Phytosterols are the most abundant plant sterols, and their structure is highly related to cholesterol(Reference Weihrauch and Gardner1). Phytostanols are saturated forms of phytosterols which are poorly absorbed in the intestine(Reference Salen, Ahrens and Grundy2, Reference Ostlund, McGill and Zeng3). A cholesterol-lowering effect for both of them has been demonstrated in human subjects and animals(Reference Moghadasian and Frohlich4–Reference Calpe-Berdiel, Escola-Gil and Blanco-Vaca8), and the most recent guidelines of the National Cholesterol Education Program recommend dietary consumption of phytosterols or phytostanols as a therapeutic option to decrease LDL-cholesterol(9).
Disodium ascorbyl phytostanol phosphate (FM-VP4), derived from sitostanol and campestanol (natural stanols), is a synthetic compound produced to obtain a cholesterol-lowering molecule with superior solubility characteristics compared with other phytosterols and stanols(Reference Burnett and Huff10). It primarily comprises two molecular entities, campestanol and sitostanol (34·62:62·41, % w/w), each covalently linked to ascorbic acid by a phosphodiester bond. Accordingly, FM-VP4 administration reduced intestinal cholesterol absorption in rats(Reference Wasan, Peteherych and Najafi11, Reference Wasan, Zamfir and Pritchard12) and has been proven to be more efficient than other plant stanols or sterols in decreasing plasma total cholesterol in hamsters and apoE-deficient mice, respectively(Reference Ebine, Jia and Demonty13, Reference Lukic, Wasan and Zamfir14). In this context, the mechanism of action of FM-VP4 seems to be dependent on its whole chemical structure, since ascorbic acid alone did not show any effect on intestinal cholesterol absorption and FM-VP4 was more active than the concomitant combination of its parent phytostanol compound and ascorbate(Reference Lukic, Wasan and Zamfir14). Other studies showed that FM-VP4 is efficient regarding its cholesterol-lowering effect in experimental animals and in clinical studies(Reference Wasan, Peteherych and Najafi11, Reference Ebine, Jia and Demonty13–Reference Vissers, Trip and Pritchard17). Further, other effects have been ascribed to FM-VP4, including a putative anti-obesity and anti-diabetic action(Reference Wasan, Zamfir and Pritchard12, Reference Looije, Risovic and Stewart18, Reference Thornton, Warburton and Wasan19).
The mechanism of action of plant sterols and stanols remains largely unknown. One mechanism that could explain their effects would be competition with cholesterol for incorporation into mixed micelles(Reference Ikeda, Tanabe and Sugano20). In this context, it is noteworthy that FM-VP4 presents increased solubility in micelles than other stanols. However, plant sterols do not need to be present simultaneously with cholesterol to inhibit its intestinal absorption(Reference Calpe-Berdiel, Escola-Gil and Blanco-Vaca8). Therefore, an increased activity of ATP-binding cassette transporter (ABC)-A1 and ABCG5/G8 heterodimer or a decreased activity of Niemann-Pick C1-like 1 (NPC1L1) protein was proposed as a mechanism underlying the hypocholesterolaemic effect of phytosterols and stanols(Reference Calpe-Berdiel, Escola-Gil and Blanco-Vaca8). However, several reports have demonstrated that the phytosterol-mediated inhibition of intestinal cholesterol absorption does not depend on these ABC transporters nor on changes in NPC1L1 expression in genetically engineered mice(Reference Calpe-Berdiel, Escola-Gil and Blanco-Vaca8). It is also currently unknown what mechanisms explain the apparently higher hypocholesterolaemic action of FM-VP4 compared with other phytostanols.
The impact of dietary phytosterol or phytostanol supplementation on liver cholesterol homeostasis remains unclear. The reduction in intestinal cholesterol absorption caused by dietary phytosterol or phytostanol treatment reduced liver cholesterol levels in human subjects and hypercholesterolaemic mice(Reference Calpe-Berdiel, Escola-Gil and Ribas7, Reference Plat and Mensink21, Reference Volger, van der Boom and de Wit22) and this usually led to a compensatory increase in whole-body endogenous cholesterol synthesis(Reference Plat and Mensink21–Reference Ntanios and Jones23). However, the main cholesterogenic liver enzyme hydroxymethylglutaryl coenzyme A reductase (HMGCoA-R) mRNA expression has not been found consistently increased in response to plant sterol- or stanol-enriched diets(Reference Calpe-Berdiel, Escola-Gil and Ribas7, Reference Plat and Mensink21, Reference Volger, van der Boom and de Wit22, Reference Xu, Le and Moghadasian24). In contrast, the large accumulation of plant sterols, seen both in sitosterolaemic patients caused by mutations affecting ABCG5/G8(Reference Shefer, Salen and Nguyen25) and ABCG5/G8-deficient mice(Reference Yu, von Bergmann and Lutjohann26), disrupted cholesterol homeostasis, presumably due to a stigmasterol interference in sterol regulatory element binding protein-2 cleavage(Reference Yang, Yu and Li27). Liver cytochrome P450 family 7 subfamily A polypeptide 1 (CYP7A1) mRNA expression, the rate-limiting enzyme in the classic bile acid biosynthetic pathway, was also inhibited in sitosterolaemic patients(Reference Shefer, Salen and Nguyen25, Reference Nguyen, Shefer and Salen28). However, phytosterol or phytostanol consumption did not seem to affect bile acid excretion in men(Reference Gylling, Puska and Vartiainen29–Reference Weststrate, Ayesh and Bauer-Plank31) or mice(Reference Calpe-Berdiel, Escola-Gil and Ribas7, Reference Volger, van der Boom and de Wit22, Reference Plosch, Kruit and Bloks32).
The main objective of the present study was to test the ability of FM-VP4 to alter the enterohepatic circulation of cholesterol and bile acids and to study the expression profile of genes related to their metabolism.
Materials and methods
Mice and diets
C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and maintained in a temperature-controlled (20°C) room with a 12 h light–dark cycle. Feed and water were provided ad libitum. Female mice, aged 8–10 weeks, were randomised in two groups and fed either a control chow-type diet (TD 00588; Harlan Teklad, Madison, WI, USA) or a 2 % (w/w) FM-VP4-enriched chow-type diet. FM-VP4 (provided by Forbes Medi-tech Inc., La Jolla, CA, USA) primarily comprises two molecular entities, campestanol and sitostanol, each covalently linked to ascorbic acid by a phosphodiester bond (campestanol–sitostanol, 34·6:62·4, % w/w)(Reference Ng, Lukic and Pritchard33). Mice were euthanised by an overdose of inhalant anaesthetic isofluorane. The physical method of euthanasia, cervical dislocation, was performed to ensure that they were in fact euthanised. All animal procedures were in accordance with published recommendations for the use of laboratory animals(34) and approved by the Institutional Animal Care Committee of the Hospital de la Santa Creu i Sant Pau.
Net in vivo intestinal cholesterol absorption
Net cholesterol absorption was measured in treated and untreated mice at the end of the study by a faecal dual-isotope ratio method as previously described(Reference Calpe-Berdiel, Escola-Gil and Ribas7). Briefly, mice were intragastrically administered a mixture of 73 992 Bq (2 μCi) [5,6-3H]sitostanol (American Radiolabeled Chemicals Inc., St Louis, MO, USA) and 36 996 Bq (1 μCi) [4-14C]cholesterol (NEN Life Science Products, Boston, MA, USA). Mice were individually housed in metabolism cages and feed consumption was calculated over the following 2 d. Stools were collected over those 2 d. Lipids were extracted from stools with isopropyl alcohol–hexane (2:3, v/v) and the 14C:3H ratio in each sample was determined. These data were used to calculate the percentage of intestinal cholesterol absorption. Plasma [4-14C]cholesterol and [5,6-3H]sitostanol were also determined at 48 h by scintillation counting.
Total cholesterol analyses of plasma and liver
Mice fed the two different diets were euthanised and exsanguinated by cardiac puncture at the end of the study. Livers were removed after being perfused extensively with saline. A piece of liver was obtained from each mouse and fragmented. Liver lipids were extracted with isopropyl alcohol–hexane (3:2, v/v). After the addition of Na2SO4, the hexane phase was isolated, dried with N2, reconstituted with 0·5 % sodium cholate and sonicated for 10 min (50 Hz) before lipid measurements. Plasma and liver total cholesterol was determined enzymically by the CHOD-PAP method with a commercial kit adapted to a BM/HITACHI 911 autoanalyser (reference 11491458; Roche Diagnostics Boehringer GmbH, Mannheim, Germany). A calibrator for automated systems, specified by the manufacturer, was used for calibration.
Bile acids in liver, small intestine and stools
Stools from individually housed mice were collected over 2 d. Mice were euthanised and small intestines were cut from the duodenum to ileum and washed extensively with sterile saline to eliminate feed and faecal matter. Liver, intestine and stool total bile acids were extracted in 4 ml ethanol (100 %, v/v) and measured by the 3α-hydroxysteroid dehydrogenase method (Sigma Diagnostics, St Louis, MO, USA)(Reference Calpe-Berdiel, Escola-Gil and Ribas7).
Distribution of intragastrically administered [3H]taurocholic acid
In a different experiment, each participant mouse received an intragastric load consisting of 184 980 Bq (5 μCi) [3H(G)]taurocholic acid (PerkinElmer Las Inc., Boston, MA, USA) dissolved in 97 μl saline and 3 μl ethanol. After 48 h, mice were euthanised and bled by cardiac puncture and target tissues (liver, small intestine) were perfused extensively with saline and collected, as were faeces and gallbladder. [3H(G)]taurocholic acid from tissues and faeces was extracted with ethanol as described above(Reference Calpe-Berdiel, Escola-Gil and Ribas7) and counted.
Quantitative real-time RT-PCR analyses
Total liver and small intestine (an equivalent segment of duodenum, jejunum and ileum) RNA was isolated from five animals per group using the Trizol RNA isolation method (Gibco-BRL, Carlsbad, CA, USA). Total RNA samples were repurified (Rneasy mini kit; Qiagen Inc., Valencia, CA, USA) and checked for integrity by agarose gel electrophoresis. Total RNA was reverse-transcribed with Oligo(dT)23 using M-MLV RT, RNase H Minus, Point Mutant (Promega Corp., Madison, WI, USA) to generate cDNA(Reference Calpe-Berdiel, Escola-Gil and Ribas7). PCR assays were performed on an Applied Biosystems Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA) and were conducted in duplicate(Reference Ribas, Palomer and Roglans35). The PCR reaction contained (final volume 20 μl): 10 μl of 2 × SYBR Green PCR Master Mix (Applied Biosystems), 40 ng reverse-transcribed RNA, 1 μl of each Assay on Demand primer and 8 μl sterile water. Primers were obtained from Applied Biosystems databases (references: liver X receptor (LXR)-α: Mm00443450_m1; ABCG5: Mm00446243_m1; ABCG8: Mm00445980_m1; ABCA1: Mm00442649_m1; HMGCoA-R: 1579156A; NPC1L1: Mm01191979_m1; scavenger receptor class BI (SR-BI): Mm00450236_m1; farnesoid X receptor (FXR): Mm00436419_m1; Na+/taurocholate co-transporter polypeptide (NTCP): Mm00441421_m1; CYP7A1: Mm00484152_m1; bile salt export pump (BSEP): Mm00445168_m1; ileal bile acid binding protein (IBABP): Mm00434316_m1; glyceraldehyde 3-phosphate dehydrogenase (GAPDH): Mm99999915_g1). Gene expression was quantified as relative to that of GAPDH. Then, control and treated mouse gene expression was compared.
Statistical analysis
All graphics are shown as box-and-whisker graphs that show the median as the middle line. The box extends from the 25th to the 75th percentile and the whiskers extend from the lowest value to the highest. Comparison of the data obtained from the two groups was performed by the Mann–Whitney U test. Statistical tests were performed using SPSS (version 15.0 for Windows; SPSS, Inc., Chicago, IL, USA). P < 0·05 was considered statistically significant.
Results
Effects of disodium ascorbyl phytostanol phosphate on intestinal cholesterol absorption and plasma and liver cholesterol
Mice appeared healthy during the study and tolerated well the diet with or without FM-VP4. Mouse weight and feed intake were controlled at the end of the 4 weeks of treatment. No significant differences were observed between the control and treated groups (Table 1). FM-VP4-treated mice showed a 2·2-fold reduction in intestinal cholesterol absorption compared with the control group (Table 1). Plasma [14C]cholesterol activity 48 h after intragastric administration was markedly lower in treated mice than in control mice (Table 1). As expected, at that time plasma [3H]sitostanol levels were very low ( < 200 counts per min/ml) with no significant differences between groups (data not shown). Plasma and liver cholesterol was 1·5- and 1·6-fold lower in the treated group than in controls (Table 1).
Table 1 Mouse weight, feed intake, intestinal cholesterol absorption and plasma and liver cholesterol in mice fed a 2 % disodium ascorbyl phytostanol phosphate (FM-VP4)-enriched or control regular chow diet for 4 weeks†
(Medians and 25th and 75th percentiles for eight mice per group)
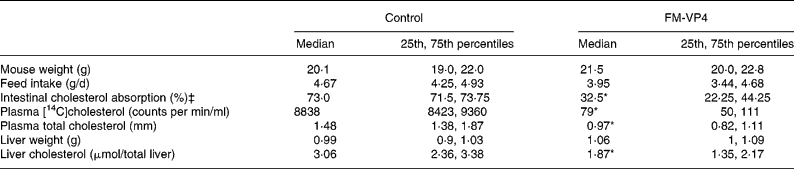
* Median value was significantly different from that of the control mice (P < 0·05).
† Details of the methods used to measure intestinal cholesterol absorption and plasma [14C]cholesterol are explained in Materials and methods.
‡ The number of mice was four per group.
Effects of disodium ascorbyl phytostanol phosphate on bile acid homeostasis
FM-VP4 increased liver and small intestine total bile acid levels by 1·3- and 2·3-fold, respectively (Fig. 1(a) and (b)). On the other hand, bile acid levels in faeces were lower (3·3-fold) in the FM-VP4-fed group (Fig. 1(c)). To determine the in vivo fate of bile acids, we further analysed the distribution of intragastrically administered [3H]taurocholic acid (Fig. 2). [3H]taurocholic acid levels were increased in the intestine of treated mice (3·6-fold, respectively). Further, there was a non-significant tendency to increased [3H]taurocholic acid levels in the plasma, liver and gallbladder of the FM-VP4-treated group. Faecal [3H]taurocholic acid levels were markedly lower than those of the liver and intestine and no differences were found in this parameter between groups (Fig. 2).

Fig. 1 Effects of disodium ascorbyl phytostanol phosphate (FM-VP4) treatment on liver (a), small intestine (b) and faeces (c) bile acid concentrations. The box-and-whisker graphs show the median (of eight mice per group) as the middle line. The box extends from the 25th to the 75th percentile and the whiskers extend from the lowest value to the highest. † Median value was significantly different from that of the control mice (P < 0·05).

Fig. 2 Distribution of intragastrically administered [3H]taurocholic acid in the plasma, liver, small intestine, faeces and gallbladder in control mice (□) and disodium ascorbyl phytostanol phosphate (FM-VP4)-treated mice (![]() ) 48 h after the administration. Each animal received an oral dose of 5 000 000 counts per min (cpm) of [3H]taurocholic acid. The box-and-whisker graphs show the median (of six mice per group) as the middle line. The box extends from the 25th to the 75th percentile and the whiskers extend from the lowest value to the highest. ○, Outside values. † Median value was significantly different from that of the control mice (P < 0·05).
) 48 h after the administration. Each animal received an oral dose of 5 000 000 counts per min (cpm) of [3H]taurocholic acid. The box-and-whisker graphs show the median (of six mice per group) as the middle line. The box extends from the 25th to the 75th percentile and the whiskers extend from the lowest value to the highest. ○, Outside values. † Median value was significantly different from that of the control mice (P < 0·05).
Quantitative real-time RT-PCR analyses
The gene expression of several key enzymes and transporters involved in cholesterol metabolism was studied both in the small intestine (Fig. 3(a)) and liver (Fig. 3(b)). FM-VP4 significantly reduced mRNA levels of LXRα (1·4-fold), ABCG5 (1·9-fold) and ABCG8 (2·3-fold) in the small intestine. No significant changes were observed in intestinal ABCA1 and NPC1L1 expression whereas FM-VP4 increased HMGCoA-R expression (3·7-fold). In contrast, FM-VP4 significantly up-regulated the transcriptional expression of liver LXRα (2·9-fold) and some of its target genes, such as ABCG5 (2·3-fold), ABCG8 (2-fold) and ABCA1 (3·3-fold). Further, FM-VP4 significantly increased the expression of HMGCoA-R (5·8-fold) and scavenger receptor class BI (2·6-fold).

Fig. 3 Relative mRNA expression of selected genes related to cholesterol metabolism in the liver (a) and small intestine (b) of control mice (□) and disodium ascorbyl phytostanol phosphate (FM-VP4)-fed mice (![]() ). mRNA levels were quantified by real-time RT-PCR using glyceraldehyde 3-phosphate dehydrogenase as an internal control. LXR, liver X receptor; ABC, adenosine triphosphate-binding cassette transporter; HMGCoA-R, hydroxymethylglutaryl coenzyme A reductase; SR-BI, scavenger receptor class BI. The box-and-whisker graphs show the median (of five mice per group) as the middle line. The box extends from the 25th to the 75th percentile and the whiskers extend from the lowest value to the highest. Medians of control values were set at a normalised value of 100 arbitrary units. ○, Outside values; *, far-out values. † Median value was significantly different from that of the control mice (P < 0·05).
). mRNA levels were quantified by real-time RT-PCR using glyceraldehyde 3-phosphate dehydrogenase as an internal control. LXR, liver X receptor; ABC, adenosine triphosphate-binding cassette transporter; HMGCoA-R, hydroxymethylglutaryl coenzyme A reductase; SR-BI, scavenger receptor class BI. The box-and-whisker graphs show the median (of five mice per group) as the middle line. The box extends from the 25th to the 75th percentile and the whiskers extend from the lowest value to the highest. Medians of control values were set at a normalised value of 100 arbitrary units. ○, Outside values; *, far-out values. † Median value was significantly different from that of the control mice (P < 0·05).
Quantitative real-time RT-PCR analyses of the main genes related to bile acid metabolism were also carried out (Fig. 4). FM-VP4 treatment significantly reduced liver CYP7A1 (2·2-fold) and Na+/taurocholate co-transporter polypeptide (1·4-fold) mRNA levels and tended to up-regulate liver bile salt export pump expression. No changes were observed in liver cytochrome P450 family 7 subfamily B polypeptide 1 (CYP7B1), cytochrome P450 family 27 subfamily A polypeptide 1 (CYP27A1), pregnane X receptor and multidrug-resistant protein 3 mRNA expression (data not shown). In the intestine, FM-VP4 tended to increase ileal bile acid binding protein gene expression but the change did not reach statistical significance.

Fig. 4 Relative mRNA levels of selected genes related to bile acid metabolism in the liver and small intestine of control mice (□) and disodium ascorbyl phytostanol phosphate (FM-VP4)-fed mice (![]() ). mRNA levels were quantified by real-time RT-PCR using glyceraldehyde 3-phosphate dehydrogenase as an internal control. CYP7A1, cytochrome P450 family 7 subfamily A polypeptide 1; FXR, farnesoid X receptor; BSEP, bile salt export pump; NTCP, Na+/taurocholate co-transporter polypeptide; IBABP, ileal bile acid binding protein. The box-and-whisker graphs show the median (of five mice per group) as the middle line. The box extends from the 25th to the 75th percentile and the whiskers extend from the lowest value to the highest. Medians of control values were set at a normalised value of 100 arbitrary units. ○, Outside values; *, far-out values. † Median value was significantly different from that of the control mice (P < 0·05).
). mRNA levels were quantified by real-time RT-PCR using glyceraldehyde 3-phosphate dehydrogenase as an internal control. CYP7A1, cytochrome P450 family 7 subfamily A polypeptide 1; FXR, farnesoid X receptor; BSEP, bile salt export pump; NTCP, Na+/taurocholate co-transporter polypeptide; IBABP, ileal bile acid binding protein. The box-and-whisker graphs show the median (of five mice per group) as the middle line. The box extends from the 25th to the 75th percentile and the whiskers extend from the lowest value to the highest. Medians of control values were set at a normalised value of 100 arbitrary units. ○, Outside values; *, far-out values. † Median value was significantly different from that of the control mice (P < 0·05).
Discussion
It is well known that phytosterols and phytostanols reduce intestinal cholesterol absorption(Reference Calpe-Berdiel, Escola-Gil and Blanco-Vaca8). In the present study, FM-VP4-treated mice presented a 2·2-fold decrease in intestinal cholesterol absorption measured by the faecal dual-isotope ratio method (Table 1). This is consistent with other studies in rats in which FM-VP4 led to a dose-related decrease in orally administered micellar [3H]cholesterol absorption(Reference Wasan, Peteherych and Najafi11). Further, FM-VP4 also reduced serum and liver cholesterol levels (Table 1). These latter findings are, however, somewhat surprising considering that the C57BL/6 mouse is not an LDL-sensitive species and that other phytosterols and phytostanols, or even ezetimibe, did not markedly reduce plasma and liver cholesterol levels(Reference Calpe-Berdiel, Escola-Gil and Ribas7, Reference Plosch, Kruit and Bloks32, Reference Repa, Dietschy and Turley36, Reference Calpe-Berdiel, Escola-Gil and Blanco-Vaca37). The effects of FM-VP4 seem to be dependent on its particular chemical structure(Reference Lukic, Wasan and Zamfir14). One possible mechanism contributing to these effects may be the greater ability of FM-VP4 to inhibit intestinal cholesterol absorption compared with other plant sterols and stanols and ezetimibe in animal studies(Reference Calpe-Berdiel, Escola-Gil and Ribas7, Reference Plosch, Kruit and Bloks32, Reference Repa, Dietschy and Turley36). However, these differences in intestinal cholesterol absorption do not seem sufficient to explain such a different impact on cholesterol levels and, therefore, other mechanisms may also be acting. In this context, it should be noted that intragastrically administered [14C]cholesterol presented plasma concentrations that were up to 100 times lower in treated mice (Table 1), an observation which could be explained through both a greater intestinal cholesterol absorption reduction and an accelerated incorporation of cholesterol into tissues. In fact, FM-VP4 up-regulated the expression of genes that operate in liver cholesterol homeostasis, such as LXRα, scavenger receptor class BI, ABCG5 and G8 (Fig. 3(b)), and whose overexpression has been reported to increase the hepatobiliary excretion of cholesterol and to decrease plasma cholesterol levels(Reference Yu, Li-Hawkins and Hammer38–Reference Naik, Wang and Da Silva43). These changes did not occur when other phytosterols, phytostanols or ezetimibe were used(Reference Calpe-Berdiel, Escola-Gil and Ribas7, Reference Plosch, Kruit and Bloks32, Reference Repa, Dietschy and Turley36). Thus, these effects on gene expression could be specific to FM-VP4. Previous studies have shown that FM-VP4 oral bioavailability is of 6·5 % in rats, a higher proportion than other stanols(Reference Wasan, Peteherych and Najafi11). Moreover, a clinical study in dyslipaemic men showed that FM-VP4 was detectable in plasma after oral administration(Reference Vissers, Trip and Pritchard17). It is possible, then, that FM-VP4 could mediate a direct action on liver metabolism, taking into account that some phytosterols, as stigmasterol, can activate LXR in vivo (Reference Yang, Yu and Li27). FM-VP4 treatment up-regulated the liver expression of the HMGCoA-R gene 6-fold. Previous studies did not show differences in similar feeding periods with other phytosterols or stanols(Reference Calpe-Berdiel, Escola-Gil and Ribas7, Reference Plat and Mensink21, Reference Volger, van der Boom and de Wit22, Reference Xu, Le and Moghadasian24), although different types of mice and diets were used. This hepatic HMGCoA-R gene expression change is likely to indicate an attempt of compensatory up-regulation of liver cholesterol biosynthesis. This situation could be due to both the higher inhibition in intestinal cholesterol absorption promoted by FM-VP4 together with, perhaps, an increased rate of hepatobiliary cholesterol excretion.
In contrast, decreases were found in intestine ABCG5 and ABCG8 gene expression, together with a non-significant tendency of the intestinal ABCA1 gene, in FM-VP4-treated mice (Fig. 3(b)). This observation is in line with the results of other phytosterol and phytostanol studies(Reference Calpe-Berdiel, Escola-Gil and Ribas7, Reference Plosch, Kruit and Bloks32, Reference Field, Born and Mathur44) and may be due to decreased induction of LXRα by oxysterols secondary to the decreased intestinal cholesterol content. Finally, inhibition of intestinal cholesterol absorption after FM-VP4 treatment is not mediated by transcriptional changes in intestinal NPC1L1, as previously reported for other phytosterols and phytostanols(Reference Calpe-Berdiel, Escola-Gil and Ribas7, Reference Plosch, Kruit and Bloks32, Reference Field, Born and Mathur44).
Somewhat unexpectedly, FM-VP4 also influenced bile acid metabolism in mice in the present study. Results in Fig. 1 strongly suggest an increase in the intestinal reabsorption of bile acids. In previous reports, phytosterol or phytostanol treatments did not affect bile acid pool size, biliary acid levels or faecal excretion of bile acids in mice(Reference Calpe-Berdiel, Escola-Gil and Ribas7, Reference Volger, van der Boom and de Wit22, Reference Plosch, Kruit and Bloks32). The distribution of intragastrically administered [3H]taurocholic acid also indicates that the FM-VP4 treatment promoted enhanced intestinal bile acid reabsorption. We speculate that the great hypocholesterolaemic effect of FM-VP4 promoted a compensatory mechanism that consisted of an increase in intestinal bile acid reabsorption, since bile acid synthesis is a major pathway of cholesterol consumption.
FXR is a nuclear receptor activated by bile acids that regulates the expression of a number of target genes critical for bile acid homeostasis. Thus, FXR plays a protective role in the liver against bile acid accumulation(Reference Chiang45, Reference Russell46). Although FM-VP4 did not significantly alter FXR gene expression, several of its target genes were affected by the treatment, probably due to increased bile acid intestinal reabsorption. Thus, FM-VP4 down-regulated liver CYP7A1, the key enzyme in the classic bile acid biosynthetic pathway, and Na+/taurocholate co-transporter polypeptide expression, the main liver transporter involved in bile acid uptake(Reference Chiang45, Reference Chiang47, Reference Stedman, Liddle and Coulter48). Of note, although liver CYP7A1 expression is up-regulated by LXRα, FXR action seems to be more efficient when both nuclear receptors are overexpressed(Reference Chiang45). The ‘crosstalk’ of FXR with other nuclear receptors could have attenuated the induction of other FXR-targeted genes such as liver bile salt export pump, which exports bile acids to the biliar canaliculi(Reference Chiang45), and intestine ileal bile acid binding protein expression, which is thought to be the main transporter involved in bile acid uptake from the small intestine(Reference Alrefai and Gill49). Moreover, the alternative bile acid biosynthetic pathway seemed to remain unchanged, at least as judged by transcriptional analysis, since no differences in cytochrome P450 family 7 subfamily B polypeptide 1 (CYP7B1) and cytochrome P450 family 27 subfamily A polypeptide 1 (CYP27A1) expression (data not shown) were observed(Reference Chiang45). On the other hand, no evidence was found of liver toxicity due to FM-VP4 administration, since no changes were observed in either multidrug-resistant protein 3 expression, one of the major protective pathways against cholestatic liver injury, or in pregnane X receptor, its main regulator(Reference Teng and Piquette-Miller50) (data not shown).
In conclusion, to our knowledge FM-VP4 is the first phytosterol or stanol compound to lower plasma cholesterol levels in normocholesterolaemic mice and, probably as a consequence of this action, increase bile acid enterohepatic recirculation.
Limitations of the study
A limitation of the present study is the absence of a stanol (other than FM-VP4) control group. Nevertheless, as previously stated, other studies have shown that FM-VP4 exerts a higher capacity of inhibiting intestinal cholesterol absorption compared with other plant stanols(Reference Ebine, Jia and Demonty13).
Furthermore, it must be taken into account that changes in gene expression are not necessarily related to changes in protein mass or activity. It cannot be determined either whether these changes are due to decreased cholesterol levels or to a direct FM-VP4 action. However, we used these data for discussion when the transcriptional changes seemed to be regulated through a known transcriptional factor activated by a ligand, such as LXR or FXR.
Acknowledgements
We are grateful to Christine O'Hara for editorial assistance. The present study was funded in part by Forbes Medi-Tech Inc. CIBER of Diabetes and Enfermedades Metabólicas Asociadas is an ISCIII project.
J. M.-G. participated in the design of the study, carried out the liver RT-PCR and bile acid analyses, performed statistical analysis and drafted the manuscript. S. S.-C. carried out the organ lipid studies and intestine RT-PCR analyses. L. C.-B. carried out the intestinal cholesterol absorption experiments. N. R. carried out the plasma lipid studies. M. V.-C. participated in the design of the study. J. C. E.-G. and F. B.-V. conceived of the study, participated in its design and coordination and edited the manuscript. J. M.-G., S. S.-C., L. C.-B., N. R. and J. C. E.-G. were responsible for the animal experiments. All authors read and approved the final manuscript.
There are no conflicts of interest.







