin was measured by the ACTIVE® Human Leptin ELISA kit (DSL-10-23100; Diagnostic Systems Laboratories) with a lower detection threshold of 1·5µg/l. Adiponectin was measured by Quantikine® Human Total Adiponectin/Acrp30 Immunoassay (R&D Systems). Based on the recommendations of the American Heart Association, hs-CRP values ≥10 mg/l were excluded as they are considered to represent acute inflammation due to injury or sickness, rather than chronic inflammation(Reference Pearson, Mensah and Alexander41).
Ethics
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki. Ethics approval for the Raine Study was granted by the committees of King Edward Memorial Hospital for Women and Princess Margaret Hospital for Children, Perth, Western Australia. Informed written consent was obtained from the adolescent and their primary caregiver.
Analytical methods
After removing participants who were taking inflammation-linked medications or had hs-CRP > 10 mg/l, 602 participants were included in the analysis. Associations between dietary fibre intake (per 5 g/d increases) and hs-CRP and leptin were estimated using tobit regression. Tobit regression was developed for analysis of datasets where insufficient range in the measurement scale results in a large number of observations clustering at one end of the scale(Reference McBee42). In this case, the lower detection threshold of the leptin and hs-CRP measurement assays led to partial information loss (censoring) where the true levels below that threshold were unknown. Tobit regression uses a modified maximum likelihood estimation method which considers whether the data are censored when calculating estimates to give an unbiased outcome(Reference McBee42). Both hs-CRP and leptin were log transformed due to skewed distribution. Associations between dietary fibre intake (per 5 g/d increases) and adiponectin concentrations were estimated with linear regression. Confounders were chosen based on biological plausibility and a significant (P < 0·1) association with hs-CRP, leptin or adiponectin, determined by univariable tobit and linear regression (see online Supplementary Table S2). Inverse probability weighting was applied to analysis due to the large numbers of participants who did not have the required data. Weighting variables were measured for the full population at birth and consisted of maternal age at birth, family income and sex of the participant. There were three multivariable models tested in addition to the unadjusted analysis (model 1). Model 2 included sex only, while model 3 further adjusted for WHtR, BMI, energy intake and dietary misreporting. Model 4 adjusted for the factors included in model 3 with the addition of ethnicity and lifestyle factors (screen time, physical activity, smoking and alcohol intake). Sensitivity analysis including only participants categorised as plausible reporters was performed with results consistent with the main analysis (see online Supplementary Table S3). Sex-stratified analysis was also performed due to a significant interaction between dietary fibre intake and sex. In sex-stratified analysis, models 1, 3 and 4 were used (minus the sex variable), and oral contraceptive usage was added to model 4 in females only. Statistical analysis was performed with R version 3.5.0(43) and R studio version 1.2.1335(44).
Results
Table 1 shows the characteristics of adolescents included in the analysis and those excluded due to incomplete data. Proportions in the excluded adolescents were calculated for those without missing data for the relevant variable (valid %). The excluded population had a higher proportion of males (51 %) than the included population (47 %). Excluded adolescents were less active, smoked more and drank more alcohol than those with complete data. A higher proportion of excluded participants had elevated hs-CRP (>3 mg/l) compared with those included in the study (16 and 11 %, respectively). Of the female adolescents included, 34 % were taking oral contraceptives compared with 25 % in those who were excluded (Table 1). Excluded adolescents also had higher hs-CRP and leptin concentrations (Table 2).
Table 1. Characteristics of those included and excluded from the study (Numbers and percentages)

hs-CRP, high-sensitivity C-reactive protein; WHtR, waist:height ratio.
* Non-Caucasian ethnicity includes Aboriginal, Asian and Polynesian. Valid % is the proportion calculated excluding those with missing data for the relevant variable. Percentages were rounded to the nearest 1 %.
Table 2. Diet variables, physical measurements and markers of inflammation in those included and excluded from the study
(Mean values, standard deviations and ranges)
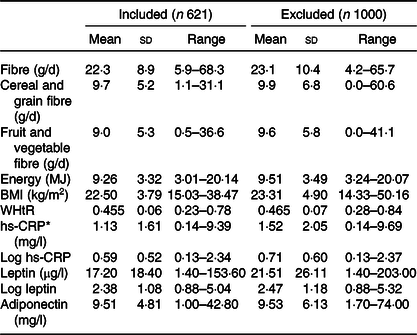
WHtR, waist:height ratio; hs-CRP, high-sensitivity C-reactive protein.
* Participants with hs-CRP > 10 mg/l are excluded from mean hs-CRP.
Mean concentrations of hs-CRP were significantly higher in females (1·33 (sd 1·75) mg/l) than in males (0·92 (sd 1·43) mg/l, P = 0·002). Concentrations of leptin differed substantially, with a mean concentration of 27·77 (sd 19·07) µg/l in females and 5·40 (sd 7·37) µg/l in males (P < 0·001). Likewise, adiponectin levels were higher in females (11·88 (sd 5·19) mg/l) than in males (7·98 (sd 3·86) mg/l, P < 0·001). Conversely, daily dietary fibre intake was significantly lower in females (20·7 (sd 7·5) g/d) than in males (24·1 (sd 9·8) g/d, P < 0·001) (Fig. 2).

Fig. 2. Daily dietary fibre intakes and concentrations of high-sensitivity C-reactive protein (hs-CRP), leptin and adiponectin in 17-year-olds from the Raine Study separated by sex. Values are means, with standard deviations represented by vertical bars. ![]() , Female;
, Female; ![]() , male.
, male.
In unadjusted analysis (model 1), a 5 g/d higher dietary fibre intake was significantly associated with lower levels of leptin and adiponectin (Table 3). The association between dietary fibre intake and lower leptin remained after adjustment for sex (model 2) but was no longer significant after further adjustment (models 3 and 4). In analyses that examined males and females separately, there was a significant association between higher dietary fibre intake and lower leptin in males (see online Supplementary Table S4) and between dietary fibre intake and lower hs-CRP in females (see online Supplementary Table S5), but neither persisted after adjustment for confounders.
Table 3. Weighted associations between dietary fibre intake and inflammatory markers measured at 17 years of age in the Raine Study*
(β Coefficients and 95 % confidence intervals)

hs-CRP, high-sensitivity C-reactive protein.
* Model 1 – dietary fibre (per 5 g/d higher intake). Model 2 – model 1 + sex. Model 3 – model 2 + waist:height ratio, BMI, energy intake and dietary misreporting. Model 4 – model 3 + ethnicity, screen time, physical activity, smoking and alcohol intake. Weighted by inverse probability of having complete data and being included in the study.
When dietary fibre from different food groups was examined separately, a 5 g/d higher cereal and grain fibre intake was significantly associated with lower hs-CRP, leptin and adiponectin in unadjusted analysis (model 1). The association of cereal and grain fibre with leptin remained significant after adjusting for all confounders (model 4), although the effect was small (Table 3). Similar results were seen in males, with a significant association between higher cereal and grain fibre and lower leptin in all models (see online Supplementary Table S4). There were no associations between cereal and grain fibre and hs-CRP, leptin or adiponectin in females (see online Supplementary Table S5). Likewise, fibre sourced from fruits and vegetables was not associated with hs-CRP, leptin or adiponectin overall (Table 3) or in females (see online Supplementary Table S5). In males, fruit and vegetable fibre was associated with lower adiponectin in unadjusted analysis only (see online Supplementary Table S4).
Discussion
In our population cohort of adolescents at 17 years of age, there were no associations between a higher dietary fibre intake and hs-CRP, leptin or adiponectin after adjustment for sex, anthropometry and a number of lifestyle factors. However, a 5 g/d higher fibre intake from cereals and grains had a small but significant association with lower levels of leptin in fully adjusted analysis. In sex-stratified analysis, the same association between cereal and grain fibre and leptin was seen in males but not females. There were no associations between hs-CRP, leptin or adiponectin with fruit and vegetable fibre intake.
The association between cereal and grain fibre and lower leptin concentrations concurs with existing research showing lower levels of leptin in mice fed cereal fibres(Reference Zhang, Jiao and Zhang45). A potential explanation for this association is that a high wholegrain intake has been linked to lower overweight and obesity(Reference Liu, Willett and Manson46) which may in turn reduce leptin(Reference Lopez-Jaramillo, Gomez-Arbelaez and Lopez-Lopez47). In our study, however, the observed association of cereal and grain fibre with lower leptin persisted after adjustment for WHtR and BMI. Similarly, no significant changes in weight or BMI were seen in a study of overweight and obese children where an intervention consisting of increasing wholegrain intake resulted in lower leptin concentrations compared with controls who refrained from consuming wholegrains(Reference Hajihashemi, Azadbakht and Hashemipor48). However, it may be that a more direct measure of fat mass such as body composition measured by dual-energy X-ray absorptiometry is required to determine the impact of adiposity, as dietary fibre may impact visceral adipose tissue independent of subcutaneous adipose tissue(Reference Davis, Alexander and Ventura49). BMI and WHtR may not accurately represent the effect of different types of adipose tissue on inflammation. Beyond overweight and obesity, nutrients such as polyphenols which are found in foods high in cereal and grain fibre may also play a role in the association between cereal and grain fibre and leptin(Reference Fardet50).
The importance of leptin to health is highlighted by the regulatory role it plays in many processes in the body including the immune system(Reference Iikuni, Lam and Lu51). Leptin can stimulate immune cells and has been shown to upregulate production of inflammatory compounds such as TNF-α (Reference Shen, Sakaida and Uchida52). Therefore, through lowering leptin levels, a higher cereal and grain fibre intake may help to reduce chronic inflammation and improve health.
In our study, we showed that at age 17 years, males had higher intakes of dietary fibre than females, and that females had higher concentrations of hs-CRP, leptin and adiponectin than males, consistent with previous work in this cohort at 14(Reference Huang, Mori and Burke53) and 17 years(Reference Le-Ha, Beilin and Burrows54,Reference Ayonrinde, Olynyk and Beilin55) . Similar higher levels of hs-CRP in women than men were also observed in a national study of young American adults(Reference Ford, Giles and Myers56). Concentrations of hs-CRP, leptin and adiponectin are well known to be linked with adiposity(Reference Galic, Oakhill and Steinberg57,Reference Ebrahimi, Heidari-Bakavoli and Shoeibi58) . It is speculated that the sex differences in leptin and adiponectin may be due to higher body fat percentages in females(Reference Christen, Trompet and Noordam59) or through differences in sex hormones(Reference Blum, Englaro and Hanitsch60). The association between male sex and lower hs-CRP, leptin and adiponectin remained significant after adjustment for BMI and WHtR (data not shown).
The lack of an association between total dietary fibre intake and hs-CRP in our study is in contrast to previous studies in healthy adult populations(Reference Lie, Brown and Forrester8,Reference Mazidi, Kengne and Mikhailidis61–Reference Ma, Griffith and Chasan-Taber65) and in adolescents(Reference Parikh, Pollock and Bhagatwala9). However, it is not without precedent, with studies in female young adult and female postmenopausal populations finding no association between dietary fibre intake and hs-CRP(Reference Murakami, Sasaki and Takahashi66,Reference Ma, Hebert and Li67) . A possible explanation for the discrepancy in results relates to how dietary fibre intake was determined. Most studies have used a 24-h dietary recall method to measure diet, in contrast to the FFQ used in our study. Studies using FFQ in healthy populations are scarce, and only one known study reported a significant association between hs-CRP and dietary fibre intake(Reference Bo, Durazzo and Guidi62). FFQ may not accurately represent diet at the time that inflammatory markers were measured, and therefore may not capture the effects of short-term dietary changes on hs-CRP concentrations. Additionally, studies on the impact of dietary fibre intake on hs-CRP are primarily conducted in adult populations. There is insufficient research in adolescents to determine whether age may play a role in the relationship between dietary fibre and inflammation. Adolescents in our study had lower concentrations of hs-CRP than are reported in most studies of adults(Reference Lie, Brown and Forrester8,Reference Mazidi, Kengne and Mikhailidis61,Reference Ajani, Ford and Mokdad63–Reference Ma, Griffith and Chasan-Taber65) . Although an association between higher dietary fibre intake and lower hs-CRP was found in another adolescent population with similar concentrations of hs-CRP, that study used a 24-h dietary recall method to measure dietary fibre intake(Reference Parikh, Pollock and Bhagatwala9).
Strengths and limitations
Strengths of our study include the use of a large population-based pregnancy cohort with comprehensive participant data, allowing for testing of a wide range of confounders. Our dietary data were taken from the Commonwealth Scientific and Industrial Research Organisation FFQ, which includes questions on a wide range of foods, and reflects usual intake over the past 12 months. However, a limitation of the FFQ is that it can be affected by recall bias and does not reflect transient changes in diet which may impact inflammation in the short term. One of the major proposed mechanisms for an effect of dietary fibre on inflammation is through the gut microbiota(Reference Cummings10). The composition of the microbiota responds rapidly to changes in diet, with differences in diversity seen within days of dietary intervention(Reference David, Maurice and Carmody68). Therefore, dietary fibre intake as measured over the previous year with an FFQ may not reflect the current state of the microbiota and its impact on hs-CRP concentrations.
Another limitation is that due to our cross-sectional study design, we could not determine causality. However, we judged cross-sectional analysis to be more appropriate than longitudinal analysis to examine the impact of dietary fibre on inflammation as current diet is a more likely modifier of inflammation than diet from several years earlier(Reference David, Maurice and Carmody68). Additionally, data on leptin and adiponectin concentrations were not available at the previous adolescent follow-up (year 14). Other limitations lie in the availability of adiposity variables; we were unable to adjust for fat mass, which is a more direct measure of overweight and obesity and has been correlated with leptin concentrations(Reference Considine, Sinha and Heiman69).
The study may have been subject to exclusion bias. The number of 17-year participants with complete data was relatively low (621 of 1621 people), with those excluded having less healthy lifestyles and higher levels of hs-CRP and leptin. The exclusion of adolescents with higher inflammation may contribute to the relatively low levels of hs-CRP in our population, potentially impacting the strength of the study to identify associations between dietary fibre intake and inflammatory markers. To address this potential exclusion bias, we applied weighting to the analysis of associations.
Conclusion
An understanding of the effect of low dietary fibre intake on inflammatory markers during adolescence is of benefit in designing dietary interventions to prevent inflammatory diseases in adulthood. Our study does not provide evidence of an independent effect of dietary fibre intake on plasma hs-CRP, leptin and adiponectin in adolescents. However, our results suggest that a higher intake of cereal and grain fibre specifically may contribute to lower leptin concentrations. This may improve health by reducing production of inflammatory cytokines thereby reducing inflammation. Randomised controlled trials in adolescent age groups are required to expand on and confirm our findings.
Acknowledgements
The authors are grateful to the Raine Study participants and their families and thank the Raine Study research staff for cohort coordination and data collection. The core management of the Raine Study is funded by the University of Western Australia, Curtin University, Telethon Kids Institute, Women and Infants Research Foundation, Edith Cowan University, Murdoch University, The University of Notre Dame Australia and the Raine Medical Research Foundation. The authors thank the National Health and Medical Research Council of Australia for their long-term contribution to funding the study over the last 30 years and the Telethon Kids Institute for long-term support of the Raine Study.
The present work was supported by the Heart Foundation Beyond Blue Strategic Research Programme (ID G08P4036 2009–2012). The Raine Study received funding from the Raine Medical Research Foundation at The University of Western Australia, the National Health and Medical Research Council of Australia, the Telstra Research Foundation, the Western Australian Health Promotion Foundation and Australian Rotary Health Research Fund. Data collection and biological specimens at the 17 years follow-up were funded by the National Health and Medical Research Council of Australia Programme Grant ID 353514 and Project Grant no. 403981. TAM is supported by an National Health and Medical Research Council of Australia Research Fellowship (ID 1136046).
O. G. S – formulating the research question, study design, analysis, interpretation, drafting and preparation of final manuscript. M. B. – formulating the research question, study design, analysis, interpretation, drafting and review of final manuscript. M. K. – formulating the research question, study design, interpretation, drafting and review of final manuscript. T. A. O. – study design, interpretation, review of final manuscript. T. A. M. – study design, interpretation, review of final manuscript. L. J. B. – study design, interpretation, review of final manuscript. W. H. O. – formulating the research question, study design, interpretation, drafting and review of final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520001609








