Osteoarthritis (OA) is the most common degenerative joint disease in the USA and the fastest cause of disability and pain. The prevalence of OA among American adults 20 years and older had increased from 6·6 % in 1999 to 14·3 % in 2014(Reference Park, Mendy and Vieira Edgar1).The number of OA cases in the USA is expected to increase partly due to the obesity epidemic and the ageing population. Globally, approximately 18 % of women aged over 60 and 9·6 % of men have symptomatic OA. In addition, OA has been estimated to affect 130 million people by 2050(Reference Conaghan, Porcheret and Kingsbury2).
Inflammation has been reported to play a potential role in the development of OA(Reference Hackney Alisha, Jennifer and Resnick3). This role is already recognised through several features. For example, synovitis (defined as the inflammation of the synovium) is a common early symptom in patients with OA of the joint(Reference Damman, Liu and Reijnierse4). Epidemiological studies have shown that serum C-reactive protein levels are significantly associated with the incidence and progression of OA(Reference Kondo, Takegami and Ishizuka5). Other studies have demonstrated a positive association between the serum levels of C-reactive protein and the histological evidence of synovitis before joint replacement(Reference Shadyab, Terkeltaub and Kooperberg6–Reference Yang, Ruan and Xu8). These observations strongly suggest that the systemic inflammation observed in OA reflects, at least in part, local synovial inflammation.
The dietary inflammation index (DII) is a literature-derived dietary tool that is used to assess the overall inflammatory potential of an individual’s diet(Reference Li, Zhan and Huang9). The association of high DII values with high serum IL-6 and TNF-α levels suggests a strong relationship between this index and inflammatory parameters. DII is also used to assess the association of dietary inflammation with metabolic syndrome, asthma, breast cancer, colorectal cancer and fractures(Reference Wood, Shivappa and Berthon10–Reference Morimoto, Shivappa and de Souza Genaro13). However, to our knowledge, no study has explored the relationship between DII and OA.
Physical activity (PA) is related to the regulation of the innate immune system and the treatment of OA(Reference Skou, Bricca and Roos14). For example, regular PA may increase the body’s serotonin synthesis, improve noradrenergic neurotransmission, trigger the release of endorphins and reduce the long-term increased sympathetic nervous system activity, thereby reducing systemic inflammation in the elderly(Reference Draganidis, Jamurtas Athanasios and Stampoulis15). In addition, individuals who regularly engage in PA are less likely to develop symptoms of OA in the future than those who do not. The possible mechanism is PA can prevent inflammation of chondrocytes and cartilage matrix by reducing IL-6 and TNF-α and other OA-related inflammatory markers, delaying the degeneration of articular cartilage, thereby improving joint function. At the same time, it may increase the level of protective inflammation markers in the body to inhibit the effect of OA progression(Reference Joseph Kenth, Dagfinrud and Christie16,Reference Liao, Liao and Liou17) . Therefore, regular PA may play a central role in maintaining physical and mental health. However, until now, no studies have examined whether PA acts as an intermediate mechanism in the relationship between low-grade systemic dietary inflammation and subsequent OA.
Therefore, on the basis of the data from the National Health and Nutrition Examination Survey (NHANES), the present study aims to assess the association between E-DII and OA prospectively and to evaluate the potential interactions and the mediating role of PA in this relationship.
Methods
Study population
In the present study, we analysed the data collected from NHANES. NHANES is a cross-sectional study designed to assess the health and nutritional status of adults and children in the USA, which is conducted by the Centers for Disease Control and Prevention. The data were collected through questionnaire surveys, physical examinations, household interviews including demographic, socio-economic, dietary, health-related questions and examinations, and laboratory tests. Detailed information about survey design and methodologies can be found elsewhere(18). In this study, a total of 1249 participants aged 65 years were selected from the 2011–2016 NHANES (Fig. 1).
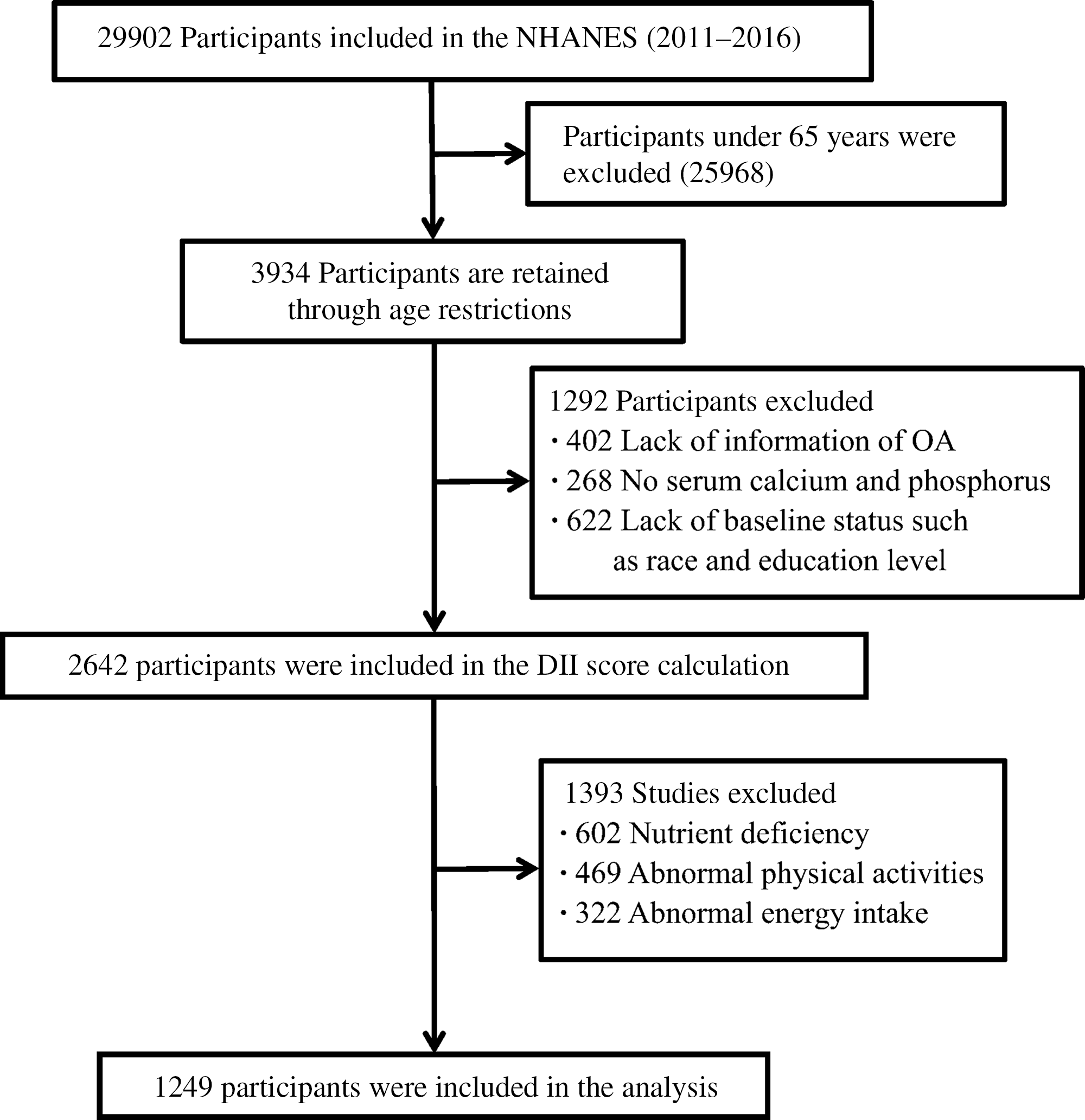
Fig. 1. Selection process of subjects.

Fig. 2. A restricted cubic spline curve was used to fit the relationship between Energy-Adjusted Dietary Inflammatory Index (E-DII) and the risk of osteoarthritis (OA) (the three nodes are located at the 25th, 50th and 75th percentiles). Risk estimates were adjusted for age, sex, BMI, education level, race, hypertension, diabetes, marital status, poverty:income ratio (PIR), serum calcium and phosphorus. The reference value for OR: median score (1·22); the solid black line represents the OR and the shaded part the lower and upper 95 % CI; P Overall < 0·001; P Nonlinear = 0·012.
Energy-Adjusted Dietary Inflammatory Index
There are some studies on the development and verification of DII in detail(Reference Shivappa, Steck and Hurley19). Research from more than 1900 peer-reviewed publications forms the basis of DII. The ‘Inflammatory Effect Score’ was created from these peer-reviewed publications for each DII food parameter, based on their impact on inflammatory cytokines. The calculation of the dietary inflammatory index links the personal dietary data obtained in each clinical study with the global average intake(Reference Shivappa, Steck and Hurley19). The world database provides standard averages and deviations of all DII food parameters. For each food parameter, create a Z-score by subtracting the individual’s estimated intake from the standard average. It is then divided by the world standard deviation and converted to a distribution centred at 0 and bounded between −1 and +1. This value is then multiplied by the inflammatory effect score for each food parameter, and then all food parameters are added together to create an overall DII score. The more positive scores mean the more pro-inflammatory diet, the more negative values, the stronger the anti-inflammatory effect(Reference Tabung, Smith-Warner and Chavarro20).
DII calculation formula:
In this study, twenty-seven of the forty-five food parameters can be obtained through NHANES data, including grams of alcohol, protein, fibre, fat, carbohydrates, cholesterol, n-3 and n-6 polyunsaturated fatty acids, saturated fatty acids/MUFA/PUFA, Mg, niacin, Zn, Fe, riboflavin, folic acid, β-carotene, caffeine, Se, thiamine and vitamins A, B6, B12, C, D and E. DII scores range from negative tail to positive tail, more negative values indicate anti-inflammatory properties and corrected scores indicate pro-inflammatory properties. E-DII represents the energy-adjusted amount of DII per 1000 energies in food intake to explain the impact of total intake on energy intake.
Physical activity
In NHANES, Participants self-reported their activity patterns through questions derived from the Global Physical Activity Questionnaire(Reference Hallal, Andersen and Bull21). Each participant was required to recall the type, frequency (exercise days per week) and duration (exercise times/d) of PA they had undertaken during the past 7 d for a minimum of 10 min, including moderate and vigorous intensity related to work, transport and recreational activities.
Moderate/vigorous intensity activities are defined as activities that require moderate/hard physical effort and cause small/large increases in breathing or heart rate. Work-related physical activities are described as paid or unpaid work, household chores and yard work. For recreational PA, the information referred to sports, fitness and recreational activities. The active transportation domain referred to all walking and bicycling activities.
The metabolic equivalents of task (MET) are a unit used to describe the energy expenditure of a specific activity, where 1 MET refers to 3·5 ml O2 kg-1/min(Reference Hallal, Andersen and Bull21). The suggested MET is 8·0 for vigorous work-related activity and vigorous leisure-time PA, while for moderate work-related activity, walking or bicycling for transportation and moderate leisure-time PA is 4·0(22). Each PA is assessed by MET-min/week by multiplying the days spent performing this activity by the mean duration by the suggested MET and sum each activity value to obtain an estimate of total PA.
Osteoarthritis status
Information regarding the assessment of OA was obtained by self-report questionnaire. A previous study demonstrated great consistency (85 %) between self-reported OA and clinically confirmed OA(Reference Loprinzi Paul23). Participants were asked, ‘Has a doctor or other health professional ever told you that you had arthritis?’ If the answer was yes, a follow-up question was ‘Which type of arthritis was it?’ The participants were classified as OA groups based on the answers to the latter question. Those who did not have or had any signs or symptoms of arthritis were placed in the no arthritis group.
Study covariates
According to previous research, the multivariable model contains latent variables that confuse the association between E-DII and OA, which include age (continuous), race/ethnicity (category), BMI category, poverty:income ratio (continuous), hypertension (category), diabetes (category) and serum Ca (continuous) and serum phosphorus (continuous). NHANES calculated the BMI categories defined as four levels: underweight (BMI < 18·5), normal weight (18·5 ≤ BMI < 24·0), overweight (24·0 ≤ BMI < 28·0) and obesity (BMI ≥ 28·0). Poverty:income ratio is calculated by dividing the family income (based on the poverty criteria specific to the size of the family) by the year and state and is used to embody the socio-economic status of the participant in the entire family. Race/ethnicity is coded as non-Hispanic white, non-Hispanic black, Hispanic (Mexican American and other Hispanics), etc.
Statistical analyses
The baseline characteristics of the study population were reported as the median (interquartile range, IQR) of continuous variables and the number (percentage) of categorical variables. We used a logistic proportional hazard model to estimate OR and 95 % CI.
DII is modelled in three different ways. For our main analysis, E-DII was included as a continuous variable, and we used an estimate of the effect of the 1-sd increase (Z-score) in E-DII. This method assumes a linear relationship between E-DII and OA. To verify the hypothesis, we then used a multivariable restricted cubic spline and placed three nodes at the 25th, 50th and 75th percentiles of the E-DII distribution node to provide a graphical representation. The spline curve allows us to evaluate whether there was a significant difference from the linear correlation. Finally, we divided E-DII into quarters and used the first quarter group as the reference category in the logical model.
Three models were proposed: the first model did not adjust for confounding factors; the second model further adjusted for known risk factors for depression and potential confounding factors, such as age, gender, BMI, education level, marital status, race, hypertension, diabetes, serum Ca and phosphorus concentrations; and the third model further adjusted PA. Confounding factors were pre-selected based on previously published articles and were associated with OA and E-DII(Reference Loeser, Beavers and Bay-Jensen24,Reference Veronese, Shivappa and Stubbs25) .
The interaction of E-DII and PA on the risk of OA was estimated by including a multiplication term between the two variables in the logistic model. Considering statistical significance, we examined the association between E-DII and OA stratified by quartile based on PA.
Regression-based mediation analysis was used to distinguish the direct effect of adherence to E-DII on the risk of depression and the indirect effect mediated by BMI. Three estimates were obtained as follows:
-
Total effect, that is, the overall association between E-DII and the risk of depression, including the association mediated by BMI.
-
Direct effect, that is, the association between E-DII and depression risk, adjusted according to BMI.
-
Indirect effect, that is, the association between E-DII and depression risk is mediated by BMI.
In addition, to effectively understand the complex relationship between E-DII, PA and OA, we used a counterfactual mediation model that allows exposure of mediators to interact. According to this model, PA represents the relationship between E-DII and OA(Reference Valeri and Vanderweele26–Reference Böhnke Jan28).
All statistical analyses were performed using the software package R (http://www.R-project.org, The R Foundation). A two-tailed P-value of <0·05 was considered statistically significant.
Results
Baseline characteristics
A total of 1249 participants were eligible for this study (median (interquartile range, IQR) age, 72 (68–78) years; 701 (56·1 %) male), of which 401 (32·1 %) cases of OA were identified based on self-report. The average E-DII in this study was +0·68 (se 0·08), and the score ranged from –5·32 (most anti-inflammatory) to +4·26 (most pro-inflammatory). Table 1 shows the distribution of characteristics across E-DII quartiles. Compared with subjects in the most anti-inflammatory E-DII category, the most pro-inflammatory subjects were more likely to be young, male, college degree or above, non-Hispanic white, normal weight, had lower serum Ca and phosphorus concentrations (P < 0·05) (Table 1).
Table 1. Distribution of characteristics across quartiles of Dietary Inflammatory Index (DII) in National Health and Nutrition Examination Surveys, USA, 2011–2016*
(Mean values and standard deviations; numbers and percentages)

PIR, poverty:income ratio.
* Quartile 1: −5 32 to −0 08; Quartile 2: −0 07, 1 22; Quartile 3: 1 23 to 2 26; Quartile 4: 2 27 to 4 26.
Energy-adjusted Dietary Inflammatory Index and osteoarthritis
Table 2 shows the rough and adjusted logistic regression results, describing the odds of OA compared with non-OA. In the first two models, E-DII was positively associated with the risk of OA in the second quartile (OR: 1·16 (95 % CI: 1·06, 1·68)) to the fourth quartile (OR: 1·64 (95 % CI: 1·13, 2·37)) compared with the first quartile. The analysis using E-DII to add 1-sd produced similar results (model 2: OR: 1·12 (95 % CI: 1·05, 1·35)). The spline variable confirmed that E-DII was non-linearly related to the risk of OA (P = 0·012), and the graph shows that the increase in E-DII was accompanied by an increase in the risk of OA (as shown in Fig. 2). After including PA (model 3), all associations weakened and became invalid (P > 0·05) (Table 2).
Table 2. Risk of osteoarthritis according to quartile groups of Energy-Adjusted Dietary Inflammatory Index (E-DII) (n 1249)
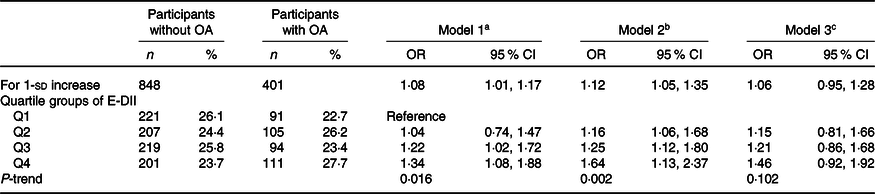
OA, osteoarthritis; PIR, poverty:income ratio; PA, physical activity.
a Model 1: unadjusted.
b Model 2: age, sex, BMI, education level, race, hypertension, diabetes, marital status, PIR, serum Ca and phosphorus.
c Model 3: further adjusted for PA.

Fig. 3. Mediation analyses without (a) and with (b) an interaction between Energy-Adjusted Dietary Inflammatory Index (E-DII) and physical activity (PA) on the risk of osteoarthritis (OA). (a) Logistic model adjusted for age, sex, BMI, education level, race, hypertension, diabetes, marital status, poverty:income ratio (PIR), serum calcium and phosphorus. (b) The 95 % CI of these estimates was computed using the bootstrap method (1000 samples).
Energy-adjusted Dietary Inflammatory Index and osteoarthritis risk stratified by physical activity category
Table 3 shows the association between E-DII and OA stratified by quartile categories of PA. An interaction between E-DII and PA on the risk of OA was observed (P Interaction < 0·001). When stratified by PA category, among people with low levels of PA, the risk of E-DII and OA were significantly positively correlated (model 2: OR: 2·12 (95 % CI: 1·46, 3·26), fourth quartile of E-DII v. the first quartile). At the same time, in people with high levels of PA, low anti-inflammatory levels showed a protective effect on OA (model 2: OR: 0·75 (95 % CI: 0·51, 0·90), the second quartile of E-DII v. the first quartile), and no significant association was observed in other populations.
Table 3. Risk of osteoarthritis by quartile of dietary inflammatory index stratified according to physical activity
(Numbers and percentages, n 1249)

OA, osteoarthritis; Q, quartile; PIR, poverty:income ratio; E-DII, Energy-adjusted Dietary Inflammatory Index; PA, physical activity.
a Model 1: unadjusted.
b Model 2: age, sex, BMI, education level, race, hypertension, diabetes, marital status, PIR, serum Ca and phosphorus.
c P Interaction was calculated using the multiplicative interaction term (E-DII × PA).
The mediating role of BMI
The result of the mediation analysis is shown in Fig. 3. First, we hypothesised a simple mediation model without the effect of the E-DII × PA interaction on the risk of OA. An increase in E-DII was associated with an increased risk of OA. This effect (22·82 %) could be explained by the significant indirect effect of PA (natural indirect effect: OR: 1·058 (95 % CI: 1·012, 1·126), as shown in Fig. 3(a)). Then, based on the aforementioned interaction of E-DII and PA on the risk of OA, we used a mediation model that allows exposure–media interaction. According to this model, PA was both a mediating factor and an influencing factor in the relationship between E-DII and OA. An increase in E-DII was also associated with an increased risk of OA (natural indirect effect and natural indirect effect OR: 1·056 (95 % CI: 1·012, 1·092) and OR: 1·032 (95 % CI: 1·016, 1·068)), but the proportion explained by the indirect influence of PA was low (20·08 %, shown in Fig. 3(b)).
Discussion
On the basis of the baseline data of 1249 elderly people in the NHANES, we found a positive and nonlinear relationship between the baseline inflammation characteristics of diets and the risk of OA and that this relationship was independent of most known or potential risk factors or confounding factors. Participants with a higher DII (corresponding to the increased proinflammatory potential of diets) had a higher risk of OA than participants with a lower DII (corresponding to anti-inflammatory diets). This association was partly mediated by PA and its interaction with DII.
Observational studies on single dietary ingredients have not been confirmed through randomised trials. For example, compared with placebos, vitamin E supplements are ineffective, and vitamin D does not have a clinically important effect on OA joint pain(Reference Liu, Machado Gustavo and Eyles Jillian29). However, people do not consume nutrients or food in isolation. In the past few years, people have increasingly emphasised the importance of diet as a whole(Reference Hu Frank30,Reference Sacks, Obarzanek and Windhauser31) . Using the dietary index to quantify the inflammatory potential of the entire diet may increase the robustness and effectiveness of detecting the relationship between diseases(Reference Hu Frank30). DII and logically expanded E-DII explain the relationship between various nutrients and foods and their underlying inflammation, and evidence showing that these nutrients and foods exert a proinflammatory or anti-inflammatory effect in diets exists(Reference Hallal, Andersen and Bull21). Our research is an improvement on previous observational studies that focused on single dietary components. This is a study that used DII to evaluate the relationship between the inflammatory potential of diets and OA. Our findings, if demonstrated in other large-scale prospective studies, may provide information for the development of prevention strategies with increased emphasis on the overall effect of diets for this highly prevalent debilitating disease.
A large number of studies have shown that DII is significantly related to serum inflammation markers(Reference Cavicchia, Steck and Hurley32–Reference Kaluza, Harris and Melhus35). Therefore, the site of OA inflammation can be assessed early before a considerable radiological change (synovitis)(Reference Sokolove and Lepus36). These findings have also been confirmed by some studies using MRI(Reference Felson David, McLaughlin and Goggins37,Reference Krasnokutsky, Belitskaya-Lévy and Bencardino38) . Therefore, chronic low-grade inflammation may be the main driver of persistent bone degeneration. Our findings indirectly confirmed the role of inflammation in the prediction of OA stage. Second, changes in the extracellular matrix may play an additional role in the association between DII and OA. Extracellular matrix breakdown is common in areas of inflammation, including bones affected by OA(Reference Krasnokutsky, Belitskaya-Lévy and Bencardino39). In addition, studies have demonstrated that extracellular matrix breakdown products themselves can promote inflammation and cartilage loss(Reference Homandberg and Hui40). Therefore, proinflammatory diets may further promote the process of cartilage loss. Finally, considering that individuals with significantly higher DII levels ate meat, sugar and fat more frequently and consumed fewer vegetables and fruits than those with lower DII levels, DII can be assumed to be negatively correlated with healthy eating patterns (such as the Mediterranean diet), which may play a role in protecting people from OA attacks.
To the best of our knowledge, this is the first study to show that weekly moderate/vigorous exercise is an important part of the mediation of the relationship between systemic low-grade dietary inflammation and the subsequent increased risk of OA. The consistency of this finding with previous results indicated that PA had a central role in bone health and the regulation of peripheral inflammatory processes(Reference Cleland Brice, Papanek and Ingraham Benjamin41,Reference Tomlinson David, Erskine Robert and Morse Christopher42) . Routine PA has been associated with a reduced risk of OA in people aged 50 years and older. Moreover, previous studies have revealed a correlation between systemic inflammation and PA. In this study, low PA was found to be significantly associated with an increased risk of OA. In addition, elevated DII was significantly associated with low PA(Reference Hamaguchi, Kurihara and Fujimoto43). Therefore, our results indicated that individuals with elevated levels of systemic inflammation may be less likely to exercise moderately or vigorously every week than those without. This situation may increase the risk of experiencing elevated levels of OA. Importantly, PA explained approximately one-fifth of the total effect of systemic dietary inflammation on the subsequent elevation of OA. Future research needs to identify other intermediate mechanisms that explain this relationship. Our findings indicated that conventional PA may not only be a valuable tool for preventing OA but may also have potential anti-inflammatory effects.
The exact biological mechanism that links the inflammatory process to OA through the action of PA is not fully understood. However, systemic low-grade inflammation and PA have been suggested to act on the same effective system of the body: the hypothalamic–pituitary–adrenal axis and the sympathetic nervous system(Reference Cotman Carl, Berchtold Nicole and Christie44). For example, elevated levels of proinflammatory cytokines are repeatedly associated with the overactivation of the hypothalamic–pituitary–adrenal axis, the excessive secretion of stress hormones (such as cortisol), the impaired production of serotonin and dopamine and the initiation of oxidative stress. In addition, regular PA can increase the body’s serotonin synthesis, improve noradrenergic neurotransmission, trigger the release of endorphins and reduce long-term increased sympathetic nervous system activity(Reference Picke, Sylow and Møller Lisbeth45,Reference Kawao, Iemura and Kawaguchi46) . PA is also related to the increase in muscle-derived IL-6. In this case, IL-6 can act as an anti-inflammatory muscle factor by inhibiting TNF-α. Importantly, these shared biological mechanisms play a major role in the pathophysiology of OA(Reference Bressi, Cagliari and Contesini47,Reference Qi, Liu and Lu48) .
Our research still has some limitations. First of all, the main limitation of the current design is that NHANES is a cross-sectional database and thus severely hindered our ability to perform causal reasoning. Second, dietary intake was self-reported, with recall bias, estimated on the basis of a 24-h history, and could not reflect changes in daily dietary intake. Among the forty-five possible food parameters, only twenty-eight can be used for the calculation of DII, and previous studies have successfully verified the modified DII (twenty-eight items) in NHANES. However, these unbalanced twenty-seven items (if they are not equally divided into proinflammatory and anti-inflammatory item) may also erroneously distort the inflammatory potential of diets. Third, the measurement of PA was based on self-reporting rather than objective methods, and the risk of self-reporting bias was present. Finally, the judgements of study participants’ OA, diabetes and hypertension were based on self-report, and there is no clinical diagnosis.
Conclusion
In summary, our findings indicated that a diet with high pro-inflammatory potential was associated with a higher risk of OA in the elderly, and PA was one of the main mediators that may occur here. Our findings may provide some evidence for understanding the underlying mechanisms of dietary inflammation, PA and OA. Considering the cross-sectional design of NHANES, it is necessary to conduct further prospective cohort studies.
Acknowledgements
The present study used data from the NHANES. We thank all of the participants and staff involved in the surveys.
The project was supported by the National Natural Science Foundation of China (Grant Nos. 81974339 and 81873988) and the Science and Technology Plan Project of Hunan Province (Grant No. 2021GK2012). The funders did not play any role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Y. H., J. X. and H. W. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Y. H., J. X. and H. W. Acquisition, analysis or interpretation of data: All authors. Drafting of the manuscript: Y. H., J. X., H. W. and R. L. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: H. W., R. L., W. T., W. S. and M. Z. Obtained funding: Y. H. and J. X. Administrative, technical or material support: H. W., R. L., J. Y. and X. F. Supervision: Y. H.
There are no conflicts of interest.









