As infants and toddlers grow up, some problematic eating behaviours perceived by caregivers may develop( Reference Bernard-Bonnin 1 – Reference Skinner, Carruth and Houck 3 ). Children who display difficulties in eating or reject certain types of foods that parents think are appropriate are often called problem feeders, picky eaters or neophobics. Children with food neophobia are reluctant to eat new foods, whereas picky children resist eating many familiar (as well as unfamiliar) foods( Reference Dovey, Staples and Gibson 4 ); both have been frequently associated with eating small meals, eating slowly and accepting a limited number of foods( Reference Mascola, Bryson and Agras 5 , Reference Reau, Senturia and Lebailly 6 ). Thus, children displaying these feeding problems are likely to avoid particular foods or food groups, which could result in a limited food selection and lack of dietary variety, and have a negative impact on their intake of essential nutrients.
Feeding problems are considered as problems that arise during infancy and early childhood related with failure of the child’s feeding learning process( Reference Aldridge, Dovey and Martin 2 ). Aldridge et al.( Reference Aldridge, Dovey and Martin 2 ) describes that there are specific risk periods of adaptations in feeding style and dietary intake, such as the introduction of complementary feeding and the self-feeding period. Most infants overcome the typical difficulties of these risky periods and their feeding style matures through practice and repeated exposures to new experiences, but some children fail to develop the skills necessary to cope with developmental demands and face persistent problems with eating( Reference Aldridge, Dovey and Martin 2 ).
Previous studies have shown that feeding problems are common in healthy young children and may compromise their future health – namely, determining a worse weight status( Reference Dubois, Farmer and Girard 7 , Reference Galloway, Fiorito and Lee 8 ), increasing the likelihood of behavioural problems during later childhood( Reference Jacobi, Schmitz and Agras 9 , Reference Winsper and Wolke 10 ) and symptoms of anorexia nervosa during adolescence( Reference Marchi and Cohen 11 ). Previous research has also documented a negative effect of feeding problems on dietary intake( Reference Dubois, Farmer and Girard 7 , Reference Galloway, Fiorito and Lee 8 , Reference Carruth, Ziegler and Gordon 12 – Reference Jacobi, Agras and Bryson 17 ) and dietary variety( Reference Mascola, Bryson and Agras 5 , Reference Carruth, Skinner and Houck 18 , Reference Falciglia, Couch and Gribble 19 ), overall suggesting that neophobia and pickiness are both associated with less healthy food choices in children. These associations have been mainly documented in relation to the consumption of specific food groups, such as fruits and/or vegetables and protein foods( Reference Dubois, Farmer and Girard 7 , Reference Galloway, Fiorito and Lee 8 , Reference Carruth, Ziegler and Gordon 12 – Reference Jacobi, Agras and Bryson 17 , Reference Falciglia, Couch and Gribble 19 ) or specific nutrients( Reference Dubois, Farmer and Girard 7 , Reference Galloway, Fiorito and Lee 8 , Reference Falciglia, Couch and Gribble 19 ). Fewer studies have documented their relation with dietary variety( Reference Mascola, Bryson and Agras 5 , Reference Carruth, Skinner and Houck 18 , Reference Falciglia, Couch and Gribble 19 ), and most of them have been conducted in children older than 5 years of age( Reference Mascola, Bryson and Agras 5 , Reference Falciglia, Couch and Gribble 19 ) or have defined dietary variety based on a single question( Reference Mascola, Bryson and Agras 5 ).
Although there is previous evidence that certain early eating behaviours may compromise later dietary intake and variety, most of the studies conducted so far are cross-sectional( Reference Galloway, Fiorito and Lee 8 , Reference Carruth, Ziegler and Gordon 12 – Reference Falciglia, Couch and Gribble 19 ), prompting to a reverse causality bias. They also frequently focused on particular aspects of eating behaviours, such as food neophobia and pickiness.
In order to compare results across different populations, and to evaluate these relationships from a prospective approach, the objective of this study was to relate eating behaviours at different age ranges (4–6, 12–15, 24 and 48–54 months) with fruit and vegetable (F&V) intake and dietary variety at 4–5 years of age in three European birth cohorts (Generation XXI from Portugal, ALSPAC from the UK and EDEN from France). Our hypothesis was that problematic eating behaviours during early childhood may be associated with poorer dietary habits, and comparing results across different populations would give consistency to these findings.
Methods
Description of cohorts
Analyses were based on three European population-based birth cohorts: the Portuguese Generation XXI birth cohort, the British Avon Longitudinal Study of Parents and Children (ALSPAC) and the French EDEN mother–child cohort.
Generation XXI is a birth cohort that recruited women from all public maternity units from Porto, Portugal, between April 2005 and August 2006. A total of 8647 children and 8495 mothers were enrolled at baseline. Of the invited mothers, 91·4 % agreed to participate( Reference Larsen, Kamper-Jorgensen and Adamson 20 ). Eating behaviours and feeding practices were assessed during intermediate follow-ups conducted in sub-samples at 6 and 15 months of age, and at 4 years of age, when 86 % of all the children were re-evaluated (70 % by face-to-face interviews). For the present analyses, 912 children with complete data were included at 6 months, 544 at 15 months and 4227 at 48 months of age (see online Supplementary Table S1).
ALSPAC is a longitudinal birth cohort study, which recruited pregnant women resident in a geographically defined area in the south-west of England with an expected delivery date between April 1991 and December 1992. A cohort of 14 541 pregnant women was established, resulting in 13 988 children alive at 12 months of age. More details can be found on the ALSPAC website (http://www.bristol.ac.uk/alspac/). Eating behaviours and feeding practices were assessed by questionnaires sent to the main caregiver when the child was 6, 15 and 54 months of age. For the present analyses, 5239 children with complete data were included at 6 months, 7685 at 15 months, 7418 at 24 months and 7620 at 54 months of age (see online Supplementary Table S1).
The EDEN mother–child cohort is a longitudinal study, which recruited 2002 pregnant women from two French university hospitals, in Nancy and Poitiers. Maternal perception of the child’s appetite was assessed at 4, 12 and 24 months of age, difficulty to feed the child was assessed at 24 and 48 months of age and the child’s food neophobia was assessed at 24 and 48 months of age. For the present analyses, 1034 children with complete data for both eating behaviour in early childhood and diet at 5 years were included in the 4-month analyses, 1002 in the 12-month analyses, 953 in the 24-month analyses and 892 in the 48-month analyses (see online Supplementary Table S1).
In each cohort, the procedures were carried out in accordance with the local and national ethical standards.
Subjects with missing values on variables of interest (eating behaviours; F&V intake; the variety score; maternal age, education level and smoking; breast-feeding duration; and the child’s BMI at 48–60 months) were excluded from the analyses. In cohorts with twins, one of them was selected at random to be included in the analyses. The different steps of sample selection are presented in online Supplementary Table S1.
Fruit and vegetable intake and the Healthy Plate Variety Score
Dietary information was assessed using FFQ at, respectively, 48, 54 and 60 months of age in the Generation XXI, ALSPAC and EDEN cohorts. Each cohort used their own FFQ, which varied in the number of items and frequency categories investigated. Table 1 provides descriptive data for each of the FFQ used in children.
Table 1 Description of the FFQ used in children at 4–5 years of age in each of the three European birth cohorts
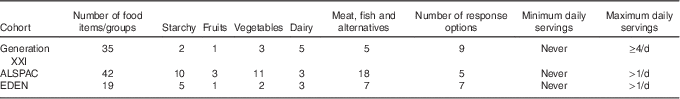
The FFQ data were harmonised by grouping questions about similar foods together in the same way in each cohort. The definition of fruits was the same in all cohorts – that is, exclusive of jams/jellies and fruit juice – and the definition of vegetables was exclusive of legumes and potatoes, but included the vegetable soup (typical in Portugal). Common cut-offs for fruits and for vegetables were used across the three cohorts, whenever possible (>1 v. ≤1 serving/d for fruits and for vegetables). In Generation XXI, the number of children in the ≤1 serving/d category of vegetables was very low (5·4 %), mainly due to the high daily consumption of the vegetable soup in Portugal. Therefore, in Generation XXI, the cut-off used for vegetable frequency was >3 v. ≤3 servings/d.
To assure the dietary data validity, some procedures were undertaken in each cohort. In Generation XXI, for a sub-sample of 2373 children, FFQ data was compared with 3-d food records completed at 4 years of age. In order to assess the validity of the FFQ, Pearson’s correlation coefficients were calculated for key food groups as measured by both methods. For those food groups most frequently consumed, weak-to-moderate correlations and fair-to-moderate agreement were observed. Significant positive moderate Pearson’s correlations were found for vegetable soup (r=0·54, P<0·001), fruit (r=0·42, P<0·001), milk (r=0·46, P<0·001) and yoghurts (r=0·48, P<0·001), and not-so-strong correlations were found for cheese (r=0·35, P<0·001), fish (r=0·27, P<0·001), vegetables eaten on a plate (r=0·26, P<0·001), eggs (r=0·21, P<0·001) and non-carbonated sugar-sweetened beverages (iced tea: r=0·29, P<0·001; juices: r=0·22, P<0·001).
The ALSPAC children’s FFQ was a modified version of the mother’s FFQ, which was based on an FFQ developed by Yarnell et al.( Reference Yarnell, Fehily and Milbank 21 ). The FFQ administered when the children were 38 months old has been compared with 3-d food records collected in a sub-sample of children at 41 months of age, and the results were very similar.
The EDEN FFQ for children was a shortened version of the mother’s validated FFQ( Reference Deschamps, de Lauzon-Guillain and Lafay 22 ), but no specific validation was made for children.
To assess dietary variety, we calculated the Healthy Plate Variety Score (HPVS), which is a modified version of the food variety index for toddlers developed by Cox et al.( Reference Cox, Skinner and Carruth 23 ). The HPVS developed here uses FFQ data and is based on the food groups and the number of servings as recommended in the food plate model (former pyramid model) according to healthy eating guidelines promoted by the US Departments of Agriculture and Health and Human Services( 24 ).
To calculate the HPVS, food items were allocated to one of the following five food groups: (1) starchy foods (including potatoes); (2) fruits; (3) vegetables; (4) meat, fish and alternatives; and (5) dairy foods. The purpose of the HPVS is to measure variety both within and between food groups – that is, it aims to capture variety between the different food groups established, but also to ensure that the child does not always eat the same food within each food group (e.g. it should vary between milk, yoghurts, cheese, etc.).
Thus, truncations were applied, as advised by Cox et al.( Reference Cox, Skinner and Carruth 23 ), to assure that a high intake of one food group cannot compensate mathematically for a low intake of another food group, and that within each food group variety is ensured. Within each food group, the contribution of a particular food item was truncated at 33 %. Foods within a food group that are similar (e.g. pasta and noodles) were grouped together and count as a single food, so that they do not contribute more than 33 %. Owing to limited questions in some of the FFQs about types of fruits and vegetables, it was not possible to assess variety within these two groups; the score instead reflects whether children ate the recommended number of servings. After the groupings and truncations were applied, the number of servings for each food group was totalled. Food group scores were calculated by dividing the total number of servings by the recommended number of servings per d for each food group (starchy foods=7, fruit=2, vegetables=3, meat, fish and alternatives=2 and dairy foods=3). To ensure variety between the food groups, each food group score was truncated at 1·00 (e.g. if a child ate eight different types of starchy food, this was divided by seven, thus giving a potential score of 1·14, which was truncated to 1·00).
Foods such as condiments, sweets/candy, herbs/spices, soft drinks, oil, butter/margarine and salty snack foods were excluded from the analysis( Reference Cox, Skinner and Carruth 23 ). In addition, we also excluded cakes, biscuits/cookies, puddings and fried potatoes.
The final HPVS score is the sum of scores of the five food groups and can range from 0·0 to 5·0. A higher HPVS represents higher variety between and within food groups and also higher dietary adequacy. The HPVS was dichotomised by the median score in each cohort (possible range variation 0·0–5·0): 3·9 in Generation XXI, 3·1 in ALSPAC and 2·6 in EDEN cohorts.
Assessment of eating behaviours
Eating behaviours were reported as the perception of the main caregiver (usually mothers) on eating problems of their child. Only similar aspects of eating behaviours available in at least two cohorts and food neophobia (in EDEN only) were considered for this analysis, resulting in four main domains: feeding difficulties, poor eating, food refusal (for Generation XXI and ALSPAC)/food neophobia (for EDEN) and irregular eating. The questions used in each cohort are described in Table 3 (a more detailed explanation for food refusal and food neophobia are described in the table’s footnote).
Each eating behaviour was dichotomised into ‘Yes’ (a considered aspect of eating behaviour was reported) v. ‘No’ (reference category). In Generation XXI, to define food refusal at 48 months of age, a combined variable was created to summarise refusal to take milk, F&V, soup or fish (‘Yes’ was considered if at least one was reported). In the EDEN cohort, to define food neophobia, an index was created based on the mean of three questions and then dichotomised into ‘≥median’ (more neophobic) v. ‘<median’ (considered as reference category).
Specific age ranges were selected, in which comparable data between cohorts were available: 4–6 months, 12–15 months and 48–54 months of age (with data available for Generation XXI, EDEN and ALSPAC) and 24 months of age (with data available for EDEN and ALSPAC).
Statistics
Associations of eating behaviours with F&V intake and the HPVS were estimated by odds ratio and 95% confidence intervals obtained from unconditional logistic regression models. Associations were estimated prospectively, except at 48–54 months of age for Generation XXI and ALSPAC, where the associations were assessed cross-sectionally.
Four models were used with the indicated covariates: (i) model 1, including as confounders those characteristics significantly associated with eating behaviours and dietary outcomes under study in at least two cohorts: maternal age, maternal education (baseline assessments), maternal smoking during pregnancy and any breast-feeding duration; (ii) model 2, including all variables from model 1 plus the child’s World Health Organization( 25 ) BMI z-score at 4–5 years of age (as a continuous variable); (iii) models 3 and 4, including all variables from models 1 and 2 plus age at introduction of fruits or vegetables, age at introduction of any food other than F&V and maternal F&V intake (assessed during pregnancy in EDEN and ALSPAC). As the final results were similar in models 2–4, final tables only present results from model 2, which was considered as the final model.
All the analyses were performed using STATA/SE (StataCorp) version 10.0 in Generation XXI; SPSS 19.0 (SPSS Inc.) in ALSPAC; SAS software version 9.2 (SAS Institute Inc.) in EDEN.
Results
Table 2 shows the characteristics of mothers and children at 4–5 years of age, by cohort. These data suggest a similar distribution of some key variables across cohorts; more than 70 % of mothers were between 25 and 35 years of age, approximately 20 % smoked during pregnancy and 50 % of the children were boys. Maternal education and child’s breast-feeding duration differed across cohorts, which could be explained by country-specific socio-cultural differences. Generation XXI had the highest F&V intake: 84 % of the Portuguese children presented a daily intake of F&V above three servings compared with 62 % in ALSPAC and 20 % in EDEN cohorts.
Table 2 Characteristics of mothers and children at 4–5 years of age by cohort (Counts and percentages, mean values and standard deviations)

HPVS, Healthy Plate Variety Score.
* Defined according to the WHO z-scores( 25 ).
† In Generation XXI, this % combines the sample size for never and less than once a month.
‡ In Generation XXI, the cut-off point used was three servings per d instead of one serving per d due to a higher vegetable intake in this population.
Table 3 presents the prevalence of the selected eating behaviours at the different ages and cohorts. The non-establishment of a daily routine by the child and the need of stimulation to eat were the eating behaviours less frequently reported by parents. Poor eating, defined as the child eating small quantities of each meal or not eating enough in Generation XXI and ALSPAC, was frequent (varying from 25 % at 4–6 months to 66 % at 24 months of age). Food refusal was reported more frequently by parents of older children (65 % in Generation XXI and 68 % in ALSPAC at 48–54 months of age), compared with less than 35 % at 4–6 months of age.
Table 3 Prevalence of eating behaviours by age and cohort

G21, Generation XXI.
* In Generation XXI at 48 months, this item was based on four separate questions on refusal of milk, fruit and vegetables, soup or fish.
† Score available only in the EDEN cohort and calculated based on three questions: (i) your child does not like to taste novel foods; (ii) your child accepts easily to change meals; (iii) your child refuses to taste something that he/she does not know. High score is defined at or above the median score.
Tables 4–6 describe the associations between the eating behaviours at the different ages and fruit intake (Table 4), vegetable intake (Table 5) and dietary variety, as defined by the HPVS (Table 6).
Table 4 Association between eating behaviours and high fruit intake (>1 serving/d) at 48 months in Generation XXI, 54 months in ALSPAC and 60 months in EDEN (Odds ratios and 95 % confidence intervals)
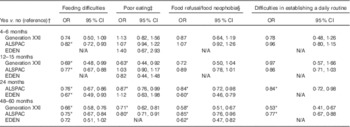
N/A, eating behaviour not available at that age.
* P<0·05.
† OR and respective 95 % CI adjusted for maternal age, education, smoking during pregnancy, any breast-feeding and child’s z-score BMI.
‡ Poor eating corresponding to eats small quantities at each meal (at 4–6 months), does not eat enough (at 12–15 months) in ALSPAC and Generation XXI and needs to be stimulated in EDEN (at 4, 12 and 24 months).
§ Refusal to milk at 4–6 months and solids at 12–15 months in Generation XXI, refusal to milk at 4–6 months and to the right food at 12–15 months and 24 months in ALSPAC, refusal to the right food at 48–54 months in Generation XXI and ALSPAC. Food neophobia score available only in EDEN and calculated based on the mean of three questions (≥median (more neophobic) v. <median (reference category)).
Table 5 Association between eating behaviours and high vegetable intake (>1 serving/d in ALSPAC and EDEN and >3 servings/d in Generation XXI) at 48 months in Generation XXI, 54 months in ALSPAC and 60 months in EDEN (Odds ratios and 95 % confidence intervals)
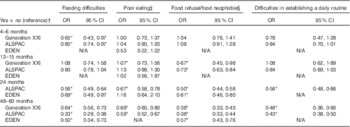
N/A, eating behaviour not available at that age.* P<0·05.
† OR and respective 95 % CI adjusted for maternal age, education, smoking during pregnancy, any breast-feeding and child’s z-score BMI.
‡ Poor eating corresponding to eats small quantities at each meal (at 4–6 months), does not eat enough (at 12–15 months) in ALSPAC and Generation XXI and needs to be stimulated in EDEN (at 4, 12 and 24 months).
§ Refusal to milk at 4–6 months and solids at 12–15 months in Generation XXI, refusal to milk at 4–6 months and to the right food at 12–15 months and 24 months in ALSPAC, refusal to the right food at 48–54 months in Generation XXI and ALSPAC. Food neophobia score available only in EDEN and calculated based on the mean of three questions (≥median (more neophobic) v. <median (reference category)).
Table 6 Association between eating behaviours and a higher score in the Healthy Plate Variety Score (>median in the three cohorts) at 48 months in Generation XXI, 54 months in ALSPAC and 60 months in EDEN (Odds ratios and 95 % confidence intervals)
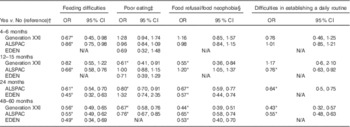
N/A, eating behaviour not available at that age.* P<0·05.
† OR and respective 95 % CI adjusted for maternal age, education, smoking during pregnancy, any breast-feeding and child’s z-score BMI.
‡ Poor eating corresponding to eats small quantities at each meal (at 4–6 months), does not eat enough (at 12–15 months) in ALSPAC and Generation XXI and needs to be stimulated in EDEN (at 4, 12 and 24 months).
§ Refusal to milk at 4–6 months and solids at 12–15 months in Generation XXI, refusal to milk at 4–6 months and to the right food at 12–15 months and 24 months in ALSPAC, refusal to the right food at 48–54 months in Generation XXI and ALSPAC. Food neophobia score available only in EDEN and calculated based on the mean of three questions (≥median (more neophobic) v. <median (reference category)).
Children with more eating difficulties, as reported by parents at the different ages, were less likely to have fruit intake >1 serving/d when compared with children with no eating difficulties (Table 4). This association was consistent across cohorts and ages. Similarly, children presenting refusal to foods or higher food neophobia scores and difficulties in establishing a daily routine, particularly at 24 and 48–60 months of age, had lower fruit intake at 4–5 years of age compared with children with no such eating difficulties. The association of poor eating with fruit intake was less consistent.
When compared with fruits, the results for vegetable intake were very similar, but slightly stronger; inverse associations were found between vegetable intake and eating difficulties – namely, at early stages of life – and also poor eating, food refusal/neophobia and difficulties in establishing a daily routine, particularly when reported at 12 months of age and thereafter (Table 5).
Children whose parents reported these eating difficulties at 4–6, 12–15 and 24 months of age also presented a lower variety score at 4–5 years of age than children with no eating difficulties (Table 6). The association with food refusal/neophobia and difficulties in establishing a daily routine were in the same direction, but only significant when eating behaviours were reported after 12 months of age.
Discussion
Feeding problems in infants and toddlers were frequently reported by mothers from the three European birth cohorts, and were shown to be prospectively associated with lower F&V intake as well as with less dietary variety at 4–5 years of age.
This study, based on population-based samples, has shown that the prevalence of feeding problems varied from 3 to 66 %. Previous studies have already described that feeding problems are common in children; approximately 20–50 % of normally developing children are reported to experience some type of feeding problems( Reference Reau, Senturia and Lebailly 6 , Reference Carruth, Ziegler and Gordon 12 , Reference Jacobi, Agras and Bryson 17 , Reference Benjasuwantep, Chaithirayanon and Eiamudomkan 26 , Reference Wright, Parkinson and Shipton 27 ). Most of these feeding problems are likely to be transient, and because some evidence suggests that the nature and extent of feeding problems decline as a function of the age of the child( Reference Koivisto and Sjoden 28 ) the present study examined whether differences would be evident across development. We found that feeding problems seemed to be more prevalent at older ages and that the associations reported were also more evident at older ages (i.e. after 12 months of age). As previously shown in a prospective study of 120 children and their parents, followed-up from 2 to 11 years of age( Reference Mascola, Bryson and Agras 5 ), incidence of picky eating declined over time, whereas point prevalence increased indicating that picky eating is often a chronic problem with 40 % having a duration of more than 2 years of age.
In fact, feeding problems in infants appear to be highly persistent throughout childhood( Reference Dahl, Rydell and Sundelin 29 ), and the persistence of these eating behaviours over time can have long-term effects that are not evident until children are older; they might be precursors of unhealthy eating and may worsen their weight status( Reference Dubois, Farmer and Girard 7 , Reference Galloway, Fiorito and Lee 8 ) later in life, which highlights the need of prospective analysis. In the present study, associations were tested prospectively, except at 4–5 years of age, in Generation XXI and ALSPAC when eating behaviours were reported at the same time than dietary intake.
Most studies conducted so far linking feeding problems with dietary intake( Reference Dubois, Farmer and Girard 7 , Reference Galloway, Fiorito and Lee 8 , Reference Carruth, Ziegler and Gordon 12 – Reference Jacobi, Agras and Bryson 17 ) were, however, cross-sectional( Reference Galloway, Fiorito and Lee 8 , Reference Carruth, Ziegler and Gordon 12 – Reference Jacobi, Agras and Bryson 17 ). They suggest that neophobia and pickiness are both associated with less healthy food choices in children. In the study by Galloway et al.( Reference Galloway, Fiorito and Lee 8 ), 9-year-old girls who were picky eaters consumed significantly fewer servings of fruits, vegetables, fats and sweets as well as less fibre than girls who were not picky eaters. These results suggest that picky eaters did not consume more fats and sweets to compensate for lower F&V intake. In a naturalistic mealtime situation, Cooke et al.( Reference Cooke, Carnell and Wardle 13 ) have also concluded in children aged 4–5 years that food neophobia impacts differentially on consumption of different food types. Specifically, it appears that children who score highly on the Child Food Neophobia Scale eat less fruit, vegetables and protein foods than their less neophobic peers.
Other studies have also attempted to investigate the impact of neophobia on dietary variety( Reference Mascola, Bryson and Agras 5 , Reference Carruth, Skinner and Houck 18 , Reference Falciglia, Couch and Gribble 19 ). Falciglia et al.( Reference Falciglia, Couch and Gribble 19 ) in 9- to 10-year-old children found that the Healthy Eating Index scores were lower for the neophobic group than for the other children. Carruth et al.( Reference Carruth, Skinner and Houck 18 ) also showed that toddlers perceived by their mothers as picky eaters had significantly lower dietary variety and diversity scores. Mascola et al.( Reference Mascola, Bryson and Agras 5 ) reported that parents of picky eaters were more likely to report that their children consumed a limited variety of foods, evaluated through a single question of the Stanford Feeding Questionnaire.
In a Canadian study, mothers’ perception of children’s eating behaviours was prospectively linked to higher body weight and less dietary adequacy at 4·5 years of age( Reference Dubois, Farmer and Girard 7 ). This study indicated that children with picky eating at 2·5, 3·5 and 4·5 years of age are under-consuming – at 4·5 years – some macronutrients (less fat, protein and total energy) and food groups, such as F&V and meat and alternatives, whereas overeaters are over-consuming grain products and meat and alternatives. To our knowledge, this is the only study presenting prospective data, but dietary adequacy was evaluated by comparison with the national recommendations – Canada’s Food Guide – and no summary measure of adequacy was provided. Nonetheless, data suggest that picky eaters have less dietary variety. Our data enlarged these findings, supporting that other eating behaviours such as food refusal/neophobia and difficulties in establishing a daily routine perceived at early ages are prospectively associated with lower F&V intake and less dietary variety, measured by a composite score that takes into account variety within and across food groups, when children were 4–5 years old. Our data also showed that the prospective and cross-sectional associations were quite comparable in magnitude after 12 months, which is again in favour of an actual association.
In the present study, the associations of eating behaviours with fruits and vegetables were in the same direction compared with dietary variety. This is in agreement with the notion that less varied diets have also lower intake of more healthy foods, such as F&V, and higher intake of unhealthy foods( Reference Murphy, Foote and Wilkens 30 ). Moreover, the inverse associations of these eating behaviours were slightly stronger with vegetables than with fruits. Cooke et al.( Reference Cooke, Wardle and Gibson 15 ) have already described that food neophobia is more strongly correlated with vegetables than with fruit intake. Vegetables are bitter and infants are born with an innate liking for sweet tastes and a dislike of sour or bitter ones( Reference Steiner 31 ), thus they have more difficulties in accepting bitter-tasting vegetables, for which they have to learn to accept them. In addition, when children’s vegetable consumption was explored in more detail, the sweeter vegetables tend to be preferred( Reference Ahern, Caton and Bouhlal 32 ).
Feeding problems, such as those reported in this study, are liable to have a strong behavioural aetiology( Reference Aldridge, Dovey and Martin 2 ), thus they can be overcome. There is good evidence that modelling and repeated exposure are strategies that enhance acceptance of a variety of foods( Reference Birch and Marlin 33 – Reference Cooke 35 ). Therefore, as suggested by Cooke et al., ‘guiding parents in the technique of regular and repeated taste exposure has the potential to improve diet’s quality of young children at what may be a sensitive period for developing lifelong healthy eating patterns’( Reference Cooke, Wardle and Gibson 15 ).
Definitions for eating behaviours vary across studies. Our findings highlight a lack of clarity that persists concerning classification of children’s problematic eating behaviours and the need of better instruments with which to measure them. Even in the present study, for some aspects of eating behaviours (especially for poor eating and food refusal), the questions differed between the ages and cohorts. However, consistent associations were reported, suggesting that these methodological differences would not influence our results.
This study has used the data collected from three different European countries in parallel analyses using the same confounders, and an attempt at data harmonisation was made. Each cohort used their own FFQ, which varied in the number of items and frequency categories; however, the core foods that most children’s diets consist of were covered. To ensure comparability between the countries, the daily number of servings was calculated in each country and, whenever possible, the same cut-off points were used. This was not possible in Generation XXI concerning vegetables, as there is a much higher frequency of vegetable consumption in Portugal than in France and the UK, as previously shown( Reference de Lauzon-Guillain, Jones and Oliveira 36 ). In addition, the dietary variety score was calculated in the same way in each country. Although we have assumed that the dietary variety score is a marker for diet quality, we are unable to validate this hypothesis, yet the foods recommended in the healthy plate model are based on guidelines for healthy eating and should reflect a diet of high quality.
In the present study, bias attributable to selective loss to follow-up cannot be discounted and may have weakened our results. In addition, in some age ranges and cohorts, the effect sizes were small and the CI large; however, the comparisons performed between different cohorts and age ranges provide more consistency to our results, and again the consistent results found across cohorts suggest a low likelihood of bias.
Conclusions
Children with eating difficulties, food refusal/neophobia and difficulties in establishing a daily routine, as reported by their mothers, presented lower F&V intake and less dietary variety at 4–5 years of age. These associations were consistent across three European birth cohorts and more evident when eating behaviours were reported after 12 months of age, and were slightly stronger for vegetables than fruits.
Efforts to improve dietary quality in early childhood could incorporate strategies aimed at reducing these problematic eating behaviours, such as modelling and taste exposure( Reference Birch and Marlin 33 – Reference Cooke 35 ).
Acknowledgements
We are grateful to Sylvie Issanchou for her coordination of the HabEat project. We are indebted to all participants for providing the data used in the three birth cohorts, as well as to all the members of the research team and coordinators (Henrique Barros from Generation XXI; George Davey Smith from ALSPAC and Barbara Heude from the EDEN Mother–Child Study Group).
This study was supported by the European Community’s Seventh Framework Program (FP7/2007-2013) under the grant agreement no. FP7-245012-HabEat. Generation XXI was funded by Programa Operacional de Saúde – Saúde XXI, Quadro Comunitário de Apoio III and by Administração Regional de Saúde Norte. For follow-up assessments Generation XXI received funding from Fundação para a Ciência e a Tecnologia, co-funded by FEDER through COMPETE and from Fundação Calouste Gulbenkian. The UK Medical Research Council (grant ref: 74882), the Wellcome Trust (grant ref: 076467) and the University of Bristol provided core support for ALSPAC. Support for the EDEN (Etude des Déterminants pre et postnatals précoces du développement et de la santé de l’ENfant) Study was provided by the Foundation for Medical Research, the French Ministry of Research (IFR programme), the Institut National de la Santé et de la Recherche Médicale (Human Nutrition Research Program), the Diabetes National Research Program (via a collaboration with the French Association for Diabetes Research), the French Ministry of Health Perinatality Program, the French Agency for Environmental Security, the French National Institute for Population Health Surveillance, the Paris–Sud University, the French National Institute for Health Education, Nestlé, the National Education Health Insurance, the French Speaking Association for the Study of Diabetes and Metabolism, the National Agency for Research (non-thematic programme) and the National Institute for Research in Public Health (TGIR health cohort 2008 programme). Study sponsors were not involved in the study design, data collection or data analyses.
Study concept and design: O. A, J. L., d. L.-G. B., E. P., M. P., C. M. A. and L. C. Acquisition of data: O. A., J. L., d. L.-G. B., E. P., M. P., C. M. A. and L. C. Analysis of data: O. A., J. L. and d. L.-G. B. Drafting of the manuscript: O. A. Critical revision of the manuscript and final approval: O. A., J. L., d. L.-G. B., E. P., M. P., C. M. A. and L. C.
There are no conflicts of interest.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515002287









