CVD is the leading cause of death worldwide, with more women than men dying each year due to CVD( Reference Coulter 1 ). In the USA, CVD accounts for almost a third of female deaths, and an estimated 390 000 women develop new or recurrent myocardial infarction (MI) and/or CHD annually( Reference Garcia, Mulvagh and Bairey Merz 2 , Reference Pagidipati and Peterson 3 ). Despite these alarming trends, CVD is largely preventable with lifestyle modifications, including the consumption of a healthy diet( Reference Benziger, Roth and Moran 4 ).
It is clear that the best treatment for CVD is prevention, and diets containing greater quantities of vegetables appear effective at reducing the risk of CVD( Reference He, Nowson and Lucas 5 ). Previous findings from the Nurses’ Health Study (NHS) indicate that green leafy vegetable consumption was significantly associated with a lower risk of CHD (relative risk (RR)=0·78, 95 % CI 0·69, 0·88; P=0·0004)( Reference Bhupathiraju, Wedick and Pan 6 ). Notably, green leafy vegetables (and root vegetables) are one of the richest sources of dietary nitrate and account for approximately 60–80 % of daily intake( Reference Lidder and Webb 7 , Reference Sindelar and Milkowski 8 ). This may be physiologically important because dietary nitrate can be metabolised in the body via the enterosalivary nitrate–nitrite–NO pathway to produce cardio-protective NO( Reference Lidder and Webb 7 , Reference Lundberg, Weitzberg and Gladwin 9 ).
NO is a major signalling molecule within the cardiovascular system and has a primary role as a vascular vasodilator( Reference Moncada and Higgs 10 – Reference Landmesser and Drexler 12 ). In addition, NO can facilitate many other anti-atherosclerotic functions by preventing blood clot formation and inflammation and by promoting the formation of new blood vessels( Reference Li and Förstermann 13 , Reference Strijdom, Chamane and Lochner 14 ). Therefore, reductions in NO bioavailability in humans have been implicated in the pathophysiology of CVD( Reference Napoli and Ignarro 15 ).
While hypertension has long been a major public health target for reducing CVD events, it is increasingly clear that additional CVD risk factors must be targeted concurrently( Reference De Backer, Ambrosioni and Borch-Johnsen 16 , Reference Mosca, Banka and Benjamin 17 ). In a 4-week randomised controlled trial of sixty-eight hypertensive patients, Kapil et al. ( Reference Kapil, Khambata and Robertson 18 ) found nitrate-rich beetroot juice significantly reduced resting blood pressure (systolic: –7·7 mmHg; diastolic: –2·4 mmHg) and improved other mediators of vascular health as evidenced by improved endothelial function and reduced arterial stiffness, compared with a placebo group. These findings are consistent with a recent systematic review and meta-analysis of randomised controlled trials, indicating an acute consumption of nitrate led to significantly lower blood pressure (systolic: –4·8 mmHg; diastolic: –1·7 mmHg), improved endothelial function, reduced arterial stiffness and reduced platelet aggregation( Reference Jackson, Patterson and MacDonald-Wicks 19 ). These observed vascular improvements if sustained are of a clinically significant magnitude, which could reduce the incidence of deaths from CHD by approximately 9 % and CVD risk by 10 %( Reference MacMahon, Peto and Collins 20 ). These findings suggest that dietary nitrate, as an effective NO donor in humans, may reduce major CVD risk factors and the development of atherosclerosis, MI and CHD( Reference Jackson, Patterson and MacDonald-Wicks 19 ).
Recent observational evidence indicates that higher long-term habitual nitrate intake are associated with a lower risk of CVD mortality( Reference Blekkenhorst, Bondonno and Lewis 21 , Reference Liu, Bondonno and Russell 22 ). However, to the authors’ knowledge no previous study assessed the long-term impact of dietary nitrate intake in relation to the risk of CHD specifically. Therefore, the aim of the present study was to examine whether higher usual dietary nitrate intake in the NHS cohort are associated with reduced risk of CHD over 26 years of follow-up.
Methods
Study population
The NHS began in 1976 and included 121 700 US registered nurses( Reference Belanger, Hennekens and Rosner 23 ). Participants were female registered nurses aged 30–55 years, from eleven states in the USA. The NHS participants completed a baseline questionnaire that included assessment of lifestyle and medical history. Follow-up questionnaires have been sent biennially to participants to collect updated health, lifestyle and disease information; dietary data were first collected in 1980. A follow-up rate of approximately 90 % was achieved in most follow-up cycles( Reference Colditz, Manson and Hankinson 24 ).
For the current investigation, the baseline year was 1986 as this included a more comprehensive semi-quantitative FFQ than was used in 1980. We excluded participants with missing baseline age information (n 48), if responses were only to the 1986 questionnaire (n 700), if death occurred before baseline (n 5), and if there was a diagnosis with diabetes (n 2892), CVD (n 3351) and cancer (n 4464) at baseline. Participants with invalid (<600 or >3500 kcal/d) responses on the 1986 FFQ, or missing dietary nitrate data were also excluded (n 1525). Thus, the baseline population consisted of 62 535 women (online Supplementary Fig. S1). The study was approved by the Human Research Committee of the Brigham Women’s Hospital. Informed consent was implied upon the return of a completed questionnaire.
Ascertainment of diet and dietary nitrate exposure
A validated semi-quantitative FFQ was used to collect dietary data in 1986 and subsequently every 4 years after. The FFQ asked participants to report their usual daily intake (never to ≥6 times/d) of a standard portion size (e.g. 1/2 cup cooked vegetables, 1 cup raw vegetables) for 126 food items. The reproducibility and validity of the FFQ responses were previously evaluated in a subset of NHS participants (n 127) using 7-d food records. The validation study found the FFQ correlated reasonably well with dietary record values, the correlation coefficients for all vegetables was 0·46 and ranged from 0·25 for kale, mustard or chard greens, to 0·73 for lettuce( Reference Salvini, Hunter and Sampson 25 – Reference Willett, Sampson and Stampfer 27 ). The validity of this questionnaire has been recently confirmed in a larger sample using both diet record and multiple biomarkers of diet( Reference Yuan, Spiegelman and Rimm 28 ). Responses from the FFQ were converted into average daily intake by combining frequency information with nitrate content information obtained from the updated US Department of Agriculture food composition tables( 29 ).
Serum and urinary nitrate levels are affected by both dietary nitrate and endogenous NO production, additionally nitrate has a short half-life (approximately 5–8 h); therefore, currently there is no reliable biomarker to assess long-term dietary nitrate intake( Reference Blekkenhorst, Prince and Ward 30 ). However, experimental trials have demonstrated high nitrate intake lead to higher urinary nitrate concentrations( Reference Pannala, Mani and Spencer 31 ). Therefore, urinary nitrate levels have been used to evaluate the validity of capturing nitrate intake assessed by FFQ. This validation study was conducted in fifty-nine individuals who responded to an FFQ( Reference Lajous and Willett 32 ). The correlation coefficient between dietary nitrate intake reported from the FFQ and urinary nitrate intake was 0·59, after adjustment for within-person variation (sex, sex and body mass)( Reference Lajous and Willett 32 ). This relatively high correlation corresponds with the findings of similar validation studies and indicates that the FFQ was reasonably accurate at capturing nitrate intake( Reference Lajous and Willett 32 ).
Intake of dietary factors were evaluated using updated cumulatively averaged intakes, by averaging all available information up to the start of a 2-year follow-up interval (e.g. the average of the 1986, 1990 and 1994 values were used for the 1994 to 1996 follow-up period and so on), which better represents long-term habitual intake and reduces random error( Reference Hu, Stampfer and Manson 33 ).
The Alternative Healthy Eating Index (AHEI) is a diet quality score based on specific foods and nutrients, in which a higher score has consistently been associated with lower risk of chronic diseases in clinical and epidemiologic investigations( Reference Chiuve, Fung and Rimm 34 ). Specifically, a higher AHEI score reflects higher intakes of vegetables, fruit, wholegrains, nuts, unsaturated fatty acids and lower intake of sugar-sweetened beverages, red/processed meat and NaCl, while moderate alcohol is positively ranked compared with no or heavy alcohol consumption( Reference Chiuve, Fung and Rimm 34 ). All AHEI components were scored from 0 (worst) to 10 (best), and the total AHEI score ranged from 0 (non-adherence) to 110 (perfect adherence).
Ascertainment of CHD
The primary end point for this study was incidence of CHD, defined by occurrence of non-fatal MI or fatal CHD after the return of the 1986 FFQ and before 1 June 2012. Participants (or next of kin if deceased) reporting a primary end point were asked for permission to access their medical records to validate the reported event. Records were reviewed by physicians who were blinded to the participant’s risk factor status. Deaths were identified by reports from the next of kin, the US postal system, or using certificates obtained from state vital statistics departments or the National Death Index. Fatal CHD was categorised as ‘definite’ only if confirmed by hospital records or autopsy report or if CHD was listed as the cause of death on the death certificate and there was evidence of previous coronary disease. If no medical records were available, persons in whom CHD was the underlying cause on the death certificate were categorised as ‘probable’ cases.
Statistical analysis
Each participant contributed person-time of follow-up from the date of return of the baseline questionnaire to the date of CHD diagnosis, death, last return of a validated questionnaire or end of analysis follow-up, whichever came first. Each participant contributed only one end point, and the cohort at risk of 2-year follow-up period included only those who remained free from CHD at the beginning of each follow-up period.
For analyses of nitrate intake, participants were divided into quintiles for cumulatively updated daily dietary nitrate consumption, with the lowest quintile representing the reference category. Median values of dietary nitrate intake for each quintile were used to test for a linear trend across the quintiles.
Cox proportional hazards models with time-varying covariates with age in months as the time scale were used for all analyses using SAS 9.4 statistical software (SAS Institute Inc.) to estimate RR and corresponding 95 % CI. Statistical significance was defined as P<0·05.
In our multivariable models, a variety of factors were considered, based on the criteria outlined by Jager et al. ( Reference Jager, Zoccali and Macleod 35 ). For example, to prevent over-adjustment, confounding variables must (1) have an association with the disease, in that, it should be a risk factor for the disease; (2) be associated with the exposure, that is, it must be unequally distributed between exposure groups and (3) not be an exposure effect( Reference Jager, Zoccali and Macleod 35 ). Vegetables and fruits are the two major sources of dietary nitrate (contributing to approximately 80–90 % of the total nitrate intake), indicating that vegetable and fruit intake principally influence nitrate intake. For this reason, the AHEI without the vegetable and fruit component score was used in our multivariable models, to prevent the risk of over-adjustment. Our models also considered a variety of potential covariates. Covariates were self-reported data updated biennially from baseline including smoking status (never, past or current 1–14, 14–25 or ≥25 cigarettes/d), physical activity level (metabolic equivalents (MET)/week)( Reference Wolf, Hunter and Colditz 36 ), race (White, Hispanic, Asian, African) and BMI (calculated as weight in kg divided by height in m2 (kg/m2))( Reference Rimm, Stampfer and Colditz 37 ) (Table 3). In a sensitivity analysis, we also adjusted our multivariable models for hypertension, high cholesterol, diabetes and family history of MI (self-reported physician diagnosis of disease, collected biennially). Further, stratified analyses were conducted to explore effect modification based on smoking status (smoker v. non-smoker), activity levels above or below the median (<18 v. ≥18 MET/week), alcohol consumption (consumer v. non-consumer) and obese BMI (BMI <30 kg/m2 v. BMI ≥30 kg/m2).
Results
During 1 473 035 person-years of follow-up (n 62 535), 2267 incident cases of CHD were identified.
At baseline, highest consumers of dietary nitrate also consumed more total fruit and vegetables and therefore were more likely to have higher AHEI scores. Highest consumers were also more likely to use multivitamin supplements, have greater intake of antioxidants (vitamin C, vitamin E), exercise more and were less likely to smoke (Table 1).
Table 1 Age-standardised baseline characteristics in the Nurses’ Health Study (1986) by quintile of nitrate intake (Mean values and standard deviations, and percentages)
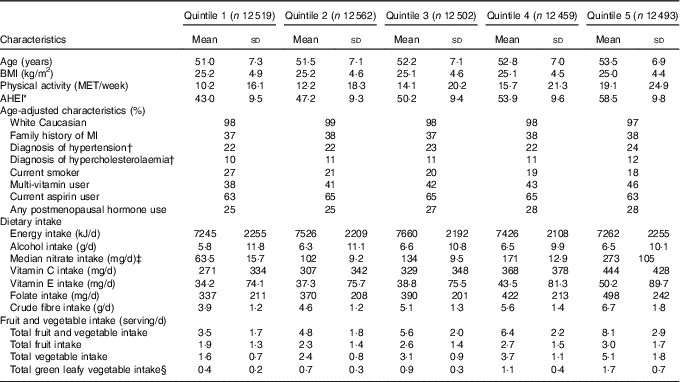
MET, metabolic equivalents; AHEI, Alternative Healthy Eating Index; MI, myocardial infarction.
* AHEI represents a total dietary score. Lowest=0, highest=110.
† Self-reported diagnosis.
‡ Values are the median dietary nitrate intakes for each quintile.
§ Total green leafy vegetables include cabbage, spinach (cooked and raw), kale and lettuce (romaine and iceberg).
The overall mean intake of nitrate was 152 (sd 75) mg/d. Lettuce (iceberg and romaine) was the primary dietary source of nitrate intake, followed by other green leafy vegetables including spinach, celery and broccoli (Table 2). Nitrate intake were significantly associated with the AHEI scores (r 0·54, P<0·01), but this association was weakened after excluding the vegetable and fruit components from the score (r 0·38, P<0·01), as vegetables and fruits are the primary sources of dietary nitrate.
Table 2 Top ten food sources of dietary nitrate in the study populationFootnote * (Mean values and standard deviations)
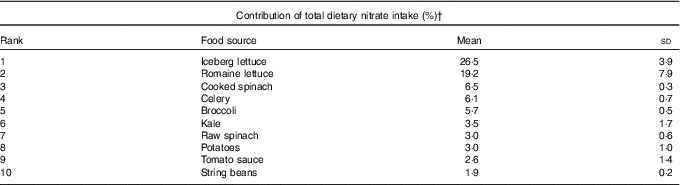
* Average contribution to total dietary nitrate from 1986 to 2010 FFQ.
† Total percentage of dietary nitrate contribution of the food listed: 78 %.
Compared with quintile 1 (lowest dietary nitrate (median: 63·5 mg/d)), the age-adjusted RR of CHD for quintile 5 (highest dietary nitrate (273 mg/d)) was 0·77 (95 % CI 0·68, 0·88; P for trend=0·0002). This association was no longer significant once adjusted for race, smoking, BMI and activity levels (model 1) (RR comparing extreme quintiles: 0·91 (95 % CI 0·80, 1·04), P for trend=0·27). Findings were further attenuated once the AHEI score, without vegetable and fruit components, was added to the model (model 2) and adjusted for CVD risk factors including hypertension, high cholesterol, diabetes and family history of MI (model 3) (Table 3).
Table 3 Risk of CHD by quintiles of nitrate intake in the Nurses’ Health Study (1986–2012)Footnote * (Relative risks (RR) and 95 % confidence intervals)
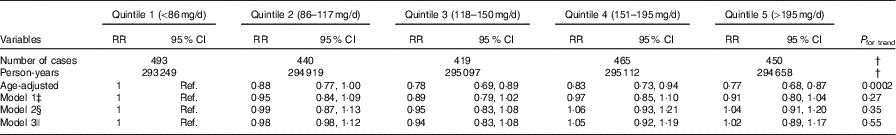
Ref., reference.
* Intake calculated using cumulative average (average of all available intake data from FFQ completed before each 2-year period at risk).
† Data not applicable.
‡ Model 1: multivariable analyses stratified by age in months and period at risk, and adjusted for smoking status, race, BMI and physical activity.
§ Model 2: model 1, adjusted for the Alternative Healthy Eating Index, excluding vegetables and fruits.
|| Model 3: model 2, adjusted for hypertension, high cholesterol, diabetes and family history of myocardial infarction.
Stratified analysis identified a possible interaction with alcohol and BMI (P<0·2) but not smoking or activity levels (online Supplementary Table S1).
Discussion
In this prospective cohort study of mid-life and older women with 26 years of follow-up, we observed that a higher long-term intake of dietary nitrate (equivalent to approximately two servings of green leafy vegetables per d) was not associated with a lower risk of CHD, once adjusted for lifestyle factors including smoking status, race, BMI, physical activity and overall diet quality (AHEI). These findings were unexpected, given that previous analysis of the NHS cohort discovered a significant 30 % risk reduction for CHD in women consuming on average 1·5 serves of green leafy vegetables per d( Reference Bhupathiraju, Wedick and Pan 6 ). However, it is important to recognise that green leafy vegetables contain a variety of beneficial components required for vascular health including, fibre, folate and K, and that other foods including potatoes and to a lesser extent processed meats, can also contribute to total dietary nitrate intake( Reference Threapleton, Greenwood and Evans 38 – Reference D’Elia, Barba and Cappuccio 40 ).
Few studies have prospectively investigated the relationship between dietary nitrate and cardiovascular-related outcomes such as CHD and MI. To our knowledge, the only previous investigation of this relationship was conducted in much smaller (n 1226–2229) Australian-based cohorts, with shorter follow-up periods( Reference Blekkenhorst, Bondonno and Lewis 21 , Reference Liu, Bondonno and Russell 22 , Reference Bondonno, Blekkenhorst and Prince 41 ). Blekkenhorst et al. ( Reference Blekkenhorst, Bondonno and Lewis 21 ) were the first to investigate prospectively the association of dietary nitrate (measured only at baseline) with atherosclerotic vascular disease (ASVD) mortality including ischaemic heart disease, heart failure, cerebrovascular disease (excluding haemorrhagic stroke) and peripheral arterial disease, in 1226 Australian women aged 70–85 years, followed up for 15 years. Finding from Blekkenhorst et al. ( Reference Blekkenhorst, Bondonno and Lewis 21 ) indicated that participants in the highest (>76·4 mg/d) compared with the lowest (<52·7 mg/d) tertile of total vegetable nitrate intake had a lower risk of ASVD mortality (HR: 0·79, 95 % CI 0·68, 0·93; P=0·004). However, similar to our findings this association was attenuated following adjustment for a Nutrient-Rich Foods Index (HR: 0·85, 95 % CI 0·72, 1·01; P=0·072)( Reference Blekkenhorst, Bondonno and Lewis 21 ). Within the same cohort, Bondonno et al. ( Reference Bondonno, Blekkenhorst and Prince 41 ) found that for every 1sd (31 mg/d) higher intake of total nitrate, there was an associated 18 % lower risk of 14·5-year ischaemic cerebrovascular disease events (P=0·017). More recently, Liu et al. ( Reference Liu, Bondonno and Russell 22 ) prospectively investigated the association of nitrate intake with CVD mortality in a sample of 2229 Australian men and women aged ≥49 years, followed up for 14 years. Participants consuming the highest (>137·8 mg/d) compared with the lowest (<69·5 mg/d) quartile for vegetable nitrate intake were observed to be at a 37 % lower hazards of CVD mortality (HR: 0·63, 95 % CI 0·41, 0·95)( Reference Liu, Bondonno and Russell 22 ).
In light of these previously published findings, it is perplexing to note that despite observing a similar trend to Blekkenhorst et al. ( Reference Blekkenhorst, Bondonno and Lewis 21 ) in the age-adjusted model, the fully adjusted model was not statistically significant. However, important differences exist between these studies which may account for this finding. First, different methods were used to estimate dietary nitrate intake. The previous Australian-based studies have drawn upon published nitrate databases to calculate intake, as nitrate is not included as part of national food composition tables in Australia. This study on the other hand calculated nitrate intake using the updated US Department of Agriculture food composition tables, which is most appropriate given that this study has been conducted in an American population. In light of these differences, it is not surprising that the nitrate intake in the NHS have been estimated at levels markedly higher (mean nitrate intake: 152 mg/d) than those reported in the Australian-based cohorts (Blekkenhorst et al. ( Reference Blekkenhorst, Bondonno and Lewis 21 ) mean nitrate: 80 mg/d; Liu et al. ( Reference Liu, Bondonno and Russell 22 ) mean nitrate: 130 mg/d). However, our mean nitrate intake estimations are similar to those previously reported in the NHS by Kang et al. ( Reference Kang, Willett and Rosner 42 ) (mean nitrate intake: 142 mg/d), in which higher dietary nitrate intake were associated with a lower primary open-angle glaucoma risk. In addition, experimental data indicate that dietary nitrate intake of at least 130 mg/d are enough to exert cardiovascular benefits, including reductions in blood pressure and improved vascular function( Reference Jackson, Patterson and MacDonald-Wicks 19 ). Second, the CVD outcomes differed between studies. This study defined CHD as those with fatal and non-fatal CHD, while the Australian studies by Blekkenhorst et al. ( Reference Blekkenhorst, Bondonno and Lewis 21 ) and Liu et al. ( Reference Liu, Bondonno and Russell 22 ) focused on CVD mortality. This may account for the different findings; however, it is important to highlight that the aim of this study was to determine whether the development of CHD, as an accumulation of atherosclerosis, and not just survival following the development of the disease (or a disease-related event) was associated with dietary nitrate intakes. This is important from the view point of prevention since dietary nitrate intake targeted to prevent the development of CHD may be important.
Previously the AHEI score was shown to strongly predict the risk of CHD within women from the NHS( Reference Chiuve, Fung and Rimm 34 ). In addition, women in this cohort with a higher AHEI tended to be higher consumers of dietary nitrate (r s 0·54, P<0·001); however, this correlation was weakened when we considered the AHEI excluding vegetable and fruit component scores (r s 0·38, P<0·001), as vegetables, followed by fruit are the primary dietary sources of nitrate, an association which is consistent with international cohorts( Reference Jackson, Patterson and MacDonald-Wicks 43 ). Particular components including carotenoids, vitamin C, fibre, polyphenols, Mg and K have been identified as important components of vegetables and fruit responsible for these beneficial effects( Reference Bhupathiraju, Wedick and Pan 6 ). Although long-term dietary nitrate intake may not yet have a clear independent effect, future studies must attempt to clearly identify whether long-term dietary nitrate could contribute to the apparent cardio-protective benefits of vegetable consumption, given that acute intake of dietary nitrate have consistently shown beneficial effects for minimising CVD risk factors in small human clinical trials( Reference Jackson, Patterson and MacDonald-Wicks 19 ).
It is worth noting, however, in a stratified meta-analysis conducted by Jackson et al. ( Reference Jackson, Patterson and MacDonald-Wicks 19 ), a high-nitrate diet (rich in green leafy vegetables) was found to influence CVD risk factors including blood pressure (systolic: –2·4 mmHg; P=0·2; diastolic: –0·6 mmHg, P=0·5) and endothelial function (flow mediated dilatation: 0·5 %, P=0·01) to a lesser extent than those observed with beetroot juice (systolic blood pressure: –5·7 mmHg; P<0·0001; diastolic blood pressure: –2·4 mmHg; P<0·0001; flow-mediated dilatation: 0. 8%, P<0·0001). These findings indicate that other dietary components may be responsible for dampening the beneficial effects of dietary nitrate. This notion was recently supported by findings from Dewhurst-Trigg et al. ( Reference Dewhurst-Trigg, Yeates and Blackwell 44 ) that in high to moderate nitrate containing vegetables including cabbage and broccoli, the cardiovascular benefits of nitrate were completely blocked in the co-presence of thiocyanate, which is thought to block the enterosalivary metabolism of dietary nitrate to NO via the nitrate–nitrite–NO pathway. Thus, it is possible such mechanisms are at play in our cohort.
On the other hand, dietary nitrate is thought to produce greater bioactive effects when consumed within the context of a healthful diet (including fruits, vegetables, nuts and fish) as antioxidants, polyphenols and PUFA interact with nitrate to enhance the potency of its effects( Reference Nadtochiy and Redman 45 , Reference Jackson, Patterson and MacDonald-Wicks 46 ). For example, vitamin C and polyphenols are abundant in a vegetable-rich diet, and their combination with nitrate has been shown to favour the formation of NO via the nitrate–nitrite–NO pathway and even prolong the half-life of NO in the stomach( Reference Mowat, Carswell and Wirz 47 , Reference Gago, Lundberg and Barbosa 48 ). More recently, it was suggested that NO and unsaturated fatty acids (e.g. oleic, linoleic and arachidonic acid) from extra-virgin olive oil, nuts, fatty fish and lean meat can react in the stomach to produce nitroalkenes( Reference Lundberg and Weitzberg 49 ). Nitroalkenes are potent cardio-protective mediators, largely because they can stimulate enzymatic NO production, which is important for preventing hypertension, atherosclerosis, blood clotting and inflammation( Reference Zhang, Villacorta and Chang 50 ).
The strengths of our study include the large sample size, long follow-up period and presence of updated dietary and covariate data. However, a limitation of this study is its observational nature, and we, therefore, cannot exclude the possibility that findings are the result of residual confounding. However, we have controlled for many known and potential risk factors related to the development of CHD in a prospective manner. In addition, long-term prospective cohort studies are the strongest observational study design, as their prospective nature makes them less prone to biases, including reverse causation, recall bias and selection bias, common to retrospective or cross-sectional studies( Reference Satija, Stampfer and Rimm 51 ).
In our study, participants who consumed greater quantities of dietary nitrate were also more likely to have a higher AHEI, be higher consumers of vegetables and have healthier lifestyles (e.g. more physical activity, less likely to smoke cigarettes). In addition, due to the complex nature of diet, self-reported dietary assessment methods including FFQ are prone to random and systematic error. This systematic error is likely to bias results towards the null; therefore, it is possible that the association we reported is underestimating the true effect. However, FFQ are useful for assessing usual intake over long periods of time, and the use of FFQ in this study has been validated against multiple diet records( Reference Lajous and Willett 32 , Reference Satija, Stampfer and Rimm 51 ). In addition, the use of repeated measures of diets to calculate cumulative averages would minimise any potential random measurement error caused by within-person variation and accommodate for diet changes over time. Further, many other dietary variables assessed by FFQ have predicted higher or lower risks of CHD in this cohort( Reference Bhupathiraju, Wedick and Pan 52 ).
Similar to other dietary constituents, external factors including farming practices, seasonal changes, storage conditions, transportation, processing and cooking practices are known to dramatically influence the nitrate content of fruits and vegetables; and therefore, accurate and reliable estimation of dietary nitrate is difficult( Reference Jackson, Patterson and MacDonald-Wicks 46 ). In particular, in the past, there has been increasing pressures placed on farmers to alter cultivating practices in order to lower the nitrate content of vegetables (e.g. apply fewer N based fertilisers) due to concerns of possible health risks( Reference Colla, Kim and Kyriacou 53 ). This is despite the European Food Safety Authority concluding that the estimated exposure to nitrate from vegetables is unlikely to result in appreciable health risks( 54 ). However, it may be possible that such pressures have reduced dietary nitrate exposures from vegetables over time. On the other hand, it appears that the intake of nitrate rich green leafy vegetables have increased over time, with US data indicating that fresh spinach consumption has increased from 0·3 kg/person in 1995 to 1·0 kg/person in 2005, likely driven by the increased availability of convenient, prewashed and pre-packaged spinach( Reference Correll, Bluhm and Feng 55 ). Thus, it is possible that changes in food environments and availability have driven higher dietary nitrate intake over time.
As previously discussed, nitrate intake estimated using databases could have led to measurement error, thus limiting our findings. However, nitrate intake captured from FFQ were previously found to be highly correlated with urinary nitrate, indicating that the average nitrate content of food does not vary substantially among individuals( Reference Lajous and Willett 32 ). In fact, Ahluwalia et al. ( Reference Ahluwalia, Gladwin and Coleman 56 ) suggest dietary nitrate databases used in epidemiological research could be improved if ‘local’ estimates of nitrate contents of vegetables were considered; however, such specific databases do not currently exist. In addition, it is possible dietary nitrate intakes have been underestimated in our analysis, given that water nitrate intake were not considered because of the limitation that the nitrate content of the water supply can vary dramatically by geographical region, and the NHS includes thousands of participants from across eleven states in the USA( Reference Fewtrell 57 ). Although nitrate is thought to be particularly high in well water sources in rural areas, this represents very few participants, and even bottled water is yet to have specific legislation around specifying acceptable water nitrate levels. Thus, any attempt to estimate water nitrate would be based on too many assumptions to be considered reliable. In addition, the omission of water nitrate from the analysis is consistent with the methodology of previously published literature( Reference Blekkenhorst, Bondonno and Lewis 21 , Reference Liu, Bondonno and Russell 22 , Reference Kang, Willett and Rosner 42 ).
Participants in the NHS were mostly non-Hispanic white women and may not be representative of the general population. For example, mean fruit and vegetable intake in our population (5·7 servings/d) were much higher than national estimates (3·0 servings/d)( Reference Moore and Thompson 58 ); and despite the average energy intake of our cohort matching US female energy intakes, the average BMI of the NHS is approximately 25·1 kg/m2, which is lower than that of the average US female population (BMI: 26·5 kg/m2)( Reference Wright and Wang 59 ). Also, the overall prevalence of CHD in our sample was only 3·5 %, which is significantly lower than the US average for middle-aged women (approximately 5·8 %)( Reference Coulter 1 ). Therefore, results need to be interpreted within this context, and they provide support for the need to explore this relationship in other cohorts, including males and more diverse populations.
Conclusion
In this large prospective study of US women, we found that higher habitual intake of dietary nitrate were not associated with a lower risk of developing CHD, after accounting for established lifestyle and non-vegetable/fruit dietary factors. Although nitrate has been shown to have a short-term beneficial effect on CVD risk factors, we did not identify a clear independent long-term effect of dietary nitrate for CHD prevention. For future investigations, it will be important to further understand the role of dietary nitrate as a component of vegetables and overall diet quality. Dietary nitrate may represent an important component of vegetables, however, continued research in diverse cohorts including males are required to understand whether findings are generalisable across populations.
Acknowledgements
The authors would like to acknowledge all the women who participated in the NHS, and the Channing Laboratory from the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School. Authors would also like to acknowledge the support of the Hunter Medical Research Institute, Australia.
This research was supported by a Hunter Medical Research Institute, Project Grant. J. K. J. is supported by a Postgraduate Research Scholarship and Greaves Family Postgraduate top-up Scholarship in Medical Research. This study was supported by grants UM1 CA176726, ROI CA050385 and R01 HL34594 from the National Institute of Health.
All authors contributed to the formulation of the research question and design of the study. J. K. J. and G. Z. carried out the analysis. J. K. J. was principally responsible for the drafting of the written manuscript. All authors contributing to the writing and approving the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114519000096






