Attention-deficit/hyperactivity disorder (ADHD) is the most common developmental disorder in childhood(Reference Sawyer, Arney and Baghurst1). The main symptoms include poor impulse control, hyperactivity and inattention(2). These problems can adversely affect school performance, family relationships and social interactions(Reference Richardson and Puri3, Reference Richardson4). Children with ADHD often experience co-morbidity with other behavioural disorders including anxiety, conduct, oppositional and/or mood disorders and antisocial personality(Reference Doyle, Faraone and DuPre5). The prevalence rates are estimated at 4–15 % in USA(Reference Costello, Mustillo and Erkanli6) and 11 % in Australia(Reference Sawyer, Arney and Baghurst1). The variation in prevalence is probably due to the differences in diagnostic criteria. Boys are more commonly diagnosed with ADHD than girls(Reference Barbaresi, Katusic and Colligan7). It is now clear that problems associated with ADHD also persist into adulthood(Reference Root and Resnick8).
The aetiology of ADHD appears to be multifactorial with genetic and environmental influences. A review has supported an association between ADHD and polymorphisms(Reference Thapar, O'Donovan and Owen9). Cigarette smoking during pregnancy has been found to increase the risk of ADHD in children(Reference Milberger, Biederman and Faraone10). Focus has also been placed on diet, including emerging evidence that ADHD may be associated with low levels of dietary and erythrocyte long-chain n-3 PUFA (LC n-3 PUFA)(Reference Richardson and Puri3, Reference Richardson4, Reference Richardson11). The LC n-3 PUFA, including EPA (20 : 5n-3) and DHA (22 : 6n-3), can be converted from α-linolenic acid (18 : 3n-3) by endogenous desaturation and elongation(Reference Holman12); however, only negligible amounts are converted(Reference Li, Sinclair and Wilson13, Reference Burdge14). LC PUFA are critical in normal brain and nervous system development and function. 22 : 6n-3 is highly concentrated in brain and retina, while LC n-6 arachidonic acid (20 : 4n-6) together with 22 : 6n-3 plays a major structural role in neuronal membranes(Reference Haag15).
Therefore, there has been growing interest in the role of n-3 PUFA in psychiatric illness across the lifespan(Reference Haag15–Reference Young and Conquer17). It has been well established that 22 : 6n-3 is critical for infant brain development, and 22 : 6n-3 may be associated with enhanced cognitive performance in childhood(Reference Lauritzen, Hansen and Jùrgensen18). A number of studies have found lower blood n-3 levels in children with ADHD compared with control groups(Reference Stevens, Zentall and Deck19, Reference Mitchell, Aman and Turbott20)and there is evidence for alleviation of ADHD symptoms with n-3 PUFA supplementation(Reference Richardson4). Results of trials have been conflicting; although the three largest, randomised placebo-controlled studies have reported improvement in ADHD symptoms in children following supplementation with 732 mg/d of LC n-3 PUFA and 60 mg/d of γ-linolenic acid (18 : 3n-6) over 24–30 weeks(Reference Sinn and Bryan21–Reference Johnson, Östlund and Fransson23).
However, no specific studies to date have investigated dietary intake of n-3 PUFA in children with ADHD to investigate whether these children are consuming low levels of LC n-3 PUFA in their diet. Therefore, the aims of the present study were to assess dietary PUFA intakes and food sources in children with ADHD, to compare these intakes to the previously published estimates of children's PUFA intake using the data from the Australian National Nutrition Survey (NNS)(Reference McLennan and Podger24), to compare these intakes to the Nutrient Reference Values (NRV)(25) and other recommendations(Reference Colquhoun, Ferreira-Jardin and Udell26, 27), and to determine whether dietary n-3 PUFA intakes and ADHD symptoms are related.
Methods
Participants
A total of 182 children with parent-reported ADHD symptoms above the 90th percentile on the 12-item ADHD Index from Conners' Parent Rating Scales(Reference Conners28) were recruited via media releases, newspaper advertisements and school newsletters, in South Australia and took part in an n-3 supplementation trial that has been published(Reference Sinn and Bryan21). Children were included if they were 6–13 years old and had ADHD or parent-reported ADHD symptoms, were not taking fish-oil supplements and were not taking stimulant medication. Eighty-six participants consented to the subsequent analysis of the dietary data. The present study focused on the baseline dietary analysis of these children from the previously published trial(Reference Sinn and Bryan21).
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Human Research Ethics Committees of the Commonwealth Scientific and Industrial Research Organisation and University of South Australia, and the South Australian Department of Education and Children's Services and Catholic Education Centre. Written informed consent was obtained from all subjects/patients.
Anthropometric measurements
During their first clinic visit, height was measured using a portable stadiometer to the nearest 0·1 cm and weight was measured using standard scales to the nearest 0·1 kg, both with the children barefoot. BMI was calculated by dividing the weight (in kilograms) by the height (in metres) squared.
Attention-deficit/hyperactivity disorder index from Conners' Parent Rating Scales
The ADHD index from Conners' Parent Rating Scales(Reference Conners28) was used to assess parent ratings of ADHD symptoms; from this, inattention, hyperactivity and total ADHD scores were used for analysis(Reference Conners28). The ADHD index is an abbreviated form of the long rating scales, essentially the key subscale that measures the core ADHD symptoms of inattention and impulsivity/hyperactivity. The index has twelve statements (e.g. ‘inattentive, easily distracted’, ‘fidgets with hands or squirms in seat’) that are scored from 0 (not at all) to 3 (very much). The maximum possible score is therefore 36.
Food records
Three-day-weighed FR were collected at baseline from the main study referred to earlier(Reference Sinn and Bryan21). Children and their parents/caregivers were asked to complete the FR on three different days; two non-consecutive weekdays and one weekend day. Children and their parents/caregivers were shown a sample FR that showed them how to fill it in. They were told to record the brand and type of foods, and to estimate the amount of all eaten foods as accurately as possible. The importance of maintaining normal diets was emphasised.
The Australia Standard database within the FoodWorks nutrient analysis software package (version 5 service pack 1, Xyris software) was used to determine the energy, macronutrients and micronutrients intakes of each child from their FR. If the foods in the FR could not be exactly matched in the FoodWorks database, these foods were substituted by other similar types of foods. For example, roma tomato was substituted with ordinary tomato.
Validation of food records
Diet reports of poor validity were identified using the Goldberg cut-off(Reference Black29). The ratio of energy intake to BMR(Reference Schofield30) of each participants was calculated, values equal to or less than 1·06 were classed as under-reporters and hence eliminated from the analysis. A 3-d-weighed FR is long enough to assess PUFA intake as shown by repeat FR assessments by Sullivan et al. (Reference Sullivan, Brown and Williams31).
PUFA intakes
The Royal Melbourne Institute of Technology Australian Fatty Acids database(Reference Mann, Sinclair and Percival32) within the FoodWorks nutrient analysis software package was used to analyse the children's PUFA intakes. Fat content of foods was obtained from the Australia Standard database(Reference Mann, Sinclair and Percival32). Again, when the foods in the FR could not be matched in the fatty acids' database, they were substituted by similar types of foods, for example muffin was substituted by cake because they have a similar fat content. Some foods that could not be found in database were entered manually as a new food from manufacturers' information, for instance Tip Top® Up™ Omega-3 Bread.
If ambiguous quantity of meat in the FR was given, where the precise weight is not recorded, for example ‘one piece of chicken breast’, a child serving size of meat was used, which is 65 g(33). If the size of meat was given, for example ‘one large piece of chicken breast’, the amount used was 65 g multiplied by 1·5. If ‘one small piece of chicken breast’ was given, the amount used was 65 g divided by 1·5.
The average daily PUFA intakes were calculated by dividing the total PUFA intakes by three (as 3-d-weighed FR were collected). The average daily 18 : 2n-6, 20 : 4n-6, total n-6 PUFA, 18 : 3n-3, 20 : 5n-3, 22 : 5n-3, 22 : 6n-3, total LC n-3 PUFA and total n-3 PUFA were determined, in addition to the ratio of n-3 PUFA to n-6 PUFA, 20 : 5n-3 to 22 : 6n-3, 20 : 4n-6 to 20 : 5n-3, n-3 PUFA to total PUFA, LC n-3 PUFA to PUFA and LC n-3 PUFA to n-3 PUFA.
Statistical analysis
All variables were tested for normality using the Shapiro–Wilk test before further analysis. All variables were not normally distributed, except age, height, weight, BMI and total ADHD score.
The Mann–Whitney U test was used to compare difference between PUFA intakes (including ratio of n-3 PUFA to n-6 PUFA, n-6 PUFA to n-3 PUFA, 20 : 5n-3 to 22 : 6n-3, 20 : 4n-6 to 20 : 5n-3, n-3 PUFA to total PUFA, LC n-3 PUFA to PUFA and LC n-3 PUFA to n-3 PUFA) and food sources in children with ADHD and estimation of PUFA intakes and food sources from the 1995 NNS(Reference McLennan and Podger24).
Non-parametric Spearman's correlation was used to determine the existence of linear relationships between PUFA intakes (including ratio of n-3 PUFA to n-6 PUFA, n-6 PUFA to n-3 PUFA, 20 : 5n-3 to 22 : 6n-3, 20 : 4n-6 to 20 : 5n-3, n-3 PUFA to total PUFA, LC n-3 PUFA to PUFA and LC n-3 PUFA to n-3 PUFA) and food sources (weight of fish/seafood and meat/egg) and ADHD symptoms.
Participants were ranked in ascending order of their PUFA intakes and divided into two groups, where lower PUFA intakes' group refers to participants consumed lowest 50 % PUFA intakes, and higher PUFA intakes' group refers to participants consumed highest 50 % PUFA intakes. The Mann–Whitney U test was used to compare any difference in ADHD scores between the two groups.
Difference between PUFA intakes in children with ADHD and recommended dietary intakes – NRV(25) was compared using Wilcoxon signed-rank test.
All data were expressed as the means and standard deviations and range and/or median ± interquartile range. Statistical analysis was performed using SPSS statistical analysis program (version 15.0.0, SPSS Inc., Chicago, IL, USA). Statistical significance was set at P < 0·05 for all analyses, except for the multiple correlation analyses where the statistical significance was set at P < 0·01.
Results
Eighty-six baseline 3-d-weighed FR were available to analyse from the previous study(Reference Sinn and Bryan21), and seven participants were eliminated as under-reporters. One thousand four hundred and eighty dietary data were available from the 1995 NNS(Reference McLennan and Podger24), 137 participants were eliminated as under-reporters.
Participant characteristics and ADHD symptoms for this group are shown in Table 1. Participant characteristics in the 1995 NNS(Reference McLennan and Podger24) are shown in Table 2. No significant difference in ADHD symptoms was found between genders (P>0·05; Table 1). Age, height, weight and BMI were not significantly different between the children with ADHD and the children from the NNS (P>0·05; Tables 1 and 2).
Table 1 Subject characteristics and attention-deficit/hyperactivity disorder (ADHD) symptoms
(Mean values and standard deviations with ranges)
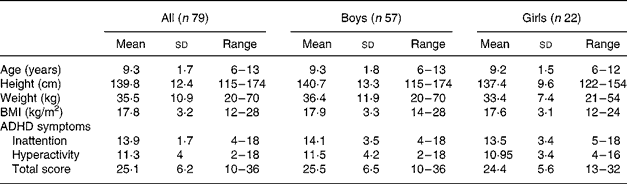
Table 2 Subject characteristics in 1995 National Nutrition Survey(Reference McLennan and Podger24)
(Mean values and standard deviations with ranges)
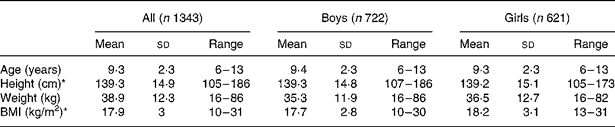
* All (n 1342), boys (n 722), girls (n 620).
PUFA intakes and comparison to the National Nutrition Survey
Daily PUFA intakes and comparison to the NNS data(Reference McLennan and Podger24) are shown in Table 3. Values are expressed as median and interquartile range rather than mean and sd because of the skewed distribution of PUFA intakes, especially LC n-3 PUFA.
Table 3 Daily PUFA intake and comparison to the National Nutrition Survey (NNS)(Reference McLennan and Podger24)
(Median values and interquartile ranges (IQR))

ADHD, attention-deficit/hyperactivity disorder; 18 : 2n-6, linoleic acid; 20 : 4n-6, arachidonic acid; 18 : 3n-3, α-linolenic acid; 20 : 5n-3, EPA; 22 : 5n-3, docosapentaenoic acid; 22 : 6n-3, DHA; LC n-3, long-chain n-3 PUFA (sum of 20 : 5n-3, 22 : 5n-3 and 22 : 6n-3).
* ADHD group (n 76), NNS (n 1195).
† ADHD group (n 73), NNS (n 1212).
In comparison of children PUFA intakes with the NNS data, there were significant differences in certain fatty acids. Intakes of 18 : 3n-3 (P < 0·001) and hence total n-3 PUFA intakes (P < 0·001) were significantly higher and 20 : 4n-6 intakes (P < 0·05) were significantly lower in children with ADHD. The ratio of total n-3 PUFA to total n-6 PUFA (P < 0·001) and total n-3 PUFA to total PUFA (P < 0·001) was significantly higher than the NNS, while the ratio of 20 : 5n-3 to 22 : 6n-3 (P < 0·05), total n-6 PUFA to total n-3 PUFA (P < 0·001) and total LC n-3 PUFA to total n-3 PUFA (P < 0·01) was significantly lower compared to the NNS.
There were no significant differences in total energy intake between the children from the NNS and the children with ADHD, and hence expressing the fatty acid intakes data as mg/d/energy showed no significant difference between these two groups (data not shown).
Fish/seafood and meat/egg consumption and the National Nutrition Survey comparison
The amount of fish/seafood and meat/egg (g/d) consumption with comparison to the NNS(Reference McLennan and Podger24) is shown in Table 4. Children with ADHD consumed significantly less fish/seafood (P < 0·05) and meat/eggs (P < 0·01) compared to the NNS. It is of interest to note that less than 25 % of children in the ADHD group and the NNS had consumed fish and/or seafood.
Table 4 Fish, seafood and meat, egg consumption and the National Nutrition Survey (NNS)(Reference McLennan and Podger24) comparison
(Mean values, medians and interquartile ranges (IQR))

ADHD, attention-deficit/hyperactivity disorder.
Correlation between PUFA intakes/food sources and attention-deficit/hyperactivity disorder symptoms
Correlation coefficient for ADHD scores and PUFA intakes/food sources is shown in Table 5. There were no significant correlations found between any of the fatty acids and ADHD symptoms (P>0·01). Likewise, no significant correlations were found between amount of fish/seafood and meat/egg consumption and ADHD symptoms (P>0·01).
Table 5 Spearman's correlation coefficient for attention-deficit/hyperactivity disorder scores, PUFA intakes and food sources consumption
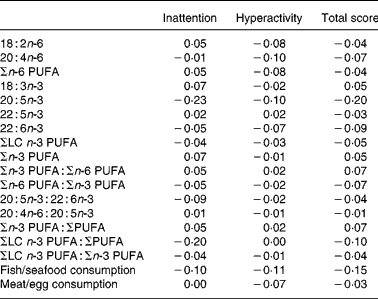
18 : 2n-6, linoleic acid; 20 : 4n-6, arachidonic acid; 18 : 3n-3, α-linolenic acid; 20 : 5n-3, EPA; 22 : 5n-3, docosapentaenoic acid; 22 : 6n-3, DHA; LC n-3, long-chain n-3 PUFA (sum of 20 : 5n-3, 22 : 5n-3 and 22 : 6n-3). P < 0·01 was used.
Comparison of attention-deficit/hyperactivity disorder scores between lower/higher PUFA intakes' groups
No significant differences in ADHD scores were found between any lower/higher PUFA intakes' groups (data not shown).
PUFA Intakes and comparison to the nutrient reference values and other recommendations
Comparison of PUFA intakes with NRV(25) and other recommendations(Reference Colquhoun, Ferreira-Jardin and Udell26, 27) are shown in Table 6. Girls consumed significantly more 18 : 3n-3 (P = 0·007) than the NRV. Males consumed significantly less 18 : 2n-6 than the NRV (P = 0·007). No significant difference was found between LC n-3 PUFA intake and NRV (P>0·05) of both sex. However, in comparison to other recommendations, the LC n-3 PUFA intake in children with ADHD was less than 13 % of those recommended intakes as suggested by the National Heart Foundation(Reference Colquhoun, Ferreira-Jardin and Udell26) and the International Society for the Study of Fatty Acids and Lipids(27) (Table 6).
Table 6 Comparison of daily PUFA intakes with recommendations from Nutrient Reference Values (NRV), National Heart Foundation (NHF) and International Society for the Study of Fatty Acids and Lipids (ISSFAL)
(Median values and interquartile ranges (IQR))

AI, adequate intake; 18 : 2n-6, linoleic acid; 18 : 3n-3, α-linolenic acid; LC n-3 PUFA, long-chain n-3 PUFA (sum of 20 : 5n-3, 22 : 5n-3 and 22 : 6n-3).
* Sum of 20 : 5n-3 and 22 : 6n-3.
Discussion
The present analysis of 3-d-weighed FR collected from seventy-nine children with ADHD symptoms indicated that these children had lower 20 : 4n-6 intake and higher 18 : 3 n-3 intake than the general population of the same age. Two previous studies that assessed the diet of children with ADHD compared to age-matched controls found no differences in PUFA intakes(Reference Chen, Hsu and Hsu34, Reference Colter, Cutler and Meckling35). A small study (n 11 ADHD and n 8 controls) from Canada showed that children with ADHD consumed more energy and saturated fat compared with children without ADHD(Reference Colter, Cutler and Meckling35). However, these authors did not take into account the differences in energy intake. If the PUFA intake was expressed as percentage of energy, the mean intake of 20 : 4n-6 and 22 : 6n-3 in children with ADHD would be approximately 40 % less than the control group(Reference Colter, Cutler and Meckling35). The other larger study (n 52) did not find any difference in 18 : 2n-6 and 18 : 3n-3 intakes in children with ADHD compared to age-matched controls(Reference Chen, Hsu and Hsu34). Again, however, if the PUFA intake is expressed as percentage of energy, it is conceivable that the 18 : 3n-3 intake would be higher than the control group.
Meat and eggs consumption was significantly lower in children with ADHD compared to the NNS, and this was consistent with lower 20 : 4n-6 intake because meat is a rich source of 20 : 4n-6(Reference Meyer, Mann and Lewis36). Children with ADHD also consumed significantly lower amounts of fish and seafood compared with children from the NNS. Fish and seafood are the richest source of LC n-3 PUFA(Reference Meyer, Mann and Lewis36), but lower consumption in the children with ADHD did not translate to significant differences in LC n-3 PUFA intake, even though they tended to be lower. Other biochemical data have shown that children with ADHD have a lower level of 20 : 4n-6 in plasma(Reference Stevens, Zentall and Deck19, Reference Burgess, Stevens and Zhang37) and in erythrocytes(Reference Chen, Hsu and Hsu34), while no differences were found in 18 : 3n-3(Reference Stevens, Zentall and Deck19, Reference Chen, Hsu and Hsu34, Reference Burgess, Stevens and Zhang37), even though dietary PUFA intake may not correlate with blood PUFA(Reference Colter, Cutler and Meckling35).
The present study showed that children with ADHD had higher n-3 : n-6 ratio, n-3 : total PUFA ratio and lower total LC n-3 : n-3 ratio, n-6 : n-3 ratio compared with age-matched children without ADHD. The reason for this is not clear, but this may partially be explained by the higher 18 : 3n-3 intake. The present study showed no correlations between any fatty acids including 20 : 5n-3 and 22 : 6n-3 and symptoms of ADHD (Table 6). However, positive clinical trials have shown some advantages over placebo for supplementing both 20 : 5n-3 and 22 : 6n-3(Reference Sinn and Bryan21–Reference Johnson, Östlund and Fransson23), as well as some n-6 PUFA(Reference Richardson and Puri38, Reference Stevens, Zhang and Peck39) in the treatment of inattention, impaired cognition or mood disorder, but no benefits from supplementation with 22 : 6n-3 alone(Reference Voigt, Llorente and Jensen40, Reference Hirayama, Hamazaki and Terasawa41). This suggests that both 20 : 5n-3 and 22 : 6n-3 may be necessary for a positive outcome.
The Australian NRV recommends an adequate intake of 70 mg LC n-3 PUFA per day for boys and girls aged 9–13 years(25). The definition of adequate intake is the average daily nutrient intake level based on observed or experimentally determined approximations or estimates of nutrient intake by a group (or groups) of apparently healthy people that are assumed to be adequate(25). This definition refers to ‘apparently healthy people’. The current NRV for LC n-3 PUFA intake of 70 mg/d for children aged 9–13 years is based on the median intake in the population(Reference Meyer, Mann and Lewis36, Reference Howe, Meyer and Record42), and assumes that the population is healthy, yet the incidence of ADHD in Australia is 11 %, which suggests our children population is not entirely healthy. There are other recommendations that suggest much higher optimal intakes of LC n-3 PUFA. The International Society for the Study of Fatty Acids and Lipids and the National Heart Foundation of Australia recommend an intake of 500 mg 20 : 5n-3 and 22 : 6n-3 per day, based on a current scientific evidence(Reference Colquhoun, Ferreira-Jardin and Udell26, 27). It is likely that the NRV recommendation of 70 mg/d is set too low and is 13 % of the recommendations set by 500 mg/d by the National Heart Foundation and International Society for the Study of Fatty Acids and Lipids. Therefore, the present study indicates that all Australian children including those with ADHD are consuming far less than optimal amounts of LC n-3 PUFA.
Although few clinical intervention studies showed some evidence of alleviation of behavioural problems on ADHD participants with LC PUFA supplementation(Reference Sinn and Bryan21–Reference Johnson, Östlund and Fransson23), not all studies agreed with this(Reference Stevens, Zhang and Peck39–Reference Hirayama, Hamazaki and Terasawa41). These studies supplemented ADHD children with DHA and other LC PUFA precursors, and no improvements in any ADHD symptoms were observed. This finding led to a hypothesis that these children may have impaired fatty acids' metabolism. A preliminary result from Brookes et al. (Reference Brookes, Chen and Xu43) showed that there is an association between fatty acid desaturase genes (FADS2) and ADHD.
It should be noted that there are limitations associated with self-reported food intake. There was some estimation of serving sizes of foods, e.g. the average child meat serving size of 65 g was used when the quantity was not specified; the food nutrient databases used do not contain all of the exact foods; hence, substitutions were necessary, e.g. roma tomato was substituted with raw tomato. Another limitation of the present study is the lack of blood analysis as dietary intakes do not necessarily correlate with PUFA levels as shown by Colter et al. (Reference Colter, Cutler and Meckling35); however 20 : 5n-3 and 22 : 6n-3 do correlate well with circulating blood levels(Reference Sullivan, Williams and Meyer44).
In summary, the present study found that children with ADHD consumed significantly less meat/egg and fish/seafood compared to the general population the same age. However, this did not translate to a statistical significantly lower consumption of LC n-3 PUFA. The only differences in dietary PUFA intake were lower 20 : 4n-6 and higher 18 : 3n-3 intakes. The median 20 : 5n-3 and 22 : 6n-3 intakes were adequate when compared with the NRV, but were only 13 % of those recommendations by National Heart Foundation and International Society for the Study of Fatty Acids and Lipids. Given the overall current evidence for the role of LC n-3 PUFA in ADHD symptoms and the lower consumption of foods rich in LC n-3 PUFA, children with ADHD are encouraged to consume more LC n-3 PUFA containing foods for mental health benefits.
Acknowledgements
The authors gratefully acknowledge the assistance from Karen Walton, Kelly Lambert, Serina Faraji, Janet Bryan and all the children and their parents who participated in the present study. The authors would like to acknowledge the University of Wollongong for funding the study. B. J. M and N. S. initiated and designed the study. N. S. collected the data. K.-H. N. G. and L. R. analysed the data supervised by B. J. M and N. S. K-.H. N. G. prepared the manuscript and B. J. M., N. S. and L. R. reviewed the manuscript. There was no potential conflict of interest for any authors of the present paper.








