It has been well demonstrated that approximately 10 % of human neonates might suffer from intra-uterine growth restriction (IUGR) at birth and remain small throughout their lives, which results in serious effects on their health( Reference Columbus, Fiorotto and Davis 1 , Reference McMillen and Robinson 2 ). Furthermore, there is some evidence that IUGR severely impairs the growth and development of skeletal muscle in neonates( Reference Rehfeldt and Kuhn 3 , Reference Yates, Macko and Nearing 4 ) and reduces the number of secondary and total muscle fibre, potentially resulting in decreased muscle mass and delayed skeletal muscle maturity( Reference Pardo, Bérard and Kreuzer 5 , Reference Alvarenga, Chiarini-Garcia and Cardeal 6 ). More importantly, neonates with IUGR have altered muscle energy metabolism and function, which have been associated with a decreased ability for glucose uptake by muscle tissue and the development of insulin resistance as adults( Reference Perruchot, Lefaucheur and Louveau 7 , Reference Vuguin, Raab and Liu 8 ). Research has documented that the growth and protein turnover of infants are higher during the neonatal period than that at any other period of life( Reference Davis, Burrin and Fiorotto 9 ), and a defect in normal muscle development during the early postnatal period can influence later growth and health, as well as muscle contractile properties and metabolic maturation( Reference Lefaucheur, Ecolan and Barzic 10 ). Therefore, the improvement of skeletal muscle development in IUGR piglets is of interest.
In recent years, considerable effort has been focused on improving the postnatal growth of IUGR neonates via nutritional regulation. Growing evidence indicates that both leucine and its metabolite (β-hydroxy-β-methylbutyrate, HMB) stimulate protein synthesis in skeletal muscle through the activation of the mammalian target of rapamycin (mTOR) signalling pathway in piglets and rats( Reference Boutry, El-Kadi and Suryawan 11 , Reference Anthony, Anthony and Kimball 12 ). In addition, studies have indicated that neonatal pigs infused with HMB (free acid) have higher skeletal muscle protein synthesis( Reference Wheatley, El-Kadi and Suryawan 13 ). In addition, Moore et al.( Reference Moore, Ferket and Mozdziak 14 ) reported that early post-hatch poultry fed with HMB had better muscle development via an increase in the mitotic activity of myogenic satellite cells. Moreover, HMB supplementation stimulated growth hormone–insulin-like growth factor (IGF) axis activity and decreased the rate of protein breakdown in a rodent model( Reference Gerlinger-Romero, Guimarães-Ferreira and Giannocco 15 , Reference Kovarik, Muthny and Sispera 16 ). However, there are only limited data on the effects of dietary exposure to HMB on skeletal muscle growth and maturity and amino acid metabolism in IUGR neonates during the early postnatal period. Sufficient skeletal muscle growth is essential for lasting metabolic health. Because of the structural and physiological similarities in skeletal muscle between pigs and humans, this study used piglets to assess the effects of dietary supplementation with β-hydroxy-β-methylbutyrate Ca (HMB-Ca) on skeletal muscle growth, the plasma amino acid profile, and the expression of muscle growth and development-related genes of IUGR neonates during the early postnatal period.
Methods
Animal care and experimental design
All procedures were in accordance with the guidelines set by the Animal Care and Use Committee of the Animal Nutrition Institute, Sichuan Agricultural University. The Ca salt (monohydrate) of HMB (purity 93 %) was purchased from Jiangyin Sanyi Chemical Co. Ltd. Neonatal piglets from parities 2 and 3 of thirty sows were selected for this study. At day 7 after birth, a total of twelve pairs of IUGR (body weight (BW) 1·85 (sem 0·36) kg) and normal-birth-weight (NBW) (BW 2·51 (sem 0·39) kg) male piglets were weaned and assigned, according to BW, to groups fed the basal diets supplemented with 0 or 0·08 % HMB-Ca. There were four treatment groups (birth weight/diet): IUGR/control (CON), IUGR/HMB, NBW/CON and NBW/HMB (six per group). All piglets were fed liquid diets every 3 h by bottle feeding from 7 and 28 d of age. All piglets were housed individually in metabolism cages (0·8×0·7×0·4 m) at an ambient temperature of 30°C in an environmentally controlled room and had free access to water.
Formula milk
The experimental diets used for the present study were prepared by adding 800 mg/kg HMB-Ca to the basal diet (Table 1). The basic milk replacement powder was formulated according to previous studies( Reference Han, Hu and Xuan 17 ). The dosage of supplemented HMB-Ca (800 mg/kg) was chosen according to previous studies on 7-d-old piglets( Reference Wheatley, El-Kadi and Suryawan 13 ). The diets were prepared by mixing 1 kg of milk replacement powder (DM, 87·5 %) with 4000 ml of water and were given to piglets via bottle feeding seven times per 24 h. The formula milk intake of the piglets was recorded daily. The average daily DM intake (ADMI) was calculated by multiplying the average daily intake of formula milk by its corresponding DM content. Formula milk intake was calculated as the difference between the offered amounts and the amount refused.
Table 1 Composition and nutrient content of the basal diet (87·5 % DM basis)Footnote *
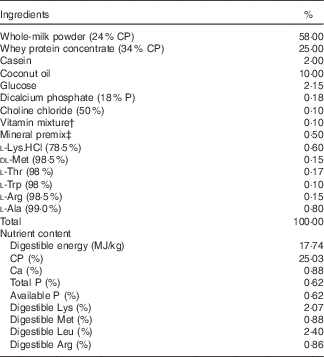
CP, crude protein; HMB-Ca, β-hydroxy-β-methylbutyrate Ca.
* The β-hydroxy-β-methylbutyrate diet was prepared by supplementing the basal diet with 800 mg/kg HMB-Ca and removing the same amount of dicalcium phosphate.
† Vitamin premix provided per kg of powder diet: vitamin A, 0·94 mg; vitamin D3, 0·01 mg; vitamin E, 20 mg; vitamin K3, 1 mg; vitamin B12, 0·04 mg; riboflavin, 5 mg; niacin, 20 mg; pantothenic acid, 15 mg; folic acid, 1·5 mg; thiamin, 1·5 mg; pyridoxine, 2 mg; biotin, 0·1 mg.
‡ Mineral premix provided per kg of powder diet: Zn, 90 mg; Mn, 40 mg; Fe, 90 mg; Cu, 6·0 mg; I, 0·2 mg; Se, 0·3 mg.
Blood and tissue samples collection
At day 29 after birth, blood samples (5 ml) were collected from the jugular vein after an overnight fast and immediately centrifuged for 10 min at 3500 g . The supernatants were stored at −20°C until analysis. All piglets were euthanised with an overdose of anaesthesia according to the method described by Han et al. ( Reference Han, Hu and Xuan 17 ). After death, the abdomen was opened, and the entire intestine was rapidly removed. Then longissimus dorsi (LD) muscle samples from the left half of the carcass were collected at the level of the twelfth/thirteenth ribs and cooled using liquid N2 and stored at −80°C until subsequent analyses. For body composition analysis of the piglets, all internal organs were weighted, and the right half of the carcass was dissected into primary cuts, such as the loin, neck and ham, which were further manually separated into muscle tissue, subcutaneous adipose tissue, skin and bones after cooling overnight at 4°C, as described previously by Rehfeldt et al. ( Reference Rehfeldt, Lefaucheur and Block 18 ). The muscle tissue of the piglets consisted of the total amount of skeletal muscle and intramuscular fat, and the whole superficial fat layer consisted of the subcutaneous fat. Muscle samples for histochemical analysis were collected from LD on the right side of the carcass and cut into 2-cm3 pieces (1·0×1·0×2·0 cm, parallel to the muscle fibres).
Biochemical analyses
The DNA of muscle samples was extracted using a QIAamp® DNA Mini Kit (Qiagen) according to the manufacturer’s instructions, and DNA quantification was performed using a NanoVue Plus spectrophotometer (GE Life Sciences). The protein concentration and the creatine kinase (CK) and lactate dehydrogenase (LDH) activities of muscle samples were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute) according to the instruction manuals. Briefly, frozen muscle samples (approximately 50 mg) were homogenised in 450 μl of 0·9 % saline and then centrifuged at 3500 g for 10 min at 4°C. The protein content in the muscle supernatant was determined with the Coomassie Brilliant Blue dyeing method using bovine serum albumin as the standard and expressed as milligram per gram. CK activity was measured in the supernatants of the muscle homogenates at 1:10 and 1:5 dilution. The optical density in microplate assays was measured at 595 and 660 nm for protein concentration and CK activity, respectively, using a biochemical analyser (Multiskan Spectrum; Thermo Scientific).The plasma insulin concentration was analysed using a porcine insulin RIA kit (Tianjin Jiuding Medical Biological Engineering Co. Ltd).
Muscle tissue samples were measured for HMB content by a modification of the plasma and milk sample analysis method previously described by Deshpande et al.( Reference Deshpande, Jie and Subbarayan 19 ) and Ehling & Reddy( Reference Ehling and Reddy 20 ). Briefly, acidified muscle tissue homogenates were extracted with methyl-t-butyl ether for 2 h, and α-hydroxy-α-methylbutyric acid (Sigma H40009; Sigma-Aldrich) was used as an internal standard. The extracting solution was centrifuged at 12 000 g and 4°C for 5 min. Then, the supernatant was transferred to a clean test tube and evaporated to dryness by N flushing at 40°C. After drying, 4 ml of 0·10 m-HCl in 90:10 (v/v) water–acetonitrile was added to the same test tube. After a brief vortex mixing, the mixture was passed through an OASIS® MCX cartridge (60 mg) (Waters) and the eluate was collected. The HMB content was analysed via HPLC with MS (Agilent).
The plasma free amino acid content was determined according to the method described by Li et al. ( Reference Li, Wan and Mercier 21 ). Briefly, 300 μl of the plasma sample and 900 μl of 10 % sulphosalicylic acid were mixed and centrifuged at 12 000 g and 4°C for 15 min. Then, the supernatant was filtered through a 0·22-μm-pore-size PTFE syringe filter (Millipore) into a 2-ml auto-sampler vial and analysed for amino acid content by an automatic amino acid analyzer (Hitachi). Amino acid standard solutions type B and AN-II (Wako Pure Chemical Industries Ltd) were used for calibration.
Histochemistry and microscopy
The LD samples were mounted on a cryostat chuck with a few drops of tissue-freezing medium, and then serial 10-μm sections were cut at −20°C. Sections were stained with a typical ATPase stain following alkalinisation (pH=9·40) and acidification (pH=4·20) to evaluate muscle morphology using a modification of the method of Guth & Samaha( Reference Guth and Samaha 22 ). All sections were photographed using a digital microscope (Nikon), and the muscle fibres were counted in five randomly selected fields of known size (1·01 mm2, 200–300 fibres). The muscle fibre cross-sectional area (CSA) in the total area analysed was measured with Image-Pro Plus version 6.0 software (Media Cybernetics).
Total RNA extraction and real-time RT-PCR
The genes detected in the muscle samples included myosin heavy-chain (MyHC) isoform (I, IIa, IIx and IIb), muscle regulatory factor 4 (MRF-4), myogenin (MyoG), IGF-1, mTOR, myostatin (MSTN) and β-actin. The information about the primer pairs for the selected genes is summarised in Table 2. Total RNA was extracted from the frozen samples using TRIzol (Invitrogen) according to the manufacturer’s protocol. Real-time quantitative PCR (qPCR) analysis was performed using the SYBR Green method, and the target genes were quantified using CFX manager version 1.1 software (Bio-Rad Laboratories). A commercial RT kit (TaKaRa) was used for the synthesis of complementary DNA (cDNA). The qRT-PCR reaction contained 5 μl of SYBR Green supermix (TaKaRa), 1 μl of cDNA, 0·5 μl of each gene-specific primer and 3 μl of double-distilled H2O. The following thermal cycling conditions were used: denaturation at 95°C for 15 s, followed by forty cycles of denaturation at 95°C for 5 s and annealing at 61·5°C for 30 s. To confirm the specificity of each product, a melting curve analysis (50–95°C with a heating rate of 0·1°C/s and continuous fluorescence measurement) was performed, and the size of the products was determined by agarose gel electrophoresis. The qPCR analysis of each sample was repeated three times. The relative mRNA abundances of the detected genes were calculated using the
![]() $${\rm 2}^{{{\minus}\Delta \Delta C_{T} }} $$
method(
Reference Li, Wan and Mercier
21
).
$${\rm 2}^{{{\minus}\Delta \Delta C_{T} }} $$
method(
Reference Li, Wan and Mercier
21
).
Table 2 Primer sequences of the target genes

MyHC, myosin heavy chain; F, forward; R, reverse; MRF-4, muscle regulatory factor 4; MyoG, myogenin; mTOR, mammalian target of rapamycin; IGF-1, insulin-like growth factor-1; MSTN, myostatin.
Tissue protein extraction and Western blot analysis
The frozen LD samples were lysed and the protein concentration was determined using a protein assay kit according to the manufacturer’s instructions. Western blot analysis of fast-MyHC content using the anti-fast myosin skeletal heavy chain antibody (ab91506, diluted 1:5000; Abcam) was carried out as described previously( Reference Wang, Li and Yang 23 ). The relative expression of the target protein was normalised to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Bio-Rich 042, BMSX, diluted 1:20 000; Beijing BMSX Technology Co. Ltd), which was used as the internal standard. The band density of fast-MyHC was normalised to that of GAPDH, and fast-MyHC protein content was presented as the fold change relative to that of the NBW piglets in the control group.
Statistical analysis
All data were checked for a normal distribution and homogeneous variance. In addition, all data were analysed using the Proc MIXED procedure (SAS Institute Inc.) according to the following statistical model Y ijk =μ+α i +β j +(αβ) ij +ε ijk (i=1,2; j=1,2; k=1,2, … , n ij ), where Y ijk is the response variable, μ the overall mean, α i the effect of BW (i=IUGR or NBW), β j the effect of HMB (j=CON or HMB), (αβ) ij the interaction between BW and HMB and ε ijk represents the random error, which was assumed to be N (0, σ2). Piglets were considered as the experimental unit. Differences between groups were analysed using the general linear model procedure followed by Duncan’s test. The results for muscle fibre CSA and fast-MyHC protein expression are presented as the mean values with their standard errors, and other data in tables are presented as the mean and pooled standard errors. Probability values <0·05 were considered statistically significant, and values between 0·05 and 0·1 were considered to indicate trends.
Results
Performance and body composition of piglets
The results of piglet performance and body composition are presented in Tables 3 and 4. At the onset of the experiment (day 7 after birth), no differences were observed in the BW of IUGR and NBW piglets between the two dietary treatments, and the final BW and BW gain of the IUGR piglets were both lower than those of their NBW counterparts (−36 %, P<0·01). However, the IUGR piglets fed HMB-Ca milk exhibited a net weight and average daily weight gain (ADG) similar to that of the CON-fed NBW piglets. Regardless of BW, piglets in the HMB group also showed an increased ADG (+14 %, P<0·10) and a decreased feed conversion rate (−7 %, P<0·01) from day 7 to 28 in comparison with that of the control pigs. In addition, HMB-Ca treatment increased (P<0·05) the relative ratio of skeletal muscle by 3·1 and 4·4 % for the IUGR and NBW piglets, respectively, compared with that of the piglets fed the control diet.
Table 3 Effect of dietary β-hydroxy-β-methylbutyrate (HMB) calcium supplementation on the growth performance of intra-uterine growth restriction (IUGR) and normal-birth-weight (NBW) piglets (Mean values with their standard errors)

CON, control; BW, body weight; ADG, average daily weight gain; ADMI, average daily DM intake; FCR, feed conversion ratio.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* FCR was calculated by dividing the ADMI by its corresponding ADG.
Table 4 Effects of dietary β-hydroxy-β-methylbutyrate (HMB) calcium supplementation on body composition of intra-uterine growth restriction (IUGR) and normal-birth-weight (NBW) piglets (Mean values with their standard errors)
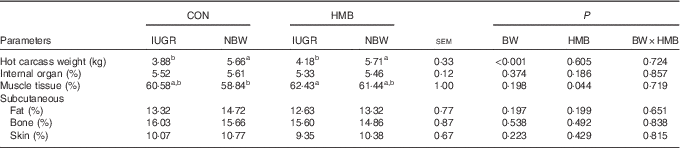
CON, control; BW, body weight.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Plasma amino acid and insulin concentrations, and muscle β-hydroxy-β-methylbutyrate levels of piglets
As shown in Table 5, irrespective of BW, piglets supplemented with HMB-Ca milk had an increased HMB concentration in their LD (P<0·01) relative to that of piglets fed CON milk. Piglets fed the HMB-Ca supplemented milk also had an elevated plasma insulin concentration (P<0·01) relative to that of their CON counterparts. In addition, in comparison with the content in the CON piglets, dietary HMB-Ca treatment increased plasma leucine and branched-chain amino acid (BCAA) content (P<0·05). Similarly, plasma essential amino acid (EAA) and non-essential amino acid (NEAA) content showed increased levels (P<0·05) in piglets from the HMB group relative to the levels in the CON groups, but no interaction (P>0·05) between BW and HMB treatment was observed. Furthermore, the plasma urea content of the IUGR piglets was lower than that of the NBW piglets (P<0·01).
Table 5 Effects of dietary β-hydroxy-β-methylbutyrate (HMB) calcium supplementation on the plasma amino acid profile and muscle HMB level of intra-uterine growth restriction (IUGR) and normal-birth-weight (NBW) piglets (Mean values with their standard errors)
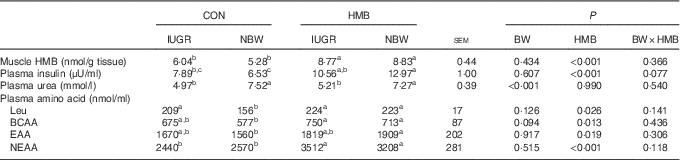
CON, control; BW, body weight; BCAA, branched-chain amino acid; EAA, essential amino acid; NEAA, non-essential amino acid.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Biochemical and histological properties of the longissimus dorsi of piglets
Data for CK and LDH activity, DNA and protein content, and the muscle fibre CSA of the skeletal muscle from the piglets are shown in Table 6 and Fig. 1. Regardless of BW, both the CK (P<0·05) and LDH activity (P<0·05) of the LD were greatly increased in piglets fed HMB-Ca milk compared with that in piglets fed CON milk. Furthermore, the mean type II fibre CSA of the LD of piglets fed HMB-Ca milk was significantly increased (P<0·01) relative to that of pigs supplemented with the CON milk, and the type I fibre CSA of the LD of IUGR piglets was lower than that of NBW piglets (P<0·01).

Fig. 1 Effects of dietary β-hydroxy-β-methylbutyrate (HMB) calcium supplementation on the muscle fibre cross-sectional area (CSA) of the longissimus dorsi of intra-uterine growth restriction (IUGR) and normal-birth-weight (NBW) piglets. Values are means, with their standard errors represented by vertical bars. a,b Mean values with unlike superscript letters were significantly different (P<0·05). □, Control; ■, HMB; BW, body weight.
Table 6 Effects of dietary β-hydroxy-β-methylbutyrate (HMB) calcium supplementation on biochemical properties of the longissimus dorsi of intra-uterine growth restriction (IUGR) and normal-birth-weight (NBW) piglets (Mean values with their standard errors)

CON, control; BW, body weight; CK, creatine kinase; LDH, lactate dehydrogenase.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Gene expression in the longissimus dorsi of piglets
As shown in Table 7, regardless of BW, piglets fed HMB milk had significantly higher MyHC-IIb mRNA levels in the LD (P<0·05) than did pigs fed CON milk. Furthermore, compared with the levels of the CON group, the mRNA levels of IGF-1, mTOR and MRF-4 in the LD were up-regulated in piglets fed HMB-Ca milk (P<0·05). However, there was no significant interaction between the effects of BW and HMB treatment on the mRNA abundance of these genes in the LD, except on mTOR (P<0·05).
Table 7 Effects of dietary β-hydroxy-β-methylbutyrate (HMB) calcium supplementation on the mRNA level of the myosin heavy-chain (MyHC) isoform and myogenic genes in the longissimus dorsi of intra-uterine growth restriction (IUGR) and normal-birth-weight (NBW) piglets (Mean values with their standard errors)

CON, control; BW, body weight; MRF-4, muscle regulatory factor 4; MyoG, myogenin; mTOR, mammalian target of rapamycin; IGF-1, insulin-like growth factor-1; MSTN, myostatin.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Protein expression of fast-myosin heavy chain in the longissimus dorsi of piglets
The data for fast-MyHC protein content in the LD of piglets are presented in Fig. 2. Regardless of BW, the fast-MyHC protein content was significantly higher in the LD of piglets fed HMB-Ca milk than in the LD of piglets fed CON milk (P<0·05). However, dietary HMB-Ca supplementation did not increase fast-MyHC protein content in the LD of IUGR piglets.

Fig. 2 Effects of dietary β-hydroxy-β-methylbutyrate (HMB) calcium supplementation on the fast-myosin heavy-chain (MyHC) protein levels in the longissimus dorsi of intra-uterine growth restriction (IUGR) and normal-birth-weight (NBW) piglets. Values are means, with their standard errors represented by vertical bars. a,b Mean values with unlike superscript letters were significantly different (P<0·05). □, Control; ■, HMB; BW, body weight; GAPDH, glyceraldehyde-3-phosphate dehydrogenase
Discussion
The primary aim of the present study was to investigate the influence of the dietary supplementation of HMB-Ca to neonates during the early postnatal period on skeletal muscle growth and development. Piglet performance, to a great extent, is influenced by milk intake, which correlates with maternal milk yield and birth weight( Reference Skok, Brus and Škorjanc 24 , Reference Fix, Cassady and Herring 25 ). Therefore, male piglets with similar low and normal BW were used for IUGR and NBW groups, respectively, in this experiment. We found no significant difference in ADMI between dietary treatments during the entire experimental period, and IUGR piglets showed lower ADMI and ADG compared with those of NBW piglets. It has been shown in previous studies that the poor growth of IUGR piglets during the neonatal period is because of a number of factors, including reduced nutrient intake( Reference Liu and He 26 ). Interestingly, IUGR piglets fed HMB-Ca milk had a net weight and ADG similar to that of CON-fed NBW piglets in the present study. HMB is a metabolite of leucine, which is used to promote the growth of lean body mass following exercise, and, in particular, it is involved in disease-related muscle wasting( Reference Wilson, Lowery and Joy 27 , Reference Eley, Russell and Tisdale 28 ). HMB has been shown to increase muscle protein synthesis through the mTOR signalling pathways while reducing proteolytic processes, which is similar to the response of skeletal muscle to leucine( Reference Wilkinson, Hossain and Hill 29 ). In the current study, HMB-Ca treatment improved the performance of both IUGR and NBW piglets, which is in agreement with the findings of Nissen et al. ( Reference Nissen, Faidley and Zimmerman 30 ), who reported that, when fed to sows at 2 g/d from day 108 of gestation to day 21 of lactation, HMB-Ca resulted in an increase in the weaning BW of piglets. In addition, in this experiment, piglets fed HMB-Ca milk had an increased percentage of skeletal muscle relative to that of CON piglets, which is consistent with the findings of Qiao et al.( Reference Qiao, Zhang and Wu 31 ), who found that, when fed to broiler chicks at a dosage of 0·1 %, HMB-Ca significantly improved breast muscle yield and resulted in less abdominal fat. Similarly, Pimentel et al.( Reference Pimentel, Rosa and Lira 32 ) reported that feeding of HMB at a dosage of 320 mg/kg BW to rats induced a significant increase in the weight of the extensor digitorum longus and soleus. Studies have also suggested that a piglet’s growth and protein turnover are higher during the neonatal period( Reference Wheatley, El-Kadi and Suryawan 13 ), and the majority of this growth comprises skeletal muscle growth, which represents 30 % of the total body mass( Reference Davis and Fiorotto 33 ). These observations indicate that increased performance might be obtained by improving skeletal muscle growth for piglets during the early postnatal period.
The secondary aim of the present study was to explore the mechanisms underlying the direct response of skeletal muscle growth in IUGR and NBW piglets to the dietary HMB-Ca supplementation, which is critical for the development of strategies to improve the growth and health of low-birth-weight neonates. It is well known that insulin and glucose, as well as amino acids, especially leucine, are the main anabolic promoters resulting in protein synthesis in the skeletal muscle of piglets( Reference Columbus, Fiorotto and Davis 34 ), and higher plasma insulin, glucose and amino acid levels can induce greater rates of muscle protein synthesis( Reference Wilson, Suryawan and Gazzaneo 35 ). In the present study, HMB-Ca supplementation significantly increased insulin levels in IUGR and NBW piglets. However, the insulin levels were not significantly different between CON and HMB-fed IUGR piglet groups. A possible reason for this is that IUGR piglets have been shown to exhibit a small degree of insulin resistance during days 2 and 28 postnatal( Reference Gondret, Père and Tacher 36 , Reference He, Dong and Xu 37 ), leading to higher fasting plasma insulin levels in IUGR piglets. Moreover, dietary HMB supplementation significantly affected the plasma amino acid metabolism of piglets, including increased plasma leucine, BCAA, EAA and NEAA content. It appears that the stimulation of protein synthesis is dependent on signals related to the concentration of extracellular, rather than intramuscular, EAA( Reference Bohé, Low and Wolfe 38 ). Interestingly, we observed that piglets fed HMB-Ca milk had increased levels of HMB in their LD in this study. This effect was also demonstrated in the study by Wheatley et al. ( Reference Wheatley, El-Kadi and Suryawan 13 ), who found that, when piglets were infused with HMB at 100 or 400 μmol/kg BW per h for 1 h, an increased HMB level was observed in the LD. This may provide the important evidence to explain why HMB enters the skeletal muscle tissue and promotes protein synthesis. In addition, the plasma urea content was not affected by dietary HMB-Ca treatment in the piglets in this study, providing further evidence that HMB might not promote protein breakdown.
In addition, we found that the LD of piglets supplemented with HMB-Ca milk showed an increase of LDH and CK activities, and had higher type II muscle fibre CSA. Lefaucheur et al. ( Reference Lefaucheur, Ecolan and Barzic 10 ) reported that early postnatal malnutrition reduced the glycolytic capacity and CSA of fast-twitch fibres, leading to a delay in muscle maturation. Previous studies have also indicated that a higher LDH activity, which is a marker for the anaerobic glycolytic fibre, is found in fast glycolytic fibres than in slow oxidative fibres( Reference Takekura and Yoshioka 39 ), and that LDH activity is related to fibre CSA( Reference Vestergaard, Oksbjerg and Henckel 40 ). Furthermore, fast glycolytic fibres exhibit both a higher specific CK activity (a marker of differentiation in skeletal muscle development) and a greater phosphocreatine content than slow-twitch fibres( Reference Conjard, Peuker and Pette 41 ). Therefore, it is possible that the switch to a more anaerobic metabolism in the LD of pigs directly contributes to muscle fibre growth and maturity in response to dietary supplementation with HMB-Ca.
It is also known that muscle development in postnatal piglets is regulated by transcription and growth factors, such as the myogenic differentiation factor (MyoD) family, which comprises four transcriptions factors (MyoD, myogenic factor 5 (Myf-5), MyoG and MRF-4), and IGF-1 and 2 (IGF-2), which might both stimulate the proliferation of myoblasts( Reference Florini, Ewton and Magri 42 , Reference Florini, Ewton and Coolican 43 ). MyoD and Myf-5 are expressed constantly up to the 3rd week after birth, and their expression is followed by a decline in 50-d-old pigs. However, the level of MRF-4 level does not show statistically significant differences between different stages of development( Reference Caliaro, Maccatrozzo and Toniolo 44 , Reference Ropka-Molik, Eckert and Piórkowska 45 ). Therefore, we used MRF-4, MyoG, IGF-1 and MSTN as the main target genes to evaluate the response of skeletal muscle fibre development to dietary HMB-Ca supplementation. It appears that the transcription factor MRF-4 regulates the final differentiation of myotubes, and this gene has a 10-fold higher postnatal expression than the other genes of the MRF family( Reference Bober, Lyons and Braun 46 ). In this study, a higher level of MRF-4 mRNA expression was observed in the LD of piglets fed HMB-Ca milk than in the LD of the CON-fed piglets. Furthermore, the feeding of milk supplemented with HMB-Ca significantly increased the IGF-1 and mTOR mRNA levels in the LD of piglets, which agrees with the results of Kim et al. ( Reference Kim, Park and Lee 47 ), who reported a significant IGF-1 mRNA increase in the soleus (+33 %) when old, female Sprague–Dawley rats were administered HMB orally at a dosage of 0·48 g/kg per d. Moreover, we found that there was a significant interaction between the effects of HMB and BW on mTOR mRNA expression in the LD in this study. It has been suggested that the activation of the mTOR signalling pathway in skeletal muscle is under the control of the arginine family of amino acids and leucine( Reference Meijer and Dubbelhuis 48 ). In the current study, an increased leucine content was observed in IUGR piglets fed the HMB-Ca diet than in CON-fed NBW piglets, which might also explain the increased expression of mTOR in IUGR pigs fed the HMB-Ca diet. However, at present, it is unclear why dietary HMB-Ca supplementation significantly increased mTOR expression in IUGR piglets but not in NBW piglets. Xu et al.( Reference Xu, Bai and He 49 ) also reported a higher mTOR protein expression in IUGR piglets than in NBW piglets in response to dietary leucine supplementation. Further studies should be conducted in relation to the specific mechanisms of protein synthesis that are mediated by HMB in IUGR piglets.
It appears that marked changes occur in the skeletal muscle growth of piglets during the neonatal period, including rapid myofibril protein accretion( Reference Suryawan, Jeyapalan and Orellana 50 ), changes in MyHC polymorphism, increases in aerobic and glycolytic metabolism and hypertrophy of muscle fibres( Reference Picard, Lefaucheur and Berri 51 ). However, limited studies exist that evaluate the direct impact of dietary HMB-Ca supplementation to piglets on the MyHC isoform mRNA levels of skeletal muscle. In this experiment, HMB-Ca treatment significantly increased MyHC-IIb mRNA and fast-MyHC protein levels in the LD of piglets. Research has suggested that high muscularity is positively related to a high abundance of the MyHC-IIb transcript( Reference Wimmers, Ngu and Jennen 52 ), and that from days 7 to 180 after birth the MyHC-IIb mRNA levels show a steadily increasing trend. Moreover, on day 30, MyHC-I and MyHC-IIb mRNA abundances are at their lowest( Reference Men, Deng and Xu 53 ). On the basis of previous reports and the data from this experiment on the CK and LDH activities, MyHC-IIb and IGF-1 mRNA levels, fast-MyHC protein content, the increased muscle growth and maturity in pigs fed HMB-Ca milk could be because of the increased levels of MyHC-IIb mRNA in fast glycolytic fibres, which enhances skeletal muscle fibre differentiation of piglets. However, dietary HMB-Ca supplementation did not increase MyHC-IIb mRNA or fast-MyHC protein expression in IUGR piglets. It has been suggested that newborn piglets exposed to IUGR have accelerated skeletal muscle development with a precocious type II to type I conversion and, as a consequence, an increase in the proportion and maturation of type I fibres( Reference Wank, Bauer and Walter 54 , Reference Bauer, Gedrange and Bauer 55 ). Similarly, Fahey et al.( Reference Fahey, Brameld and Parr 56 ) reported that fibre type shifts in the early neonatal period after maternal dietary restriction in sheep exhibited either a relative increase in type I oxidative fibres or a relative decrease in glycolytic type II fibres. The increased MyHC transition towards type I fibres during the neonatal period for IUGR piglets might result in decreased MyHC-IIb mRNA and fast-MyHC protein levels, and lower LDH activity in the LD in response to dietary HMB-Ca supplementation. The potential molecular mechanisms underlying the different effect on the growth of skeletal muscle in IUGR and NBW piglets fed an HMB diet should also be investigated further.
In conclusion, the findings of the present study suggest that dietary supplementation of HMB-Ca contributes to muscle fibre growth and maturity by accelerating fast-twitch glycolytic fibre development in IUGR and NBW pigs during the early postnatal period. The ability of HMB to stimulate gains in lean mass in young or neonatal animal has been largely ignored, and this study may have important implications for the postnatal skeletal muscle growth of IUGR and NBW piglets during the neonatal period. Future studies are needed to investigate the effects of different levels of HMB-Ca supplementation on lean mass gain in early postnatal piglets.
Acknowledgements
The authors thank John A. Rathmacher and John C. Fuller, Jr for their immeasurable help in determining the purity of HMB-Ca.
This work was supported by the National Special Research Fund for the Non-Profit Sector (Agriculture) (no. 201203015), the Academy of Kechuang Feed Industry in Sichuan (2013NZ0056), the Research Team of Youth Scientific and Technical Innovation of Sichuan (13CXTD0004) and the Program for Changjiang Scholars and Innovative Research Team in University (IRT13083).
The authors’ contributions are as follows: D. Wu designed the study; H. Wan, J. Zhu, G. Su, Y. Liu, L. Hua, C. Wu, R. Zhang, P. Zhou and Y. Shen carried out the study; H. Wan, J. Zhu and G. Su performed the analysis. H. Wan, S. Xu and B. Feng analysed the data; H. Wan wrote the paper and D. Wu, L. Che, Z. Fang, L. Hu and Y. Lin made modifications to the manuscript.
The authors declare that there are no conflicts of interest.












