The determination of the true digestibility of N and amino acids (AA) in feedstuffs is of great interest for animal production because it allows for better adjustment of the supply to the requirement and reduction of N pollution(Reference Hess, Ganier and Thibault1, Reference Zhou, Jiang and Tan2). Correction of the significant losses of endogenous N (EN) and endogenous amino acids (EAA) occurring during digestion and absorption along the gastrointestinal tract is necessary for estimating the true digestibility of N and for measuring the N and AA requirements by factorial methods. The endogenous contribution has not received much attention until recently and the estimates used have been fairly low. Several reports have acknowledged that the endogenous protein makes up a considerable fraction of the duodenal N flow(Reference Larsen, Madsen and Weisbjerg3–5). However, it is difficult to determine the losses of EN and EAA in ruminants because their N and AA supplies originate from both micro-organisms and ruminally undegraded feed protein. Prediction of the supply from each of these origins has to be based on experiments with ruminants cannulated at the start of the small intestine, and the total flows of N and AA must be separated with respect to the origin. This separation is complicated by the endogenous secretion of N and AA during digestion originating predominantly from various digestive secretions, mucoproteins, and desquamated epithelial cells shed from the gut lining and the intestinal flora(Reference Tamminga, Schulze and Van Bruchem6, Reference Gabriel, Lessire and Juin7).
Secretion and/or reabsorption of EN and EAA are influenced by many factors, including animal species(Reference Tamminga, Schulze and Van Bruchem6), body weight(Reference Nyachoti, de Lange and McBride8), DM intake (DMI)(Reference Tamminga, Schulze and Van Bruchem6, Reference Nyachoti, de Lange and McBride8), dietary protein content and quality(Reference Larsen, Madsen and Weisbjerg3) and dietary fibre content(Reference Tamminga, Schulze and Van Bruchem6, Reference Zebrowska and Kowalczyk9). Starch usually supplies energy and is an important component of the animal diet, with cereal grains serving as the primary source of starch in ruminant diets. Maize, wheat, rice and sorghum are commonly used worldwide as a starch source in all animal feeds, including ruminants(Reference Huntington10). Grain texture plays a major role in the rate and location of starch digestion in ruminants(Reference Philippeau, Le Deschault de Monredon and Michalet-Doreau11), and variations in the starch granule structure among species of cereal grains may account for distinct rates of digestion patterns(Reference Swan, Bowman and Martin12). In a previous study, we demonstrated that the dietary starch source had significant effects on the ruminal degradation and intestinal digestion in goats(Reference Wang and Tan13, Reference Wang, Jiang and Tan14). Our hypothesis was that these variations influenced the losses of EN and EAA in ruminants. The AA composition of the endogenous protein at different sites of the digestive tract has received little attention in growing goats, and accurate determination of EN and EAA along the digestive tract is essential for optimising protein nutrition in ruminants. Therefore, the objective of the present study was to investigate the effects of the dietary starch source on EN and EAA losses along the gastrointestinal tract in growing goats.
Materials and methods
Animals and management
The experiment was conducted according to the animal care and use guidelines of the Animal Care Committee, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha, China.
Four Liuyang black growing wethers (a local breed in the South of China) with initial body weights of 20 (SD 2·5) kg were each fitted with a ruminal plastic cannula (4 cm internal diameter) and proximal duodenal and terminal ileal fistulae (T-type, 1 cm internal diameter; Laboratory Factory, Yinchuan, Ninxia University). The animals were kept individually in stainless-steel metabolism cages in a temperature-controlled (21°C) and constantly lit animal house with free access to fresh water.
Experimental diets and design
The experiment was carried out in a 4 × 4 Latin square design, with four goats, four dietary treatments (the dietary starch sources were mainly from maize, wheat, paddy and sorghum) and four periods. The formal experiment for each period lasted for 24 d, consisting of 14 d for adaptation, 3 d for in situ degradability of dietary protein determination and 7 d for sample collection.
The ingredients and chemical composition of the experimental total mixed rations are shown in Table 1. The total mixed rations were offered and the refusals were collected and weighed daily for 7 d before the commencement of the formal experiment to measure the voluntary feed intake. During the formal experiment, the supplied experimental total mixed rations were fed in equal portions at 07.00 and 19.00 hours daily to meet 1·3 times the maintenance requirements of the metabolisable energy according to the nutrient requirements of Chinese goats(Reference Lu, Zhang, Lu and Zhang15). To avoid feed refusals, the amount of the diets offered to each goat was controlled at 90 % of its voluntary feed intake measured before the commencement of the experiment.
Table 1 Ingredients and chemical composition of the experimental diets (% DM)
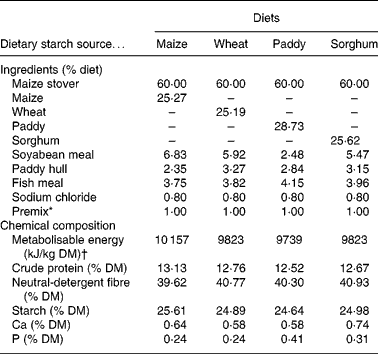
* Premix (per kg): 243·8 g MgSO4.H2O; 15·8 g FeSO4.7H2O; 3·3 g CuSO4.5H2O; 13·0 g MnSO4.H2O; 14·5 g ZnSO4.H2O; 20 mg Na2SeO3; 60 mg KI; 40 mg CoCl2.6H2O; 28·5 mg vitamin A; 0·438 mg vitamin D; 1224 mg vitamin E.
† Metabolisable energy values were reported by Zhang & Zhang(Reference Zhang, Zhang, Zhang and Zhang65).
In situ protein degradation
The in situ degradability of the dietary protein was examined from day 15 to day 17 according to the modified procedure of Tan et al. (Reference Tan, Lu and Hu16). The passage rate (Kp) was calculated according to the following equation described by the National Research Council(5): Kp (%/h) = 3·362+0·479 × (DMI, % of body weight) − 0·017 × (% neutral-detergent fibre (NDF), DM basis) − 0·007 × (% concentrate in diet, DM basis). The calculated average Kp value was 0·051 %/h in the present study. The effective rumen degradability of the dietary protein was calculated by the equation of Øskov & McDonald(Reference Øskov and McDonald17). The dietary rumen degraded protein was calculated by multiplying the effective rumen degradability by the dietary N or AA content, and the rumen undegraded protein was determined by subtracting the rumen degraded protein from the dietary protein content.
Sample collection
Feed samples were taken before feeding. From day 18 to day 24 of each period, a total of 4 g of chromic oxide (Cr2O3; 1 g every 6 h per d), as the particulate-phase digesta marker, was administered daily via the rumen fistulae at 06.00, 12.00, 18.00 and 24.00 hours(Reference Tan, Lu and Hu16). Samples (50 ml of ruminal, 50 ml of duodenal and 30 ml of ileal digesta) were collected at 05.00, 11.00, 17.00 and 23.00 hours on day 22, at 03.00, 09.00, 15.00 and 21.00 hours on day 23 and at 01.00, 07.00, 13.00 and 19.00 hours on day 24. At the end of each period, equal portions of the ruminal, duodenal and ileal samples were pooled. The pooled ruminal digesta were collected to isolate bacteria, and the pooled duodenal and ileal digesta were collected to measure the digesta flows. The ileal samples were chilled to 5°C until all twelve samples were collected in order to avoid lysis of bacteria, which would introduce cellular content into the water-soluble phase. From day 18 to day 21, the total faeces and urine were collected and subsampled for analyses for the determination of the N balance.
Sample handling
The pooled ruminal digesta were used to isolate bacteria by differential centrifugation according to the procedure of Reed et al. (Reference Reed, Lardyl and Bauer18). The isolated bacteria were freeze-dried (GLZY-0·5B; Pudong Freeze Dryer Equipment Co., Ltd, Shanghai, China), and then mashed with a mortar and pestle for analysis of microbial DM, N and AA (including diaminopimelic acid (DAPA)). The pooled duodenal and ileal digesta were separated as follows: one subsample of the duodenal and ileal digesta was freeze-dried and further analysed for DM, Cr, N and AA, respectively. The other subsample of duodenal digesta was used to isolate bacteria by differential centrifugation according to the procedure of Reed et al. (Reference Reed, Lardyl and Bauer18), and the isolated bacteria were used to analyse the microbial DM, N and AA (including DAPA). A subsample (100 g) of the pooled ileal digesta was added to 100 g of demineralised water and then shaken for 5 min, and the resulting suspension was squeezed through two layers of cheesecloth. The remaining feed particles and protozoa were removed by centrifugation (409 g; 5 min; 3°C), and the bacterial fraction in the ileal digesta was removed by centrifuging the supernatant fraction twice (17 300 g; 20 min; 3°C). The supernatant fraction was frozen for the analysis of water-soluble N and AA(Reference Larsen, Madsen and Weisbjerg19). A 50 g representative sample of each daily faeces was frozen at − 20°C for later analyses. The urine was acidified daily with 50 ml of 0·75 m-H2SO4 during its collection. Subsamples of urine were taken daily and kept at − 20°C until analysis.
Chemical analyses
The DM content of the feed, remaining feed (orts), faeces, digesta, incubated nylon bag samples and freeze-dried bacteria was determined by drying at 65°C to a constant weight. The faeces, feed, orts and digesta were ground through a 0·5 mm screen with a laboratory mill (DF-2; Changsha Instrument Factory, Changsha City, China). The total N content of the feed, orts, faeces, urine, digesta and incubated nylon bag samples was analysed according to AOAC methods(20) and that of bacteria was determined by the Dumas method according to Hansen(Reference Hansen21). The AA content, including DAPA, was determined according to Mason et al. (Reference Mason, Bech-Andersen and Rudemo22). Chromic oxide analysis on digesta was determined colorimetrically after oxidation to chromate according to Schurch et al. (Reference Schurch, Lloyd and Crampton23).
Calculations
The flows of DM, N and AA at the duodenum and ileum were calculated as described by Sun et al. (Reference Sun, Tan and Yao24). The duodenal flows of microbial N and AA were determined by the internal microbial marker DAPA(Reference Hutton, Bailey and Annison25), and the microbial passage at the duodenum was calculated from the concentration of DAPA in isolated rumen bacteria and the passage of DAPA at the duodenum. The following equations were used to calculate the duodenal flows of microbial N and AA(Reference Dufva, Bartley and Arambel26):
where R indicates the ratio of microbial N to total N or AA of digesta, DPd and Nd stand for the concentration of DAPA and N in the digesta, respectively, DPm and Nm are the concentrations of DAPA and N in isolated rumen bacteria, Mp is the flow of microbial N at the duodenum, and DFN stands for the flow of N in the digesta at the duodenum.
The calculated equation for the duodenal flows of EN or EAA is:
The duodenal flow of N and individual AA was also separated by origin by the amino acid profile (AAP) method(Reference Zhou, Jiang and Tan2, Reference Larsen, Madsen and Weisbjerg3, Reference Evans, Axford and Offer27, Reference Powell28). This mathematical method estimates the contribution of total N and AA from each origin by solving the following equation using the least squares calculation with SAS procedure PROC REG (SAS Institute, Inc., Cary, NC, USA)(29):
where AAi is the ith AA flow in the duodenum; i is the individual AA (i = 1–16); β1–4 is total AA from each origin; FeedAAi, MicAAi, AboAAi and BileAAi are the ith AA proportion in undegraded protein in total mixed rations, microbial protein, and endogenous protein in abomasum and bile, respectively. The AA profiles of abomasal fluid and bile were previously determined in our laboratory using twenty-three slaughtered goats.
The ileal EN and EAA were assumed to be located in the water-soluble phase and the apparent reabsorption of EN and EAA was calculated according to the method of Larsen et al. (Reference Larsen, Madsen and Weisbjerg19).
Statistical analyses
The results were analysed using the SAS procedure PROC GLM (SAS Institute, Inc.)(29) with the following model:
where Yijk is the response, μ is the overall mean, Periodi is the effect of the period (i = 1–3), Goatj is the effect of the goat (j = 1–3), and Treatmentk is the effect of the dietary starch source treatment (k = 1–3). The error (εijk) was assumed to be independent and N (0, σ2). Statistical effects were considered to be significant when the probabilities (P) were below 0·05, and tendencies were considered at 0·05 < P < 0·10.
Results
The duodenal flow, ileal flow and intestinal reabsorption of EN are presented in Table 2. There were no significant differences (P>0·05) for N intake, duodenal flow of total N (TN), undegraded feed N, microbial N (MN), EN, ileal flow or intestinal absorption of EN. The average ratio of MN:TN at the duodenum was from 56·0 % (maize) to 63·8 % (sorghum), and the average absorption of EN in the intestine was about 53·1 %.
Table 2 Effect of starch source on the duodenal flow, ileal flow and intestinal reabsorption of endogenous nitrogen (EN) in growing goats (g/d)
(Mean values with their pooled standard errors)
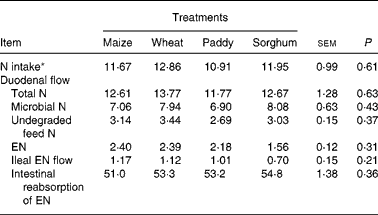
* Intake measured during the total collection period.
Table 3 presents the effects of the dietary starch source on the duodenal flow, ileal flow and intestinal reabsorption of EAA. There were no significant differences (P>0·05) in the duodenal or ileal flow of EAA among treatments. The dietary starch sources had no effect on the intestinal reabsorption of EAA, except for Leu. The intestinal reabsorption of endogenous Leu was significantly (P < 0·05) lower for the maize treatment than for the other three treatments. The average reabsorption of endogenous protein was 57·5 %.
Table 3 Effect of starch source on the flow of endogenous amino acids at the duodenum and ileum and intestinal reabsorption in growing goats (g/d)
(Mean values with their pooled standard errors)

a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Discussion
Effects of dietary starch source
Over the last few years, several studies have been conducted to test the effects of dietary factors on the loss of EN and EAA along the gastrointestinal tract on the DM intake(Reference Furuya and Kaji30, Reference Butts, Moughan and Smith31), fibre content(Reference Zhou, Jiang and Tan2, Reference Tamminga, Schulze and Van Bruchem6, Reference Ouellet, Demers and Zuur32), protein quantity and quality(Reference Butts, Moughan and Smith33) and anti-nutritive factors(Reference Barth, Lunding and Schmitz34–Reference Jansman, Enting and Verstegen36) in both ruminants and single-stomached animals. Some studies have been conducted to examine the influence of the dietary starch source on feed consumption, ruminal fermentation parameters, skeletal growth, blood metabolites, nutrient digestion, and growth performance in ruminants(Reference Wheeler, Wangsness and Muller37–Reference Khan, Lee and Lee41). To our knowledge, few studies have been conducted to evaluate the effects of dietary starch sources on the flows of EN and EAA in ruminants. The results of the present study indicated that there are no effects of dietary starch source on the intestinal flows of EN and EAA in growing goats. The chemical components with unique physical properties of dietary starch, its relationship to proteins, and the cellular integrity of starch-containing units affect the availability to microbes, nutrient digestibility(Reference Theurer, Lozano and Alio42) and produce energy in the intestine(Reference Owens, Secrist and Hill43), which may result in the variations of EN losses and EAA composition. Further studies are needed to investigate the effects of dietary starch levels and the ratios of starch:N on losses of endogenous materials along the gastrointestinal tract in ruminants.
Duodenal flows of endogenous nitrogen and endogenous amino acids
In the present study, Cr2O3 was selected as the particulate phase digesta marker. Within the last few years, considerable attention has been given to indicator methods for determining digestibility. The new methods are advantageous for saving time, labour and equipment. Hamilton et al. (Reference Hamilton, Mitchell and Kick44) used Cr2O3 to study estimated coefficients of digestibility and found that the length of the sampling period should be ≥ 3 d to reduce sampling errors. Crampton & Lloyd(Reference Crampton and Lloyd45) concluded that Cr2O3 recovery was 98 to 99 % when sheep received a hay plus grain ration, even 3 d after Cr2O3 administration had stopped. To confirm the stable marker concentrations in the rumen and intestine, the goats were fed with total mixed rations and the Cr2O3 administration was continued for 7 d in the present study. Estimates of the flows of endogenous protein in the duodenum vary from 16 % of the total protein flow(Reference Lammers-Wienhoven, Voigt and Ram46) to 56 %(Reference Hannah, Cochran and Vanzant47). Van Bruchem et al. (Reference Van Bruchem, Voigt and Lammers-Wienhoven48) used a continuous abomasal infusion of 15N-labelled, grass meal–beer yeast suspension, and reported that the endogenous contribution accounted for 12 % of the duodenal N flow in sheep. In addition, Sandek et al. (Reference Sandek, Krawielitzki and Kowalczyk49) reported that EN contributed to 3–12 % of the duodenal flow of total N determined by the 15N-labelled method for diets ranging from 15 to 25 % of crude fibre content in sheep. Ouellet et al. (Reference Ouellet, Demers and Zuur32) found that the average contribution of EN to TN at the duodenum was 14 % when estimated by the 15N dilution technique in Holstein cows fed high-fibre (containing 37·4 % NDF) and low-fibre (containing 23·3 % NDF) diets. Using an infusion of [15N]leucine in lactating cows, Lapierre et al. (Reference Lapierre, Ouellet and Berthiaume50) demonstrated that the EN contribution to the duodenal N flow averaged at 18 % when silage was fed and at 20 % when hay was offered. In the present study, the average endogenous contribution to the duodenal TN flow was 17 %, which is within the range of previously published values. Variations between the various reports could be due to differences of cannulae positions, analytical methods, diet compositions and experimental animal species.
Larsen et al. (Reference Larsen, Madsen and Weisbjerg3) found that the average duodenal flow of EN was 10·0 g/kg DMI when estimated by the difference method in lactating cows fed diets low in AA content. Using the AAP method, Larsen et al. (Reference Larsen, Madsen and Weisbjerg19) reported that the average duodenal flow of total essential amino acids (TEAA) was 25·6 g/kg DMI in dairy cows. Ouellet et al. (Reference Ouellet, Demers and Zuur32) demonstrated that the mean duodenal flow of EN was 4·4 g/kg DMI, which was estimated by the 15N dilution method in Holstein cows fed with diets containing different NDF contents. By contrast, Jensen et al. (Reference Jensen, Weisbjerg and Hvelplund51) reported that the duodenal flow of EN was 7·9 g/kg DMI using the AAP method in lactating Danish Holstein–Friesian cows fed maize silage. Zhou et al. (Reference Zhou, Jiang and Tan2) found that the mean duodenal flows of EN and TEAA were 2·1 and 11·8 g/kg DMI by estimation with the difference method and the AAP method in growing goats fed diets containing different NDF levels, respectively. In the present study, the average duodenal flows of EN and TEAA were 3·7 and 19·0 g/kg DMI, as determined by the difference and AAP methods, respectively. The differences of EN and TEAA between goats and dairy cows might result from the different computational processes among the determination methods. Furthermore, the big size and relatively large amount of protein turnover for dairy cows might result in greater losses of EN at the duodenum(Reference Lapierre, Ouellet and Berthiaume50), which needs to be further examined in future studies. The flows of EN and TEAA in the present study were higher than those of our previous study(Reference Zhou, Jiang and Tan2), probably because of the greater dietary NDF content (40·4 v. 35·6 %) in the present study, an important factor affecting the endogenous protein losses(Reference Tamminga, Schulze and Van Bruchem6, Reference Nyachoti, de Lange and McBride8, Reference Moughan, Souffrant and Hodgkinson52, Reference Hess and Sève53), or the lower level of feed intake (only 1·3 times the maintenance requirement for metabolisable energy). Some studies have shown that endogenous losses are sensitive to feed intake(Reference Furuya and Kaji30, Reference Butts, Moughan and Smith31, Reference Stein, Trottier and Bellaver54–Reference Moter and Stein56). The losses of EN and EAA (measured in g/kg DMI) in pigs fed at the maintenance metabolisable energy are twice that if pigs are fed three times maintenance metabolisable energy(Reference Moter and Stein56), and the level of feed intake also significantly influences the losses of EAA(Reference Butts, Moughan and Smith31, Reference Stein, Trottier and Bellaver54, Reference James, Butts and Koolaard55).
Ileal flows of endogenous nitrogen and endogenous amino acids
The flow of endogenous protein at the terminal ileum balances between secretion and reabsorption. With 15N marker techniques, Larsen et al. (Reference Larsen, Madsen and Weisbjerg19) found that the ileal proportion of water-soluble non-amino-N (NAN) of the total ileal NAN is similar to the proportion of the ileal EN in sheep(Reference Hamilton, Mitchell and Kick44, Reference Van Bruchem, Voigt and Lammers-Wienhoven48, Reference Sandek, Krawielitzki and Kowalczyk49), supporting the assumption that endogenous protein in the ileum is located in the water-soluble phase. Zhou et al. (Reference Zhou, Jiang and Tan2) reported that the average ileal flow of EN located in the water-soluble phase is 5·0 g/kg DMI in growing goats. In the present study, the average ileal flow of EN was 1·7 g/kg DMI, with the differences potentially ascribed to different experimental diet compositions.
It was reported that about 70–80 % of secreted endogenous protein is hydrolysed and reabsorbed before reaching the distal ileum(Reference Souffrant, Rérat and Laplace57Reference Krawielitzki, Kreienbring, Zebrowska, Souffrant and Hagemeister58). The major part of the remaining endogenous protein originates from deconjugated bile salts and mucin glycoprotein because these components are largely resistant to proteolysis and therefore escape reabsorption(Reference Taverner, Hume and Farrell59–Reference Moughan and Schuttert61). Glycine accounts for more than 90 % of the total AA content of bile, and mucin glycoprotein is rich in Thr, Asp and Glu(Reference Souffrant, Verstegen, Huisman and den Hartog62). Gardner(Reference Gardner63) provided evidence for a substantial flux of Pro and Gly from the enterocytes into the intestinal lumen. It has also been reported that Thr, Asp and Glu are more slowly absorbed from the intestinal lumen than most other AA(Reference Taverner, Hume and Farrell59). Therefore, endogenous protein usually has a high content of these AA(Reference Taverner, Hume and Farrell59, Reference Homes, Bayley and Leadbeater64). The AA composition of endogenous protein in the present study is in agreement with this hypothesis.
Larsen et al. (Reference Larsen, Madsen and Weisbjerg19) demonstrated that the small-intestinal reabsorption of EAA showed some extremes when the duodenal flows were estimated by the difference method, and pointed out that the reabsorption of EAA is related to the secretion of digestive juices with specific AA compositions. The average apparent reabsorption of EAA in the small intestine ranges from 62·3 to 82·5 % in sheep(Reference Lammers-Wienhoven, Voigt and Ram46, Reference Van Bruchem, Voigt and Lammers-Wienhoven48). The average apparent reabsorption of EAA was about 57·7 %, which was close to the lowest reported value in the present study. The significant differences of intestinal reabsorption of endogenous Leu need to be further studied.
Conclusion
In conclusion, the dietary starch sources did not significantly affect the duodenal and ileal losses of endogenous protein or the AA composition in growing goats.
Acknowledgements
We acknowledge the participation of Z. T. for direction of the experimental design, Y. P. for assistance with analyses, S. L. for paper revision, S. T., Z. S. and X. H. for help with the fistulae surgery of goats, and M. W. for guidance with the animal experiments and laboratory analyses. C. Z. participated in the animal experiment, laboratory analysis, treatment of data and writing of the manuscipt.
We wish to express our appreciation to the Chinese Academy of Sciences (no. KSCX2-YW-N-022), the Ministry of Science and Technology of China (2008BADA7B04) and the Natural Science Foundation of Hunan Province (no. 05JJ10004) for providing the financial support for the present study.
There are no conflicts of interest in the present study.





