Ulcerative colitis is a chronic inflammatory disease of the colon and rectum( Reference Lee, Wang and Cheng 1 ). Recently, the inflamed colon has received attention in both humans and animals. Butyrate is an important energy substrate for mucosal cells of the large intestine and also plays an important role in the repair of the injured gut( Reference Lee, Wang and Cheng 1 , Reference Hou, Liu and Hu 2 ). As a normal constituent of the colonic luminal contents, butyrate is produced by the bacterial fermentation of unabsorbed complex carbohydrates in the mammalian digestive tract and of amino acids released from undigested proteins( Reference Gaschott, Steinhilber and Milovic 3 ). In the normal colonic mucosa, butyrate serves as a primary energy source, promotes the growth of normal colonic epithelial cells and plays a role in the prevention of certain types of colitis( Reference Gaschott, Steinhilber and Milovic 3 , Reference Roediger 4 ). By contrast, in a wide variety of neoplastic cells, butyrate has been reported to inhibit the growth of colonocytes in vitro ( Reference Gaschott, Steinhilber and Milovic 3 , Reference von Engelhardt, Bartels and Kirschberger 5 , Reference Schröder, Hess and Caspary 6 ).
Normally, natural butyrate is largely absorbed in the stomach. Tributyrin (TBU), which is chemically synthesised from butyrate and TAG( Reference Gaschott, Steinhilber and Milovic 3 ), can bypass the stomach to reach the hind gut via oral administration( Reference Roediger 4 – Reference Kuefer, Hofer and Altug 7 ). TBU has beneficial effects on the attenuation of ulcerative colitis due to its trophic, anti-inflammatory, pro-apoptotic and anti-carcinogenic properties( Reference Leonel, Teixeira and Oliveira 8 ). Thus, TBU is a good dietary source of butyrate via the action of lipase in the intestine and has beneficial effects on the maintenance of normal intestinal morphology( Reference Hou, Liu and Hu 2 , Reference Piva, Grilli and Fabbri 9 ). In support of this view, dietary supplementation with 5 g/kg TBU has been shown to improve growth performance and intestinal morphology in weanling pigs( Reference Hou, Liu and Hu 2 ). Additionally, TBU has positive effects on colonic restructuring in experimental colitis induced by dextran sodium sulphate in mice through the inhibition of inflammation and regulation of anti-inflammatory cytokine and regulatory T-cell expression( Reference Leonel, Teixeira and Oliveira 8 ). Similarly, treatment of human mononuclear cells with butyrate, TBU, propionate or trichostatin A has been shown to inhibit TNF-α secretion and NF-κB activation( Reference Usami, Kishimoto and Ohata 10 ). At present, little is known about the effects of TBU on ulcerative colitis in pigs.
Distal colitis in rats can be induced by intracolonic administration of acetic acid (ACA)( Reference Cetinkaya, Bulbuloglu and Kurutas 11 , Reference Sharon and Stenson 12 ). This animal model shares many characteristics of human colitis( Reference Cetinkaya, Bulbuloglu and Kurutas 11 , Reference Sharon and Stenson 12 ) to investigate the acute phase of inflammation( Reference Gulec, Yasar and Yildiz 13 – Reference Myers, Dempsey and Yasar 16 ). Recently, a porcine model of colitis has been developed by intrarectal administration of 10 % ACA( Reference Cetinkaya, Bulbuloglu and Kurutas 11 , Reference Wang, Hou and Yi 17 ). The disorder is characterised by a deregulation of the colonic mucosal immune system along with the presence of architectural distortion and infiltration of neutrophils and macrophages( Reference Wang, Hou and Yi 17 ). We hypothesised that dietary TBU supplementation could alleviate intestinal inflammation by reducing oxidative stress and activating epidermal growth factor receptor (EGFR) signalling. This hypothesis was tested in the present study using an ACA-induced porcine model of colitis.
Materials and methods
Animal care and diets
The animal use protocol followed in the present study was approved by the Animal Care and Use Committee of Hubei Province. A total of eighteen cross-bred healthy female piglets (Duroc × Landrace × Yorkshire) were reared by sows and then weaned at the age of 18 d to a maize and soyabean meal-based diet (Table 1), which was formulated to meet the National Research Council (1998)-recommended requirements for all nutrients. After a 7 d adaptation period, piglets (25 d of age and average body weight of 5·8 (sd 1·17) kg) were housed individually in stainless-steel metabolism cages (1·20 × 1·10 m2) and maintained in an environmentally controlled room (at a room temperature of 28, 25 and 22°C during the first, second and third weeks of the trial, respectively). Each cage was equipped with a feeder and a nipple waterer to allow piglets free access to food and drinking-water.
Table 1 Ingredients and nutrient composition of the basal diet (air-dry basis)
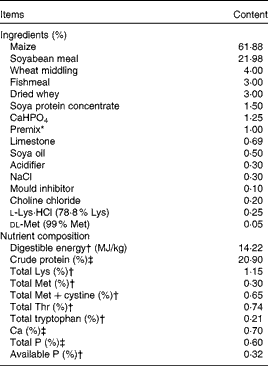
* Premix provided the following amounts of vitamins and trace minerals per kg of the complete diet: Fe, 100 mg (FeSO4·H2O); Cu, 150 mg (CuSO4·5H2O); Mn, 40 mg (MnSO4·5H2O); Zn, 100 mg (ZnSO4·7H2O); I, 0·5 mg (KI); Se, 0·3 mg (Na2SeO3·5H2O); retinol acetate, 3·72 mg; cholecalciferol, 0·10 mg; dl-α-tocopheryl acetate, 26·7 mg; vitamin K3, 4 mg; thiamin, 6 mg; riboflavin, 12 mg; pyridoxine, 6 mg; vitamin B12, 0·05 mg; biotin, 0·2 mg; folic acid, 2 mg; niacin, 50 mg; d-calcium pantothenate, 25 mg.
† Calculated value.
‡ Analysed value.
Experimental design
At 25 d of age, piglets were randomly assigned to one of the following three treatment groups: (1) control group (non-challenged control, piglets fed the basal diet and intrarectally administered with saline); (2) ACA group (ACA-challenged control, piglets fed the basal diet and intrarectally administered with ACA); (3) TBU group (ACA challenge+0·1 % TBU, piglets fed the basal diet supplemented with 0·1 % TBU and intrarectally administered with ACA). There were six piglets per group. TBU (purity 97 %; Sigma) was mixed well with the basal diet. The diets of the control and ACA groups were supplemented with 0·1 % maize starch to obtain approximately isoenergetic diets. The dosage of supplemental TBU (0·1 %) used in the study was chosen because it has been shown in previous studies that higher growth performance is achieved in pigs fed a diet supplemented with 0·1 % sodium butyrate( Reference Piva, Morlacchini and Casadei 18 , Reference Lu, Zou and Wang 19 ). On day 15 of the trial, overnight fasted piglets in the ACA and TBU groups were intrarectally administered with ACA (10 ml of 10 % ACA), whereas piglets in the control group were intrarectally administered with the same volume of saline solution. Under anaesthesia (with an intravenous injection of sodium pentobarbital at a dose of 30 mg/kg body weight), a soft catheter was introduced into the rectum (20–25 cm from the anus) for careful administration of ACA or saline. Before removing the catheter, 20 ml air was applied to spread ACA into the colon. During days 0–14 of the trial (pre-challenge), all piglets had free access to food and drinking-water. To exclude a possible effect of ACA-induced reduction in food intake on the intestine of piglets, during days 15–21 of the trial (post-challenge with ACA), piglets in the control and TBU groups were pair-fed the same amount of feed per kg body weight fed to those in the ACA group. On day 22, all piglets were killed under anaesthesia with an intravenous injection of sodium pentobarbital (50 mg/kg body weight) to collect intestinal mucosae for further analysis.
Blood sample collection
On day 22 of the trial, blood samples were collected from the anterior vena cava into heparinised vacuum tubes (Becton Dickinson Vacutainer System), as described previously( Reference Hou, Yao and Wang 20 , Reference Hou, Wang and Zhang 21 ). Blood samples (7 ml) were centrifuged at 1000 g for 10 min at 4°C to separate plasma and then stored at − 80 °C until analysis( Reference Hou, Wang and Ding 22 , Reference Yi, Hou and Wang 23 ).
Intestinal sample collection
The abdomen of piglets was surgically opened immediately from the sternum to the pubis, and the whole gastrointestinal tract was immediately exposed( Reference Hou, Wang and Ding 22 , Reference Li, Kim and Li 24 ). The small intestine and colon were dissected free of the mesentery and placed on a chilled stainless-steel tray. Segments measuring 5 and 10 cm were cut from the mid-ileum and the mid-colon, respectively( Reference Hou, Wang and Ding 22 , Reference Wang, Chen and Li 25 ). The 5 cm intestinal segments were gently flushed with ice-cold PBS (pH 7·4) and then placed in 10 % fresh, chilled formalin solution for histological measurements( Reference Hou, Wang and Ding 22 , Reference Nofrarías, Manzanilla and Pujols 26 ). The 10 cm intestinal segments were opened longitudinally and the contents were flushed with ice-cold PBS( Reference Hou, Wang and Zhang 21 , Reference Hou, Wang and Ding 22 ). Mucosa was collected by scraping using a sterile glass microscope slide at 4°C( Reference Hou, Wang and Ding 22 , Reference Hou, Wang and Zhang 21 , Reference Wang, Ou and Yin 27 ), rapidly frozen in liquid N2 and stored at − 80°C until analysis. All samples were collected within 15 min of killing.
Determination of total and differential leucocyte counts
Total and differential leucocyte counts were determined using an automated haematology analyser (Sysmex K4500, Sysmex Company). The differential leucocyte percentage was calculated as the ratio of leucocyte number:total leucocyte number( Reference Han, Liu and Fan 28 ).
Assessment of blood biochemical parameters
Blood biochemical parameters (alanine aminotransferase, aspartate aminotransferase, blood urea N and creatinine) were assessed using an automatic analyser (7020 Clinical Analyzer, Hitachi High-Technologies Company).
Determination of diamine oxidase activity in the plasma
Diamine oxidase (DAO) activity in the plasma was determined using spectrophotometry as described by Hosoda et al. ( Reference Hosoda, Nishi and Nakagawa 29 ). DAO was used as a marker of intestinal injury( Reference Hou, Wang and Zhang 21 , Reference Luk, Bayless and Baylin 30 ).
Determination of growth-related hormone and PGE2 concentrations in the plasma
Growth hormone concentrations in the plasma were determined using a commercially available porcine growth hormone 125I RIA kit (Linco Research, Inc.). The minimum detection limit was 1 ng/ml, with an intra-assay CV of 4·0 %.
Insulin-like growth factor-I concentrations in the plasma were determined using a commercially available porcine insulin-like growth factor-I IRMA kit (Diagnostic Systems Laboratories, Inc.). The minimum detection limit was 2 ng/ml, with an intra-assay CV of 3·9 %.
Epidermal growth factor (EGF) concentrations in the plasma were determined using a commercially available 125I RIA kit (Beijing Sino-UK Institute of Biological Technology). Human EGF antibody was used as the standard, and the intra-assay and inter-assay CV were < 5 and < 10 %, respectively. The detection limit was 0·1 μg/l.
Insulin concentrations in the plasma were determined using a commercially available 125I RIA kit (Beijing Sino-UK Institute of Biological Technology). The detection limit for the insulin assay was 13 pmol/ml, with the intra-assay and inter-assay CV being < 10 and < 15 %, respectively.
PGE2 concentrations in the plasma were determined using a commercially available 125I RIA kit (Beijing Sino-UK Institute of Biological Technology), which we had validated previously for pigs( Reference Hou, Wang and Yi 31 ). The detection limit for the PGE2 assay was 0·12 pg/ml. The intra-assay and inter-assay CV were < 7·5 and < 10·5 %, respectively.
Determination of the activities of antioxidative enzymes and concentrations of their products in the plasma and colonic mucosa
Plasma and colonic mucosa samples were used for the analysis of antioxidative enzymes and their products. Specifically, malondialdehyde (MDA), superoxide dismutase, catalase (CAT) and inducible NO synthase (iNOS) were analysed using commercially available kits (Nanjing Jiancheng Bioengineering Institute). Assays were carried out in triplicate( Reference Hou, Wang and Yi 31 ).
Intestinal morphometric analyses
Tissue samples used for the morphometric study were dehydrated and embedded in paraffin, sectioned at a thickness of 4 μm, and stained with haematoxylin and eosin( Reference Hou, Wang and Ding 22 , Reference Luna 32 ). Morphological measurements were carried out with a light microscope (American Optical Company). Ileal villus height and width, as well as crypt depth, were measured using a linear ocular micrometer equipped with a computer-assisted morphometric system (BioScan Optimetric, BioScan, Inc.). The villus height:crypt depth ratio and villous surface area were calculated. Colonic intraepithelial lymphocyte (IEL) number, lamina propria cell density and goblet cell number in crypts were determined( Reference Nofrarías, Manzanilla and Pujols 26 ). The number of IEL and goblet cells is expressed per 100 enterocytes. On the basis of cell morphology, differences among the nuclei of enterocytes, goblet cells and lymphocytes were clearly distinguishable at 400 × magnification. Cell density was determined by counting total visibly stained nuclei and total lymphocytes in ten fields (total area of 4000 μm2) from each section using a grid ocular. Cell density is expressed as the number of total stained cells and the number of lymphocytes per 1000 μm2. The number of lymphocytes in relation to the number of total cells was also calculated. All morphometric analyses were carried out by the same person, who was blinded to the treatments, as described by Nofrarías et al. ( Reference Nofrarías, Manzanilla and Pujols 26 ).
Determination of amphiregulin, epidermal growth factor and epidermal growth factor receptor mRNA levels using real-time PCR
Amphiregulin (AR), EGF and EGFR mRNA levels in colonic mucosae were quantified using real-time PCR. Approximately 100 mg of a frozen mucosal sample were powdered under liquid N2 using a mortar and pestle. The powdered samples were homogenised, and total RNA was isolated using the TRIzol Reagent protocol (Invitrogen). Total RNA was quantified using the NanoDrop® ND-2000 UV–VIS spectrophotometer (Thermo Scientific) at an optical density (OD) of 260 nm, and the purity was assessed by determining the OD260:OD280 ratio. All samples had an OD260:OD280 ratio above 1·8, corresponding to >95 % pure nucleic acids. The integrity of RNA in each sample was assessed using 1 % denatured agarose gel electrophoresis. RNA was used for real-time PCR analysis when it had a 28 S:18 S ribosomal RNA ratio ≥ 1·8.
Total RNA was reverse-transcribed using the PrimeScript® RT Reagent Kit with gDNA Eraser (Takara) according to the manufacturer's instructions. Complementary DNA was synthesised and stored at − 20 °C until use. Real-time PCR analysis for the determination of gene expression was carried out using primers for AR, EGF, EGFR and ribosomal protein L4 (RPL4) (Table 2) and the SYBR® Premix Ex Taq™ (Takara) on the Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Inc.). The total volume of the PCR system was 50 μl. In brief, the reaction mixture contained 0·2 μm of each primer, 25 μl of SYBR® Premix Ex Taq™ (2 × ) and 4 μl of complementary DNA in a 50 μl reaction volume. All PCR were carried out in triplicate on a ninety-six-well real-time PCR plate (Applied Biosystems) under the following conditions (two-step amplification): 95 °C for 30 s, followed by forty cycles of 95 °C for 3 s and 60 °C for 30 s. A subsequent melting curve analysis (95 °C for 15 s, 60 °C for 1 min and 95 °C for 15 s) with continuous fluorescence measurement and final cooling to room temperature was conducted. Amplification products were verified by melting curves and agarose gel electrophoresis. Results were analysed using the ΔΔC T method( Reference Fu, Stromberg and Viele 33 ). The standard curves were generated using relative concentration v. C T value. The linear correlation coefficients of all genes were >0·995. Based on the slopes of the standard curves, the amplification efficiencies of the standard ranged from 90 to 110 % (derived from the formula efficiency = 101/ − slope− 1)( Reference Nygard, Jørgensen and Cirera 34 ). Moreover, we tested other housekeeping genes (GAPDH and β-actin) by analysing gene stability as described by Vandesompele et al. ( Reference Vandesompele, De Preter and Pattyn 35 ) and found the expression of RPL4 to be more stable than that of other housekeeping genes in the colonic mucosa; therefore, we used RPL4 as the normaliser in the calculation of relative mRNA levels for target genes. Each biological sample was run in triplicate.
Table 2 Primers used for the real-time PCR analysis*

AR, amphiregulin; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; RPL4, ribosomal protein L4.
* The oligonucleotide primers were designed from pig gene sequences in the GenBank NM_214376 (for AR), NM_214020 (for EGF), NM-2140075 (for EGFR) and DQ845176 (for RPL4). To avoid amplification of potentially contaminating genomic DNA, the primers were designed to span introns and intron–exon boundaries.
Protein immunoblot analysis
Caspase-3 and claudin-1 proteins were analysed by Western blot analysis as described by Hou et al. ( Reference Hou, Wang and Zhang 21 ). Briefly, frozen intestinal mucosal samples were powdered under liquid N2 using a mortar and pestle. The powdered samples (100 mg) were homogenised in 1 ml of lysis buffer using a Polytron homogeniser. The homogenates were centrifuged at 12 000 g for 15 min at 4°C. The supernatant fluid was aliquoted into microcentrifuge tubes, to which 2 × SDS sample buffer (2 ml of 0·5 mol/l Tris, pH 6·8, 2 ml glycerol, 2 ml of 10 % SDS, 0·2 ml of β-mercaptoethanol, 0·4 ml of a 4 % solution of bromophenol blue and 1·4 ml of water) was added at a ratio of 1:1. The samples were boiled for 5 min and cooled on ice before being used for Western blot analysis. Proteins (150 μg/sample for caspase-3; 60 μg/sample for claudin-1 and β-actin) were separated by electrophoresis on a 10 % (for caspase-3 and β-actin) or 12 % (for claudin-1) polyacrylamide gel. Proteins were electrophoretically transferred onto polyvinylidene difluoride membranes. Non-fat dry milk in TBS T buffer was used to block the membranes for at least 1 h at room temperature. The membranes were incubated with primary antibodies overnight at 4°C: caspase-3 (rabbit polyclonal antibodies from Cell Signaling Technology, Inc.; dilution 1:1000); claudin-1 (rabbit monoclonal antibodies from Invitrogen Technology, Inc.; dilution 1:1000); β-actin (mouse monoclonal antibodies from Sigma Chemicals; dilution 1:5000). The primary antibody dilution buffer was 1 × TBS–0·1 % Tween 20 with 5 % bovine serum albumin. The membranes were washed three times with TBS T (1 × Tris-buffered saline including 0·1 % Tween 20) and incubated for 1 h at room temperature with anti-rabbit (mouse) IgG horseradish peroxidase-conjugated secondary antibodies (Beijing ZhongShan Golden Bridge Biological Technology Company, Limited; dissolved in 5 % non-fat dry milk in TBS–Tween 20 buffer at 1:5000 dilution). The incubation of membranes with primary antibodies was followed by three washes with TBS T buffer for 10 min, and incubation with secondary antibodies was followed by five washes for 8 min. Blots were developed using an Enhanced Chemiluminescence Western blotting kit (ECL-plus, Amersham Biosciences), visualised and quantified using an imaging system (Alpha Innotech FluorChem FC2).
Statistical analyses
Results are expressed as means and standard deviations of the means with their standard errors and statistically analysed by one-way ANOVA. The normality and constant variance of the experimental data were tested using Levene's test( Reference Wei, Carroll and Harden 36 ). If data did not have homogeneous variance, they were log-transformed to meet the necessary assumptions for ANOVA( Reference Wei, Carroll and Harden 36 ). Differences among the treatment means were determined using Duncan's multiple range tests. All statistical analyses were carried out using the SPSS 13.0 software (SPSS, Inc.). P values < 0·05 were considered to indicate statistical significance, and P values < 0·1 were considered as trends towards statistical significance.
Results
Effects of dietary tributyrin supplementation on the growth performance of piglets
Between days 0 and 14 of the trial (before the ACA challenge), average daily feed intakes of piglets were 554, 540 and 551 g/d (P= 0·804), average daily weight gains (ADG) were 314, 322 and 373 g/d (P= 0·090), and feed:gain ratios were 1·7, 1·7 and 1·5 (P= 0·096), respectively, in the control, ACA and TBU groups. Dietary TBU supplementation tended to (P< 0·1) increase ADG and decrease feed:gain ratios. Between days 15 and 21 of the trial (the ACA challenge period), average daily feed intakes of piglets were 574, 577 and 576 (P= 0·205), ADG were 306, 261 and 284 g/d (P= 0·439), and feed:gain ratios were 1·9, 2·2 and 2·1 (P= 0·425), respectively, in the control, ACA and TBU groups.
Effects of dietary tributyrin supplementation on total and differential leucocyte counts, erythrocyte counts and Hb concentrations in piglets after the acetic acid challenge
The ACA group had higher (P< 0·05) leucocyte, lymphocyte and neutrophil counts than the control group (Table 3). In comparison with piglets in the ACA group, those in the TBU group had lower lymphocyte counts (P< 0·05), without any change in leucocyte and neutrophil counts. Erythrocyte counts, Hb concentrations and lymphocyte percentage in the blood did not differ among the treatment groups.
Table 3 Total and differential leucocyte counts, erythrocyte counts and Hb concentrations in the blood of piglets (Mean values with their standard errors, n 6)
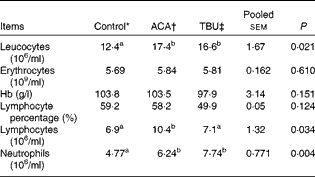
ACA, acetic acid; TBU, tributyrin.
a,bMean values within a row with unlike superscript letters were significantly different (P <0·05).
* Non-challenged control: piglets fed the basal diet and intrarectally administered with saline.
† ACA-challenged control: piglets fed the basal diet and intrarectally administered with ACA.
‡ ACA challenge+0·1 % TBU supplementation: piglets fed the basal diet supplemented with 0·1 % TBU and intrarectally administered with ACA.
Effects of dietary tributyrin supplementation on the blood biochemical parameters of piglets after the acetic acid challenge
The average mean values for alanine aminotransferase in the plasma were 52·2, 51·2 and 54·3 U/l (P= 0·414), for aspartate aminotransferase 35·2, 40·3 and 35·6 U/l (P= 0·422), for blood urea N 2·4, 2·6 and 2·9 mmol/l (P= 0·265), and for creatinine 73·9, 82·8 and 78·8 μmol/l (P= 0·005), respectively, in the control, ACA and TBU groups. (1 u/l is defined as the amount of alanine aminotransferase or aspartate aminotransferase required for catalysing 1 μmol of α-Ketoglutarate/min per litre plasma at 25°C). Compared with the control group, the ACA group exhibited an increase (P <0·05) in creatinine concentrations in the plasma. Relative to the ACA group, the TBU group exhibited a decrease (P <0·1) in creatinine concentrations in the plasma.
Effects of dietary tributyrin supplementation on growth hormone, insulin-like growth factor-I, epidermal growth factor, insulin and PGE2 concentrations in the plasma of piglets
Compared with the control group, the ACA group exhibited an increase (P <0·05) in PGE2 concentrations in the plasma. In comparison with the ACA group, the TBU group exhibited an increase (P <0·05) in insulin concentrations, but a decrease (P <0·1) in PGE2 concentrations in the plasma (Table 4).
Table 4 Growth hormone (GH), insulin-like growth factor (IGF)-I, epidermal growth factor (EGF), insulin and PGE2 concentrations in the plasma of piglets (Mean values with their standard errors, n 6)

ACA, acetic acid; TBU, tributyrin.
a,bMean values within a row with unlike superscript were letters significantly different (P <0·05).
* Non-challenged control: piglets fed the basal diet and intrarectally administered with saline.
† ACA-challenged control: piglets fed the basal diet and intrarectally administered with ACA.
‡ ACA challenge+0·1 % TBU supplementation: piglets fed the basal diet supplemented with 0·1 % TBU and intrarectally administered with ACA.
Effects of dietary tributyrin supplementation on redox status in the plasma and colonic mucosa of piglets after the acetic acid challenge
Data on the concentrations of antioxidative enzymes and related products in the plasma and colonic mucosae are summarised in Table 5. Compared with the control group, the ACA group exhibited an increase (P <0·05) in MDA concentrations and iNOS activity in the plasma. In comparison with the ACA group, the TBU group exhibited a decrease (P <0·05) in MDA concentrations and iNOS activity in the plasma.
Table 5 Diamine oxidase (DAO) activity in the plasma as well as the redox status in the plasma and colonic mucosa of piglets (Mean values with their standard errors, n 6)
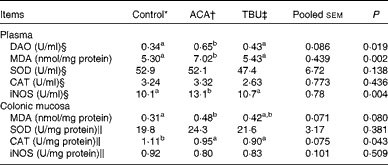
ACA, acetic acid; TBU, tributyrin; MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase; iNOS, inducible NO synthase.
a,bMean values within a row with unlike superscript letters were significantly different (P <0·05).
* Non-challenged control: piglets fed the basal diet and intrarectally administered with saline.
† ACA-challenged control: piglets fed the basal diet and intrarectally administered with ACA.
‡ ACA challenge+0·1 % TBU supplementation: piglets fed the basal diet supplemented with 0·1 % TBU and intrarectally administered with ACA.
§ 1 U/ml is defined as the amount of DAO, SOD, CAT or iNOS required for catalysing 1 mmol of cadaverine dihydrochloride, superoxide (O2 −), H2O2 or l-arginine/min per ml plasma at 25 °C, respectively.
∥ 1 U/mg (colon SOD, CAT and iNOS) is defined as the amount of SOD, CAT or iNOS required for catalysing 1 mmol of O2 −, H2O2 or l-arginine/min per mg colon protein at 25 °C, respectively.
Compared with the control group, the ACA group exhibited an increase (P <0·05) in MDA concentrations and a decrease (P <0·05) in CAT activity in the colonic mucosa. However, MDA concentrations and CAT activities in the colonic mucosa did not differ between the ACA and TBU groups.
Effects of dietary tributyrin supplementation on plasma diamine oxidase activity in piglets after the acetic acid challenge
Data on DAO activity in the plasma are summarised in Table 5. Compared with the control group, the ACA group exhibited an increase (P <0·05) in DAO activity in the plasma. In comparison with the ACA group, the TBU group exhibited a decrease (P <0·05) in DAO activity in the plasma.
Effects of dietary tributyrin supplementation on the intestinal mucosal histomorphology of piglets after the acetic acid challenge
Morphometric measurements are summarised in Table 6. Compared with the control group, the ACA group exhibited a decrease (P <0·05) in the ratio of villus height:crypt depth in the ileum. In comparison with the ACA group, the TBU group exhibited an increase (P <0·1) in the ratio of villus height:crypt depth in the ileum and in the number of goblet cells per 100 enterocytes in the colon, but a decrease (P <0·05) in the number of IEL per 100 enterocytes and lymphocyte density in the colon. Morphometric measurements in the duodenum and jejunum did not differ among the control, ACA and TBU groups (data not shown). Compared with the control group, the ACA group exhibited a decrease (P <0·05) in the number of goblet cells per 100 enterocytes and an increase (P <0·05) in the number of IEL per 100 enterocytes and lymphocyte density in the colon.
Table 6 Intestinal mucosal histomorphology of piglets after the acetic acid (ACA) challenge (Mean values with their standard errors, n 6)
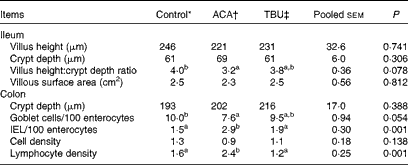
TBU, tributyrin; IEL, intraepithelial lymphocytes.
a,bMean values within a row with unlike superscript letters were significantly different (P <0·05).
* Non-challenged control: piglets fed the basal diet and intrarectally administered with saline.
† ACA-challenged control: piglets fed the basal diet and intrarectally administered with ACA.
‡ ACA challenge+0·1 % TBU supplementation: piglets fed the basal diet supplemented with 0·1 % TBU and intrarectally administered with ACA.
The colonic mucosa of the ACA group exhibited ulcerations and obvious infiltration by inflammatory cells (Fig. 1). The histological ulcers revealed necrosis in the colonic mucosa with submucosal inflammation, with neutrophils and lymphocytes as the predominant infiltrating cells. Collectively, some adverse effects of ACA were attenuated by dietary supplementation with 0·1 % TBU

Fig. 1 Morphological changes in the colon of piglets after intrarectal administration of acetic acid (ACA). (a, d) Control group (non-challenged control): piglets fed the basal diet and intrarectally administered with saline; (b, e) ACA group (ACA-challenged control): piglets fed the basal diet and intrarectally administered with ACA, with colon injury characterised by the distortion of normal crypt architecture, loss of goblet cells, desquamation of epithelium and infiltration of lymphocytes; and (c, f) tributyrin (TBU) group (ACA challenge+0·1 % TBU supplementation): piglets fed the basal diet supplemented with 0·1 % TBU and intrarectally administered with ACA, with histological changes being improved by TBU treatment. A, goblet cells; B, the denuded epithelium; and C, lymphocytes.
Effects of dietary tributyrin supplementation on amphiregulin, epidermal growth factor and epidermal growth factor receptor mRNA levels in the colonic mucosa of piglets after the acetic acid challenge
Data on AR, EGF and EGFR mRNA levels in the colonic mucosa are summarised in Table 7. Mucosal EGFR mRNA levels were lower (P <0·05) in the colonic mucosa ( − 26 %) of ACA piglets than in that of the control piglets. Compared with the ACA group, the TBU group exhibited an increase (P <0·05) in the abundance of EGFR mRNA in the colonic mucosa by 59 %. No differences in AR or EGF mRNA levels were detected in the colonic mucosa among the treatment groups.
Table 7 Effects of tributyrin (TBU) on amphiregulin (AR), epidermal growth factor (EGF) and epidermal growth factor receptor (EGFR) mRNA levels in the colonic mucosa of piglets after the acetic acid challenge (Mean values with their standard errors, n 6)

ACA, acetic acid.
a,bMean values within a row with unlike superscript letters were significantly different (P <0·05).
* Non-challenged control: piglets fed the basal diet and intrarectally administered with saline.
† ACA-challenged control: piglets fed the basal diet and intrarectally administered with ACA.
‡ ACA challenge+0·1 % TBU supplementation: piglets fed the basal diet supplemented with 0·1 % TBU and intrarectally administered with ACA.
Effects of dietary tributyrin supplementation on the abundance of caspase-3 and claudin-1 proteins in the colonic mucosa of piglets after the acetic acid challenge
The ACA group exhibited an increase (P <0·05) in the abundance of caspase-3 protein and a decrease (P <0·05) in that of claudin-1 protein in the colonic mucosa compared with the control group. Relative to the ACA group, the TBU group exhibited a decrease (P <0·05) in caspase-3 expression and an increase (P <0·05) in claudin-1 expression in the colonic mucosa (Figs. 2 and 3). The levels of caspase-3 and claudin-1 in TBU-supplemented piglets were comparable to those of the control group.

Fig. 2 Relative levels of caspase-3 protein in the colonic mucosa of piglets. Mucosal extracts (150 μg protein/sample) were separated by 10 % SDS–PAGE for the determination of caspase-3 and β-actin levels. Values for caspase-3 protein were normalised to those for β-actin. Values are means, with their standard errors represented by vertical bars (n 6). Control group (non-challenged control): piglets fed the basal diet and intrarectally administered with saline; acetic acid (ACA) group (ACA-challenged control): piglets fed the basal diet and intrarectally administered with ACA; and tributyrin (TBU) group (ACA challenge+0·1 % TBU supplementation): piglets fed the basal diet supplemented with 0·1 % TBU and intrarectally administered with ACA. a,bMean values with unlike letters were significantly different (P <0·05).

Fig. 3 Relative levels of claudin-1 protein in the colonic mucosa of piglets. Mucosal extracts (60 μg protein/sample) were separated by 12 % SDS–PAGE for the determination of claudin-1 and β-actin levels. Values for relative claudin-1 were normalised to those for β-actin. Values are means, with their standard errors represented by vertical bars (n 6). Control group (non-challenged control): piglets fed the basal diet and intrarectally administered with saline; acetic acid (ACA) group (ACA-challenged control): piglets fed the basal diet and intrarectally administered with ACA; tributyrin (TBU) group (ACA challenge+0·1 % TBU supplementation): piglets fed the basal diet supplemented with 0·1 % TBU and intrarectally administered with ACA. a,bMean values with unlike letters were significantly different (P <0·05).
Discussion
ACA-induced colitis in rats shares many characteristics with the human disease( Reference Cetinkaya, Bulbuloglu and Kurutas 11 , Reference Sharon and Stenson 12 ), and similar studies involving other models and other species have been reported( Reference Cetinkaya, Bulbuloglu and Kurutas 11 , Reference Nieto, Torres and Fernández 37 ). To our knowledge, the present study is the first to determine the effects of dietary TBU supplementation on the attenuation of ACA-induced colitis in piglets. In the present study, we used a porcine model of colitis induced by administering ACA intrarectally to determine whether dietary TBU supplementation can alleviate intestinal injury and to elucidate the underlying mechanisms.
In the porcine model, the ACA challenge increased the number of leucocytes (including that of lymphocytes and neutrophils) and dietary TBU supplementation decreased the number of lymphocytes. These results indicate that ACA induced inflammation in the colon of piglets and TBU had an anti-inflammatory effect. Creatinine is a useful indicator of kidney function. The results of the present study indicate that the ACA challenge resulted in an increase in creatinine concentrations in the plasma, and this effect of ACA tended to be alleviated by dietary TBU supplementation. Similarly, Kim et al. ( Reference Kim, Mullan and Hampson 38 ) reported that pigs had higher plasma creatinine concentrations after digestive disease. Elevated creatinine concentrations in the plasma signify renal dysfunction. When highly concentrated ACA is ingested, it can cause severe intravascular haemolysis, haemoglobinuria, acute hepatitis, coagulopathy, and acute gastrointestinal bleeding and acute renal failure( Reference Chibishev, Sikole and Pereska 39 , Reference Tong, Mak and Wong 40 ). It is of interest that we found dietary TBU supplementation to prevent the ACA-induced increase in creatinine concentrations in the plasma, suggesting that TBU may have a beneficial effect on the alleviation of renal dysfunction induced by ACA. The protection conferred by TBU was accompanied by a significant decrease in PGE2 generation and iNOS activity. Thus, intrarectal ACA administration readily resulted in systemic inflammation in piglets. This finding is consistent with an earlier report that ACA-induced colitis is associated with elevated concentrations of leukotriene B4 in the blood( Reference Gulec, Yasar and Yildiz 13 ). A decrease in iNOS activity in the TBU-treated piglets suggests that reduction in NO generation is one of the mechanisms responsible for the anti-inflammatory effect of TBU.
Ulcerative colitis is a chronic recurrent inflammatory bowel disease in which oxidative stress has been implicated( Reference Cetinkaya, Bulbuloglu and Kurutas 11 ). In the colonic tissues of animals with ACA-induced colitis, myeloperoxidase activity and MDA concentrations are elevated, while reduced-glutathione concentrations as well as superoxide dismutase and CAT activities are decreased( Reference Cetinkaya, Bulbuloglu and Kurutas 11 ). In the present study, dietary TBU supplementation suppressed the elevated MDA concentrations in the plasma of the ACA-challenged piglets. In addition, in the colonic mucosa of ACA piglets, MDA concentrations were elevated, while CAT activity was decreased, as reported previously for the rodent model( Reference Cetinkaya, Bulbuloglu and Kurutas 11 ). More importantly, dietary TBU supplementation suppressed the elevated MDA concentrations in the colonic mucosa of the ACA-challenged piglets. Thus, it appears that TBU exerts its antioxidant effects in piglets at both the systemic and intestinal levels. Oxidative stress and resultant tissue damage are the hallmarks of cell death. Of particular interest is the finding that ACA induced cell death through the activation of caspase-3 and TBU attenuated the production of active caspase-3 in the colon of the ACA-challenged piglets (Fig. 2). Caspase-3 is one of the key components of the apoptotic pathway in the small intestine( Reference Hou, Wang and Zhang 21 , Reference Tan, Yin and Kong 41 ). This protein is either partially or totally responsible for the proteolytic cleavage of many key ‘death’ proteins. These findings indicate a protective effect of TBU against ACA-induced enterocyte death and further support the conclusion that dietary TBU supplementation is effective at preventing intestinal injury and inflammatory disease.
Previous studies have demonstrated that plasma DAO is an indicator of the severity of mucosal injury( Reference Hou, Wang and Zhang 21 , Reference Hou, Wang and Ding 42 , Reference Luk, Bayless and Baylin 43 ). In mammals, DAO is abundantly expressed in the duodenal and jejunal mucosa and, therefore, DAO activity is a non-invasive marker of alterations in intestinal mucosal function and structure( Reference Hou, Wang and Zhang 21 , Reference Li, Yu and Hao 44 ). Under certain circumstances, intestinal mucosal cells undergo necrosis and slough off into the intestinal lumen, leading to a decrease in intestinal mucosal DAO levels and an increase in circulating DAO levels( Reference Hou, Wang and Zhang 21 , Reference Li, Lu and Hu 45 ). In the present study, TBU decreased DAO activity in the plasma in response to ACA administration (Table 5), indicating that TBU supplementation may alleviate the colonic injury induced by intrarectal ACA administration. Furthermore, TBU supplementation resulted in the following: (1) an increased ratio of villus height:crypt depth in the ileum; (2) an increased number of goblet cells; (3) a decreased number of IEL; (4) decreased lymphocyte density in the colon. All these results indicate that TBU can affect the intestinal immune system and epithelial morphology. Our findings also support the notion that TBU beneficially alleviates the ACA-induced damage in the intestinal structure (Fig. 1). The integrity of the intestinal epithelium ensures its normal physiological function( Reference Hou, Wang and Ding 22 , Reference Liu, Yin and Du 46 ). A damage to the mucosal epithelium can directly impair its integrity and the absorption of nutrients, thereby reducing the growth performance and compromising the health of pigs( Reference Hou, Wang and Zhang 21 , Reference Liu, Yin and Du 46 , Reference Boudry, Péron and Le Huërou-Luron 47 ). Accordingly, we observed that ACA reduced the abundance of claudin-1 in the colonic mucosa of piglets, whereas dietary TBU supplementation increased the levels of claudin-1 in the colon of the ACA-challenged piglets (Fig. 3). It is possible that TBU affects intestinal functions by promoting tight-junction formation, as previous studies have demonstrated that the claudin family of proteins plays a critical role in epithelial permeability in a variety of tissues, including the gut( Reference Hou, Wang and Zhang 21 , Reference Howe, Reardon and Wang 48 , Reference Tsukita and Furuse 49 ). Claudin-1 integrates diverse processes such as cell proliferation and mucosal integrity to affect intestinal function( Reference Hou, Wang and Zhang 21 , Reference Rhoads and Wu 50 , Reference Schneeberger and Lynch 51 ).
Volatile fatty acids play a role in the modulation of the digestive process and can be supplied by direct feed supplementation. Butyric acid is produced by the bacterial fermentation of carbohydrates and amino acids in the lumen of the large intestine and serves as a primary source of energy for colonocytes as well as a strong mitosis promoter and a differentiation agent in the gastrointestinal tract in vivo ( Reference Piva, Prandini and Fiorentini 52 , Reference Salminen, Bouley and Boutron-Ruault 53 ). It has been shown previously that TBU can improve the trophic status of the intestinal mucosa in the gut of piglets( Reference Hou, Liu and Hu 2 , Reference Piva, Prandini and Fiorentini 52 ). Taken together, these data indicate that TBU could enhance the intestinal barrier function and the ability of the intestine to absorb nutrients under inflammatory conditions. In the present study, however, dietary TBU supplementation partially alleviated the ACA-induced adverse changes in the measured intestinal variables. The dose effect of TBU was not investigated in the present study. Whether an insufficient dose of TBU is responsible for the partial alleviation effect remains to be determined.
Another significant finding from the present study is that dietary TBU supplementation increased the expression of the EGFR gene in the colonic mucosa (Table 7), which is consistent with our observations that TBU treatment resulted in reduced cell death (indicated by the decreased expression of caspase-3 protein) in the colonic mucosa of the ACA-challenged piglets. Similarly, results of previous studies have shown that EGF and its receptor (EGFR), which play important roles in the repair of the small-intestinal mucosa following damage( Reference Liu, Yin and Du 46 , Reference Helmrath, Shin and Erwin 54 , Reference Nair, Warner and Warner 55 ), are crucial for promoting enterocyte proliferation and migration as well as regeneration of the mucosal epithelium( Reference Hou, Wang and Yi 31 , Reference Engler, Gupta and Li 56 , Reference Ryan, Miller and Seydel 57 ). The beneficial outcomes of EGFR signalling include the stabilisation of the internal environment in the gut( Reference Helmrath, Shin and Erwin 54 , Reference Nair, Warner and Warner 55 ).
AR is a member of the EGF protein family( Reference Ciarloni, Mallepell and Brisken 58 ). It is an autocrine growth factor as well as a mitogen for many cell types, such as astrocytes, Schwann cells and fibroblasts. Interestingly, AR is related to EGF and transforming growth factor-α( Reference Howe, Reardon and Wang 48 ). Through interaction with the EGF/transforming growth factor-α receptor, AR promotes the growth of normal epithelial cells and inhibits the proliferation of certain aggressive carcinoma cell lines( Reference Ryan, Miller and Seydel 57 , Reference Ciarloni, Mallepell and Brisken 58 ). In the present study, we observed that TBU did not affect AR or EGF mRNA levels, but increased EGFR expression in the colonic mucosa of the ACA-challenged piglets. Therefore, it is possible that TBU may alleviate colonic injury partly via EGFR signalling.
In conclusion, dietary supplementation with 0·1 % TBU is beneficial for alleviating ulcerative colitis in piglets. Possible mechanisms for the actions of TBU may include the following: (1) reduction of oxidative stress (indicated by decreased production of MDA in the plasma); (2) alleviation of intestinal injury (indicated by alleviation of ACA-induced damage in the intestinal structure and decreased plasma DAO activity, decreased expression of caspase-3 protein and increased expression of claudin-1 protein in the colonic mucosa of the ACA-challenged piglets); (3) improvement of mucosal repair via EGF signalling (indicated by increases in EGFR expression in the colon). These findings may have important implications for treating colitis in humans and other animals.
Acknowledgements
The present study was jointly supported by the National Basic Research Program of China (no. 2012CB126305), the National Natural Science Foundation of China (no. 31372319), the Hubei Provincial Research and Development Program (no. 2010BB023), the Natural Science Foundation of Hubei Province (no. 2013CFA097, 2013CFB325, 2012FFB04805 and 2011CDA131), the National Research Initiative Competitive Grants from the Animal Growth and Nutrient Utilization Program (2008-35206-18764) of the USDA National Institute of Food and Agriculture and Texas AgriLife Research (H-82000). All these funding agencies had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: Y. H. designed the study and wrote the manuscript; L. W., D. Y., B. D., X. C., Q. W., H. Z. and Y. L. collected and analysed experimental results; Y. Y., J. G. and G. W. carried out the experiments and revised the paper. All authors contributed to data interpretation and approved the final version of the manuscript.
The authors declare that they have no conflicts of interest.












