Pancreatic cancer is a common cancer with poor prognosis, ranking as the fourth cause of cancer death in both sexes combined in the EU, and it is one of the few cancers for which mortality has not declined over the past three decades(Reference Sung, Ferlay and Siegel1,Reference Santucci, Carioli and Bertuccio2) . Tobacco smoking and diabetes are the best recognised risk factors for pancreatic cancer(Reference Kleeff, Korc and Apte3–Reference Rosato, Polesel and Bosetti5). High alcohol intake was also associated with excess pancreatic cancer risk(Reference Lucenteforte, La Vecchia and Silverman6,Reference Tramacere, Scotti and Jenab7) . Some of the risk factors for this malignancy, such as obesity, high waist circumference and diabetes, are strongly related to diet(Reference Mizrahi, Surana and Valle8–Reference Schlesinger, Neuenschwander and Schwedhelm13). A western dietary pattern, high in animal products and red meat, has been shown to increase pancreatic cancer risk(Reference Zheng, Guinter and Merchant14–Reference Di Maso, Talamini and Bosetti16).
The branched-chain amino acids (BCAA), i.e. leucine, isoleucine and valine, are a subgroup of the nine essential amino acids. They derive from protein food sources, mainly animal food products such as meat, fish and dairy products(Reference Sivanand and Vander Heiden10,Reference de la, Zazpe and Ruiz-Canela17) .
BCAA have been associated with various cardiometabolic conditions such as type 2 diabetes, insulin resistance and adiposity(Reference de la, Zazpe and Ruiz-Canela17–Reference Zheng, Li and Qi22). They have also been associated with the risk of selected cancers, including those of the colorectum and the breast(Reference Rossi, Mascaretti and Parpinel23–Reference Zeleznik, Balasubramanian and Ren25). Three epidemiological studies investigated the relationship between BCAA and pancreatic cancer risk, reporting positive associations with circulating BCAA plasma levels, but no data were available on BCAA estimates from diet to date(Reference Tobias, Hazra and Lawler24,Reference Katagiri, Goto and Nakagawa26,Reference Mayers, Wu and Clish27) . In addition, in vivo and in vitro studies support the implication of BCAA in the development and progression of pancreatic cancer(Reference Lee, Cho and Kim28–Reference Li, Yin and Wang30).
We analysed the relationship between dietary BCAA intake and pancreatic cancer risk in an Italian multicentric study.
Methods
We used data from a multicentric case–control study of pancreatic cancer, conducted between 1991 and 2008 in the province of Pordenone and in the greater Milan area, northern Italy(Reference Polesel, Talamini and Negri15). The study included 326 cases (174 men, 152 women, median age 63 years and range 34–80 years) with incident cancer of the pancreas. Eighty-four per cent of cases were interviewed within one month from diagnosis and the remaining cases within one year. Controls were 652 patients (348 men, 304 women, median age 63 years and range 34–80 years) admitted to the same teaching or general hospitals as cases for a wide spectrum of acute nonneoplastic conditions, unrelated with digestive tract diseases, smoking, alcohol consumption or long-term modifications of diet. Controls were hospitalised for traumatic orthopaedic disorders (31 %), other orthopaedic disorders (31 %), acute surgical conditions (28 %) and miscellaneous other illnesses (10 %), including eye, nose, ear, skin or dental disorders. Controls were frequency-matched to cases by study centre, sex and age (±5 years), with a control to case ratio of 2:1. Less than 5 % of the approached cases and controls refused to participate in the study. All enrolled subjects signed an informed consent, according to the recommendations of the Board of Ethics of each participating centre. All procedures were performed in accordance with the ethical standards according to the Declaration of Helsinki.
Cases and controls were interviewed in hospital by centrally trained interviewers, with the use of a standard, structured questionnaire. The questionnaire included information on socio-demographic and anthropometric factors at different ages, selected lifestyle habits, such as history of tobacco use, and physical activity, personal medical history of selected diseases including diabetes and family history of cancer in first-degree relatives.
For the dietary assessment during the years preceding cancer diagnosis or hospital admission, a validated and reproducible FFQ was used(Reference Franceschi, Negri and Salvini31,Reference Decarli, Franceschi and Ferraroni32) . Subjects were asked to indicate their average weekly consumption of seventy-eight food items, food groups or recipes. Data on history of consumption of alcoholic beverages were also collected in an additional section that includes five items (resulting in a total of eighty-three FFQ items).
We used an Italian food composition database to estimate total energy, nutrients, Ca, vitamin D and BCAA intake of study participants(Reference Gnagnarella, Parpinel and Salvini33,Reference Bravi, Polesel and Bosetti34) . Data on BCAA were available for leucine, isoleucine and valine. Given the high collinearity between leucine, isoleucine and valine (r∼1·00), we analysed total BCAA intake as the sum of their individual intakes.
BCAA intake was categorised into quartiles based on the distribution of controls, both directly on the BCAA intakes and on the residuals of the regression of BCAA on energy(Reference Willett and Stampfer35). Since both analyses yielded similar results, only findings from the first approach are presented. Using the lowest quartile as reference, OR of pancreatic cancer for BCAA quartiles and the corresponding 95 % CI were estimated by logistic regression models, conditioned on study centre, sex and age, and further adjusted for year of interview (continuous variable), years of education (< 7, 7–11, ≥ 12; categorically), BMI (< 22, 22–24·9, 25–29·9, ≥ 30 kg/m2; categorically), cigarette smoking (never smoker, former smoker, current smoker of < 15 and ≥ 15 cigarettes per day; categorically), history of diabetes (yes, no), family history of pancreatic cancer (yes, no), alcohol intake (never drinker, ever drinker of < 7, 7– 20·9 and ≥ 21 drinks per week; categorically) and total energy intake (tertiles; categorically). We also run unconditional regression models and found no appreciable changes in the OR estimates. Tests for trend were based on the likelihood ratio test between models with and without a linear term for BCAA. We further adjusted the OR by including in turn in the model terms for other dietary factors both correlated with BCAA intakes (r > 0·50) and previously associated to pancreatic cancer risk(Reference Polesel, Talamini and Negri15,Reference Bravi, Polesel and Bosetti34,36) , including vegetable protein (r∼0·69), total lipids (r∼0·77), fibre (r∼0·51), folate (r∼0·69), vitamin E (r∼0·56), vitamin D (r∼0·58), Ca (r∼0·74) intakes and red meat consumption (r∼0·64).
Sensitivity analyses were performed excluding in turn subjects with diabetes, with family history of pancreatic cancer and with outliers in energy intake (< 500 or ≥ 4000 kcal/d).
We computed the OR for BCAA quartiles in strata of sex, age (< 60 and ≥ 60 years), BMI (<25 and ≥ 25 m/kg2), smoking status (non and current smokers) and alcohol consumption (no or light: < 7, moderate: 7–20·9, heavy: ≥ 21 drinks/week). We estimated heterogeneity among strata through the likelihood ratio test comparing the models with and without interaction terms. We also computed OR for combined categories of BCAA intake and smoking status or alcohol drinking.
Results
Table 1 shows the distribution of 326 pancreatic cancer cases and 652 controls according to sex, age and other characteristics. By design, cases and controls had similar distributions for sex, age and centre. Cases were more frequently interviewed after 2000 than controls, but there was no cluster of cases and/or controls in any specific calendar year. Cases were more educated, more frequently smokers and reported more frequently a history of diabetes than controls.
Table 1. Distribution of 326 patients with pancreatic cancer and 652 control patients according to sex, age, education and other selected variables (Italy, 1991–2008)

* The sum does not add up to the total because of some missing values.
† History of diabetes one year before cancer diagnosis.
Table 2 gives the number and percentage of cases and controls, the OR and their corresponding 95 % CI according to quartiles of BCAA intake, with the lowest quartile as reference category. We found a positive association between the BCAA intake and pancreatic cancer risk from the third quartile onward (OR for the third quartile = 1·88, 95 % CI = 1·08, 3·26; OR for the fourth quartile = 2·17, 95 % CI = 1·17, 4·06), with a significant trend in risk.
Table 2. Odds ratio (OR)* of pancreatic cancer and corresponding 95 % confidence interval (CI) according to quartiles† of branched-chain amino acid (BCAA) intakes among 326 cases with pancreatic cancer and 652 controls (Italy, 1991–2008)

* Estimated through a logistic regression model, conditioned on age, centre and sex and adjusted for year of interview, education, BMI, diabetes, family history of pancreatic cancer, smoking, alcohol and total energy intake.
† Based on the controls’ distribution.
Table 3 shows the OR for BCAA intake quartiles, compared with the lowest one, after adjustment in turn for selected dietary factors. Allowance for vegetable protein, lipids, fibre, folate, vitamin E, vitamin D and Ca intake, as well as for red meat consumption, did not appreciably modify the association.
Table 3. Odds ratio (OR)* of pancreatic cancer and corresponding 95 % confidence interval (CI) according to quartiles† of branched-chain amino acid (BCAA) intake among 326 cases with pancreatic cancer and 652 controls after adjustment of selected dietary factors (Italy, 1991–2008)

* Estimated through a logistic regression model, conditioned on age, centre and sex and adjusted for year of interview, education, BMI, diabetes, family history of pancreatic cancer, smoking, alcohol, total energy intake and, in turn, intake of each dietary factor.
† Control-generated quartiles.
‡ Reference category.
After exclusion of forty-seven cases and thirty-seven controls with a history of diabetes, the OR for the highest quartile of BCAA intake compared with the lowest one slightly increased to 2·49 (95 % CI = 1·29, 4·83). Similarly, after the exclusion of ten cases and fifteen controls with family history of pancreatic cancer, the OR became 2·41 (95 % CI = 1·28, 4·55). When we excluded seventeen cases and nineteen controls with outliers in energy intake, the OR became 2·06 (95 % CI = 1·09, 3·88) (data not shown).
Table 4 shows the relation between BCAA intake and pancreatic cancer risk in strata of sex, age, BMI, smoking status and alcohol consumption. The associations were apparently stronger in men, young individuals, those with higher BMI, in smokers and subjects with higher alcohol intake, although in the absence of significant heterogeneity.
Table 4. Odd ratio (OR)* of pancreatic cancer and corresponding 95 % confidence interval (CI) for quartiles† of branched-chain amino acid (BCAA) intakes among 326 cases with pancreatic cancer and 652 controls according to strata of selected covariates (Italy, 1991–2008)

* Estimated through logistic regression models, conditioned on age, centre, sex and adjusted for year of interview, education, family history of pancreatic cancer, BMI, diabetes, smoking, alcohol and total energy intake, unless the variable was the stratification factor.
† Control-generated quartiles.
‡ The sum does not add up to the total because of some missing values.
We also evaluated the combined effect of dietary BCAA exposure and lifestyle behaviours, such as tobacco status and alcohol consumption on pancreatic cancer risk (Fig. 1). Using as reference group non-smokers in the lowest two quartiles of BCAA intake (102 cases and 259 controls), the OR in the two highest quartiles of BCAA intake were 1·44 (95 % CI = 0·91, 2·30) among non-smokers (121 cases and 258 controls) and 3·64 (95 % CI = 1·97, 6·73) among current smokers of ≥ 15 cigarettes/d (forty-seven cases and forty controls) (Fig. 1(a)). Using as reference group never/light alcohol drinkers (0–< 7 drinks/week) in the first two quartiles of BCAA intake (fourty-eight cases and 133 controls), never/light alcohol drinkers in the two highest BCAA quartiles (thirty-nine cases and eighty-six controls) had an OR of 1·68 (95 % CI = 0·88, 3·20) and heavy alcohol drinkers (≥ 21 drinks/week) in the two highest BCAA quartiles (ninety cases and 119 controls) had an OR of 4·57 (95 % CI = 2·28, 9·15) (Fig. 1(b)).
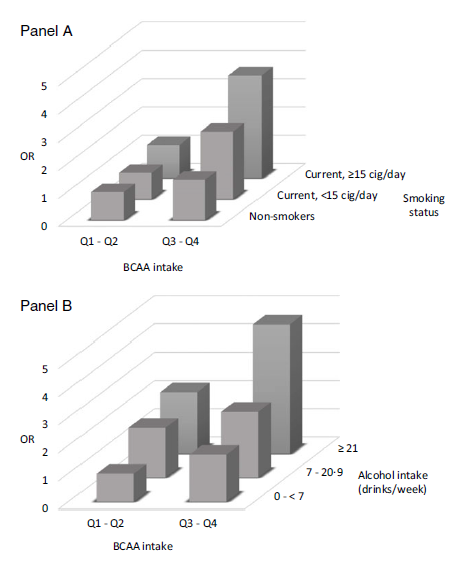
Fig. 1. Odd ratios (OR)* and corresponding 95 % confidence interval (CI) according to combination of quartiles of branched-chain amino acid (BCAA) intake and smoking status (Panel A) or alcohol consumption (Panel B) among 326 cases with pancreatic cancer and 652 controls. Italy, 1991–2008.
Discussion
Our study found a positive association between total dietary BCAA intake and pancreatic cancer risk, after adjusting for several potential confounding factors, including tobacco smoking, BMI and history of diabetes and additional nutritional factors. The excess risk became substantial in combination with high tobacco or alcohol consumption.
To our knowledge, no previous study evaluated the relation between dietary BCAA intake and pancreatic cancer risk. Higher circulating BCAA levels have been positively associated with pancreatic cancer risk in different study populations. In a combined analysis on four large US cohorts, including 454 pancreatic cancer cases, elevated plasma levels of BCAA were associated with a twofold increased risk of pancreatic cancer. The OR in the highest quintile of total plasma BCAA compared with the lowest one was 2·01 (95 % CI, 1·34, 3·03)(Reference Mayers, Wu and Clish27). When authors considered separately BCAA plasma levels, they found similar OR for leucine (OR = 1·97; 95 % CI = 1·29, 2·99), isoleucine (OR = 2·00; 95 % CI = 1·31, 3·05) and valine (OR = 1·90, 95 % CI = 1·28, 2·81). Interestingly, the strongest associations between BCAA levels and pancreatic cancer risk were found in subjects with blood samples collected 2 to 5 years before diagnosis (OR for total BCAA = 4·34; 95 % CI = 1·82, 10·35) compared with more than 5 years(Reference Mayers, Wu and Clish27). In a nested case–control study within the Japan Public Health Center-based prospective study, including 170 cases of pancreatic cancer, the OR for the highest serum BCAA quartile, compared with the lowest one, was 2·43 (95 % CI = 1·21, 4·90)(Reference Katagiri, Goto and Nakagawa26). The OR were 2·14 (95 % CI = 1·10, 4·15) for leucine and 2·89 (95 % CI = 1·43, 5·84) for valine. In the longitudinal Women’s Health Study cohort of 26 711 US women including seventy-four cases of pancreatic cancer, circulating total BCAA were marginally associated with pancreatic cancer risk (hazard ratio, per one standard deviation = 1·24; 95 % CI = 0·98, 1·57)(Reference Tobias, Hazra and Lawler24). The hazard ratio were 1·27 (95 % CI = 0·99, 1·64) for leucine, 1·31 (95 % CI = 1·01, 1·07) for isoleucine and 1·13 (95 % CI = 0·89, 1·43) for valine(Reference Tobias, Hazra and Lawler24). We were not able to investigate individual BCAA, given the high collinearity of their intakes in our data. Comparing findings from circulating BCAA levels and dietary intakes needs caution considering the low agreement found between these measures(Reference Iwasaki, Ishihara and Takachi37). However, our results are in line with previous studies on circulating BCAA, providing further evidence for a positive association between leucine, isoleucine and valine and pancreatic cancer risk.
Different mechanisms have been implicated for this association. In a murine model of pancreatic cancer, leucine supplementation reduced glucose clearance in obese mice with subsequent increase in circulating glucose enhancing pancreatic tumour growth(Reference Liu, Lashinger and Rasmussen29). Moreover, pancreatic tumour tissues showed increased BCAA uptake through solute carrier transporters(Reference Lee, Cho and Kim28). Knockdown of the key enzymes in BCAA catabolism, BCAT2 and BCKDHA inhibited pancreatic ductal adenocarcinoma cell proliferation by regulating lipogenesis that has been shown to be increased in proliferative cells, which need high levels of fatty acids for the generation of the cell membranes. In an animal study, KRAS mutation, a signature marker occurring in more than 90 % adenocarcinomas of pancreas, was found to be positively correlated with BCAT2 protein level(Reference Li, Yin and Wang30). In the same study, pancreatic cancer cells consumed 1·5 to 2·5 times more BCAA than normal cells(Reference Li, Yin and Wang30).
Major food sources of BCAA intake in our data were red meat (26 %), dairy products (13 %), poultry (12 %) and fish (7 %). In these data, red meat and cheese were positively associated with pancreatic cancer risk (OR for high v. low consumption = 1·99, 95 % CI = 1·18, 3·36 and OR = 1·90, 95 % CI = 1·12, 3·19, respectively), whereas no association was found for poultry (OR = 0·91; 95 % CI = 0·70, 1·58) and fish (OR = 1·05; 95 % CI = 0·71, 1·56)(Reference Polesel, Talamini and Negri15,Reference Di Maso, Talamini and Bosetti16) . Several studies have shown positive associations between red meat and pancreatic cancer risk(36,Reference Chan, Wang and Holly38) . Also, dietary patterns with low intake of animal protein such as the Mediterranean diet(Reference Bosetti, Turati and Dal Pont39) or a posteriori-derived healthy dietary pattern (non-western type diet)(Reference Zheng, Guinter and Merchant14,Reference Chan, Gong and Holly40) have been shown to exert a protective effect on the risk of pancreatic cancer. Since in our population BCAA intake mainly derived from animal food sources with a small contribution from fish consumption, it would be of interest to evaluate the relation between BCAA intake and pancreatic cancer risk in populations with a high consumption of fish, such as the Nordic countries or Japan. However, in this study, further allowance for dietary factors correlated with BCAA affected only weakly, if at all, the OR estimates. This suggests that BCAA are at least in part responsible for the detrimental effects observed by diets rich in these components, such as the western diet.
In a study of colorectal cancer, we reported no association with BCAA intake, but BCAA were related to a reduced risk of sigmoid cancer(Reference Rossi, Mascaretti and Parpinel23). This confirms that nutritional risk factors for pancreatic cancer were at least in part different from those of colorectal cancer(Reference Kleeff, Korc and Apte3).
Limitations of the study are generial concerns about hospital-based case–control studies(Reference Breslow and Day41). Selection bias cannot be excluded, and dietary habits of hospital controls may differ from the general population, but we excluded from the control group all subjects diagnosed with conditions associated with long-term dietary modifications. We had information on tumour, nodes and metastasis (TNM) on a limited number of cancers. Most of these were advanced cases. Thus, our study refers essentially to advanced pancreatic cancer, though there is no consistent evidence that risk factors are appreciably different for early v. late pancreatic cancer(Reference Kleeff, Korc and Apte3). Among the strengths of the study there is the large sample and the fact that cases and controls were from comparable catchment areas and interviewed in a similar setting. Moreover, the almost complete participation is reassuring in terms of potential selection bias. We used a satisfactorily reproducible and valid FFQ, with r values for BCAA food sources between 0·6 and 0·7(Reference Franceschi, Negri and Salvini31,Reference Decarli, Franceschi and Ferraroni32) . Dietary information refers to the habitual diet in the years before diagnosis or hospital admission, limiting bias due to reverse causation. Recall bias can be influenced by a recent diagnosis of cancer but remains unlikely, given the scanty knowledge by the Italian population on a link between diet and pancreatic cancer risk at the time of information collection.
We controlled for major confounders, including smoking, BMI and diabetes. All participants were Caucasian; thus, race/ethnicity cannot confound results in this study. We were not able to adjust for chronic pancreatitis, which is a known risk factor for pancreatic cancer, but it accounts for a minor proportion of cases only(Reference Kleeff, Korc and Apte3). Lack of blood samples can be another limitation, as we were not able to estimate the variability between BCAA from diet and circulating levels of BCAA, and to compare their effects on pancreatic cancer risk estimates.
The observed association was consistent in sensitivity analyses excluding in turn subjects with diabetes, family history of pancreatic cancer and outliers in energy intake. In addition, the association was consistent across strata of several covariates, though possibly stronger in heavy alcohol drinkers and tobacco smokers. The combination of high BCAA intake with heavy smoking or heavy drinking is compatible with a multiplicative effect of the two exposures, leading to excessively high OR for subjects heavily exposed to smoking or alcohol in addition to BCAA.
Taking into account the still rising rates and fatality of this aggressive cancer and the absence of non-invasive screening tools to date(Reference Kleeff, Korc and Apte3), approaches to improve primary prevention and early diagnosis, such as dietary guidelines or tests based on specific metabolites, are warranted. Our results on BCAA point in that direction, but they need further confirmation.
Acknowledgements
No funding to declare.
Conception and design: M. R. and C. L. V.; collection of data: M. F., D. S., E. N. and C. L. V.; analysis of data: M. R., F. T. and P. S.; drafting the manuscript: M. R. and P. S. All authors contributed to data interpretation, critical revision and final approval of the manuscript.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114522000939








