A wide variety of legumes such as dry grain pulses, soyabeans and peanuts are consumed in the human diet. Of these, the dry grain pulses have played a significant role throughout civilisation as a primary source of macronutrients, particularly protein, when combined with cereal grains. According to the FAO, the ideal ratio of cereal grains to pulses in the diet is 2:1, which is considerably different from the current consumption ratio of 8:1(1). Interest in pulses is reawakening because they are particularly well suited to emerging global concerns about food security in an ecologically and energy sustainable manner(2, 3). Of the four pulses that are prominent staple foods, i.e. dry beans, dry peas, chickpeas and lentils, dry bean (Phaseolus vulgaris L.) is the most widely produced and consumed non-processed legume and was the focus of the experiments reported herein(1).
Numerous studies indicate that dry bean consumption is associated with lower rates of chronic diseases such as CVD, type-2 diabetes and cancer(Reference Adebamowo, Cho and Sampson4–Reference Winham, Hutchins and Johnston13). The cardioprotective activity of dry bean consumption is primarily attributed to its effect on cholesterol metabolism(Reference Winham, Hutchins and Johnston13–Reference Pittaway, Ahuja and Cehun17). A recent meta-analysis found that the net change in total cholesterol for individuals treated with a pulse diet compared to control was − 118 mg/l; mean net change in LDL-cholesterol (LDL-C) was − 80 mg/l(Reference Bazzano, Thompson and Tees16). Consistent with these reports, cholesterol-lowering activity has also been observed in animal models of diet-induced hypercholesterolaemia using rats or pigs(Reference Costa, Walker and Low18–Reference Sarathy and Saraswathi23). In those studies, the effects of dry bean on multiple aspects of cholesterol metabolism were investigated. A reduction in plasma cholesterol of 15–20 % was observed at dietary bean concentrations of 30 % w/w on a DM basis, which is equivalent to about twice as much bean as is consumed in parts of Africa, particularly Burundi, where annual per-capita intake has been reported to be 40 kg(1). To put this observation in perspective, worldwide consumption varies considerably, ranging from 2 to 3·5 kg/capita per year in Europe and the United States to the 40 kg/capita per year in Burundi(1, Reference Mitchell, Lawrence and Hartman24). While the clinical effects on cholesterol metabolism summarised in the meta-analysis(Reference Bazzano, Thompson and Tees16) and the work conducted in animal models both indicate that the effects of dry bean and other pulses on the plasma lipid profile associated with reduced CVD risk are modest at currently recommended levels of consumption(25), differences among dry bean cultivars in cholesterol-lowering activity have been observed(Reference Bazzano, Thompson and Tees16, Reference Dabai, Walker and Sambrook20, Reference Rosa, Costa and Nunes21). This fact is largely unappreciated, and yet provides an avenue for increasing dry bean's clinical impact via the identification of cultivars of dry bean and other pulses with markedly higher levels of cardioprotective activity. That the differences among cultivars of bean in health benefits can be systematically exploited, is supported by a recent report that dry bean types have significantly different cancer inhibitory activity and that differences in activity are associated with the genetic heritage of the beans(Reference Thompson, Brick and McGinley26). As outlined in reference(Reference Thompson, Thompson and Sparks27), our laboratory has been working to encourage biomedical and agricultural scientists to work together to improve staple food crops such as pulses for their biomedical traits. For this to occur, it is essential to develop in vivo models for defining mechanisms and for translating those mechanisms to assays that can be used to screen the large number of genetically diverse dry bean varieties that are consumed in different regions of the world.
The initial objective of the present study was to determine if the effects of dietary dry bean on plasma cholesterol could be assessed in a 1-week animal feeding study. The approach reported is based on a 7 d experiment in which the safety of graded dietary levels of cooked bean were assessed in rat liver using a gene expression array(28). Transcript expression profiling revealed a dose-dependent induction of cytochrome P-450 cholesterol 7α hydoxylase (CYP7A). Since the enzyme encoded by this gene is a rate-limiting enzyme in the synthesis of bile acid from cholesterol and is involved in the regulation of cholesterol excretion by catalysing the formation of 7-α-hydroxcholesterol, we hypothesised that the same 1-week feeding study design could be used to evaluate the effects of dry bean on cholesterol metabolism. Based on initial findings consistent with this hypothesis, the work was expanded in two directions: (1) assessment of the effects of dry bean feeding on the activity of key enzymes that regulate lipid metabolism in rats; (2) the evaluation of dry bean effects on cholesterol metabolism in mice that were induced to be obese by feeding a high-fat diet.
Research design and methods
Experimental animals
Experiment 1
For this experiment, 19-d-old female Sprague–Dawley rats (n 42) were obtained from Taconic Farms and housed in a room maintained at 25°C with 30 % relative humidity and a 12 h light–12 h dark cycle. Animals were fed a standard laboratory animal diet American Institute of Nutrition (AIN)-93-G(Reference Reeves, Nielsen and Fahey29) until 27 d of age, followed by feeding of the experimental diets from 27 to 34 d of age. Rats were randomised to one of five groups that consumed diets containing: 0 % (n 18), 7·5 % (n 6), 15 % (n 6), 30 % (n 6) or 60 % (n 6) w/w dry red bean powder.
Experiment 2
For this experiment, sixteen female Sprague–Dawley rats (Taconic Farms), 27 d of age, were randomly divided into two groups (n 8) and fed one of two diets containing 0 or 60 % (w/w) dry bean for 7 d. The diet formulation was as described in Expt 1.
Experiment 3
For this experiment, forty C57BL/6J male mice fed an obesogenic high-fat diet from birth were obtained from Jackson Laboratory at 9 months of age. Mice were randomised to one of two dietary groups and fed 0 % (n 20) or 46·5 % (n 20) (w/w) dry bean in the high-fat diet (formulation in Table 1) for 12 d.
Table 1 High-fat diet composition*
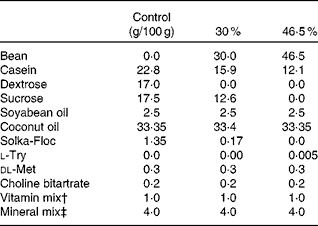
* The energy density of each diet was: 19·79, 21·92 or 23·26 kJ/g (4·73, 5·24 or 5·56 kcal/g) for the control, 30 and 46·5 % w/w fat diets, respectively.
† Vitamin mix AIN-93-VX(Reference Reeves, Nielsen and Fahey29).
‡ Mineral mix AIN-93-MX(Reference Reeves, Nielsen and Fahey29).
Experiment 4
For this experiment, eighteen C57BL/6J male mice, fed an obesogenic high-fat diet from birth were obtained from Jackson Laboratory at 9 months of age. Mice were randomised to one of three groups and assigned to the high-fat diet containing: 0 % (n 6), 30 % (w/w) dry bean (formulation in Table 1). Next, six mice were assigned to a low-fat diet (Research Diets formulation D12329). The experimental feeding was for 7 weeks. The work for all animal experiments reported was reviewed and approved by the Institutional Animal Care and Use Committee and conducted according to the committee guidelines.
Experimental diets
The diet formulation for Expts 1 and 2 was a modification of standard AIN-93-G diets (16·28 kJ/g (3·89 kcal/g)), and identical to the formulation used previously(Reference Thompson, Brick and McGinley30). The diet formulation used for Expts 3 and 4 is shown in Table 1. Dry bean (market class: small red) was provided by Archer Daniels Midland Company and was sent to Bush Brothers & Company for canning. Cooked beans were packed in standard brine without the incorporation of any additives. Beans were then sent to Van Drunen Farms where the beans were drained and freeze-dried. The freeze-dried bean was then milled into a homogeneous powder and sent to our laboratory and stored at − 20°C until incorporated into diets. Diets were formulated using specific guidelines(Reference Reeves, Nielsen and Fahey29) and adjusted using the proximate analysis of the bean powders (Warren Analytical). The diets were formulated to match macronutrient levels (i.e. protein, carbohydrate and crude fibre) across the diet groups. The differences in macronutrient composition were balanced with purified diet components. The percentage of dry bean incorporated into the diets is expressed as mass of bean powder in g/100 g of total diet. Control diets consisted of 7·5 % crude fibre to correspond to the dry bean diets. Study diets consisted of the standard diet with ground cooked red bean powder added to make up 7·5, 15, 30 or 60 % w/w of the diet for rat and 30 or 46·5 % red bean in high-fat diet formula for mice. Casein and maizestarch were adjusted to maintain similar macronutrient content across red bean dosage group as previously published(Reference Thompson, Thompson and Brick31). At all times during the study, animals had ad libitum access to food and water.
Necropsy
Necropsy occurred after the rats had received the experimental diets for 7 d in Expts 1 and 2 and after the mice had received the experimental diets for 14 d (Expt 3) or 49 d (Expt 4). Animals were killed via inhalation of gaseous CO2 followed by cervical dislocation, and the sequence in which the animals were euthanised was stratified across groups so as to minimise the likelihood that order effects would masquerade as treatment-associated effects. After the animals lost consciousness, blood was directly obtained from the retro-orbital sinus and gravity-fed through heparinised capillary tubes (Fisher Scientific) into EDTA coated tubes (Becton Dickinson). In Expts 1 and 2, the livers were immediately removed after plasma collection, freeze-clamped in liquid N2 and stored at − 80°C.
Body weight analysis
Animals in all experiments were weighed three times per week. The AUC of body weight was generated using GraphPad Prism 5 software (La Jolla, CA, USA) and analysed by Systat statistical analysis software, version 12 (Chicago, IL).
Plasma lipids analysis
Total cholesterol, HDL-cholesterol and TAG in plasma were determined enzymatically using a commercially available kit (Pointe Scientific, Inc.). Plasma LDL-C was calculated using the following formula: LDL-C = total cholesterol − (HDL-cholesterol+(TAG/5))(Reference Balal, Paydas and Inal32).
Plasma hormones and cytokines analysis
Insulin-like growth factor-1, insulin, leptin, IL-6 and TNF-α in plasma were determined using a commercially available ELISA or Multiplex kits (Millipore).
Western blotting analysis
Antibodies
Primary antibodies used in this study were anti-phospho-acetyl-CoA carboxylase, anti-acetyl-CoA carboxylase, anti-fatty acid synthase and anti-rabbit Ig-horseradish peroxidase-conjugated secondary antibody, as well as LumiGLO reagent with peroxide, all from Cell Signaling Technology. Anti-3-hydroxyl-3-methylglutaryl-CoA reductase, anti-stearoyl-CoA desaturase, anti-sterol regulatory element binding protein 1, anti-CYP7A and anti-goat Ig-horseradish peroxidase-conjugated secondary antibody were from Santa Cruz Chemicals. Mouse anti-β-actin primary antibody was obtained from Sigma Chemical Company.
Liver tissues were homogenised in lysis buffer (40 mm-Tris–HCl (pH 7·5), 1 % Triton X-100, 0·25 m-sucrose, 3 mm-ethylene glycol tetraacetic acid (EGTA), 3 mm-EDTA, 50 μm-β-mercaptoethanol, 1 mm-phenyl-methylsulfony fluoride and complete protease inhibitor cocktail (Calbiochem)). The lysates were centrifuged at 7500 g for 10 min in a tabletop centrifuge at 4°C and clear supernatant fractions were collected and stored at − 80°C. The protein concentration in the supernatants was determined by the Bio-Rad protein assay (Bio-Rad).
Western blotting was performed as described previously(Reference Jiang, Zhu and Thompson33). Briefly, protein lysate was subjected to 8–16 % SDS–PAGE and the proteins were transferred to a nitrocellulose membrane. The levels of phospho-acetyl-CoA carboxylase (Ser79), acetyl-CoA carboxylase, 3-hydroxyl-3-methylglutaryl-CoA reductase, fatty acid synthase, stearoyl-CoA desaturase, sterol regulatory element binding protein 1 and β-actin were determined using specific primary antibodies, followed by treatment with the appropriate peroxidase-conjugated secondary antibodies and visualised by LumiGLO reagent Western blotting detection system. The chemiluminescence signal was captured using a ChemiDoc densitometer (Bio-Rad) that is equipped with a charge-coupled device camera having a resolution of 1300 × 1030 and run under the control of Quantity One software (Bio-Rad) and analysed by the software. The actin-normalised scanning density data were reported.
Statistical analysis
Body weight, Lee Index (a measure of body fatness that parallels BMI in human; (body mass (g)0·33/nasoanal length (cm)) and plasma lipids, hormones and cytokines were analysed using ANOVA (repeated-measures ANOVA for body weight) followed by Bonferroni post hoc test. For Western blots, the data shown in the tables were either the actin-normalised scanning data for proteins or the ratio of the actual scanning units derived from the densitometric analysis of each Western blot for the phospho-proteins. For statistical analyses, the actin-normalised scanning density data obtained from the ChemiDoc scanner using Quantity One (Bio-Rad) were rank-transformed, an approach that is particularly suitable for semi-quantitative measurements that are collected as continuously distributed data as is the case with Western blots. The ranked data were then subjected to multivariate ANOVA(Reference Morrison34). Ratio data were computed from the scanning units derived from the densitometric analysis, i.e. the arbitrary units of optical density for variables stated and then the ratios were rank-transformed and evaluated via multivariate ANOVA. All analyses were performed using Systat statistical analysis software, version 12.
Results
Experiment 1
This study was conceived based on the observation that dietary cooked bean induced a dose-dependent increase in transcript levels of CYP7A in the liver, one of the proteins that regulates cholesterol saturation of bile acids(28). Induction of CYP7A was predicted to result in a dose-dependent reduction in plasma cholesterol. As shown in Table 2, total plasma cholesterol decreased with increasing dietary bean, although the decrease was only statistically significant at the highest two dietary concentrations. This observation is in agreement with earlier reports of cholesterol-lowering in a rat model of diet-induced hypercholesterolaemia as well as in a similar hypercholesterolaemia model that was implemented in the pig(Reference Costa, Walker and Low18–Reference Sarathy and Saraswathi23). Interestingly, the magnitude of cholesterol-lowering in the earlier studies that were of longer duration was of the order of 15–20 %. This reduction is comparable to that reported in Table 2. It is well known that the rat metabolises cholesterol differently than the human, given that it has low cholesteryl ester transport protein and high phospholipid transfer protein(Reference Cheung, Wolfbauer and Albers35, Reference Guyard-Dangremont, Desrumaux and Gambert36). Plasma HDL concentrations are regulated by and correlated inversely with plasma cholesteryl ester transport protein(Reference Tsutsumi, Hagi and Inoue37). Consequently, rats transport most of their cholesterol in the HDL fraction. This fraction is known to be refractory to alteration(Reference Turk and Laughlin38–Reference Moghadasian40), as was observed in the response shown in Table 2. On the other hand, LDL-C was reduced by bean feeding, an observation consistent with clinical findings(Reference Bazzano, Thompson and Tees16). Also noted in Table 2 is an effect of bean feeding on plasma TAG, although given the bi-phasic nature of this response, the biological significance of the changes observed is unclear.
Table 2 Effect of dietary bean (7 d) on plasma lipid profile and liver cytochrome P-450 cholesterol 7α hydoxylase (CYP7A) in rats
(Mean values with their standard errors)
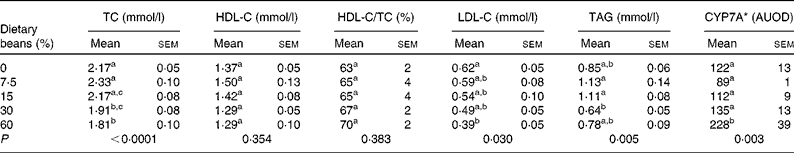
TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; AUOD, arbitrary units of optical density.
a,b,c Mean values within a column with unlike superscript letters were significantly different among those of dry beans (P < 0·05; ANOVA with post hoc comparisons by the method of Bonferroni).
* Values ( × 103) are determined by Western blotting.
Based on these observations, livers from the rats were probed for levels of CYP7A protein using a polyclonal rabbit antibody. As shown in Table 2, there was a graded increase in protein with higher levels of dietary bean, but the change was significant only at the highest dietary concentration. To further evaluate the observation of increased levels of CYP7A in the liver, plasma total cholesterol concentration was regressed on the hepatic concentration of CYP7A protein. An inverse relationship was observed, but it was not significant (r 2 0·04, P = 0·78). These results are not consistent with a causal relationship between induction of CYP7A and the observed reduction in plasma total cholesterol.
Experiment 2
Based on the results of Expt 1, the scope of mechanistic inquiry was broadened to determine if bean feeding was affecting lipid biosynthesis. The proteins selected for analysis are enzymes that control key steps in lipid biosynthesis in the liver. As shown in Table 3, while there was a pattern of protein expression consistent with the down-regulation of lipid biosynthesis, none of the differences was statistically significant. While this does not rule out the existence of differences in enzyme activity, the finding is consistent with the focus placed on cholesterol elimination and bile acid degradation in previous efforts to identify the cholesterol-lowering mechanisms associated with dry bean feeding(Reference Costa, Walker and Low18–Reference Sarathy and Saraswathi23).
Table 3 Effects of dry bean (7 d) on key regulatory proteins in lipid metabolism of rats
(Mean values with their standard errors)
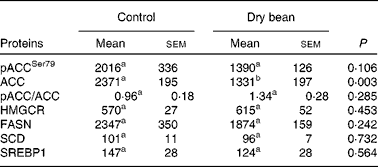
pACCSer79, phospho-acetyl-CoA carboxylase with phosphorylation at site of Ser79; ACC, acetyl-CoA carboxylase; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; FASN, fatty acid synthase; SREBP1, sterol regulatory element binding protein-1.
a,b Mean values within a row with unlike superscript letters were significantly different between control and 60 % (w/w) dry bean (P < 0·05; ANOVA).
Experiment 3
In Expts 1 and 2, there was no manipulation of cholesterol metabolism by feeding dietary cholesterol. This approach was in marked contrast to what has been reported in the literature, where the effects of bean feeding were assessed on diet-induced hypercholesterolaemia(Reference Costa, Walker and Low18–Reference Sarathy and Saraswathi23). To complement both approaches, work was extended to a diet-induced obesity model. Wild-type C57Bl/6 mice are highly susceptible to diet-induced obesity and the obesity syndrome that results is associated with alterations in lipid metabolism characteristic of metabolic syndrome, a risk factor for CVD(Reference Burrage, Baskin-Hill and Sinasac41–Reference Ley, Backhed and Turnbaugh46).
To initiate this experiment, obese mice that had been on an obesogenic high-fat diet throughout their lives were obtained from the vendor. The same high-fat diet formulation used by the vendor was modified as detailed in Table 1 to contain 46·8 % w/w bean, the maximum amount that could be incorporated into this formulation. As shown in Fig. 1, the initial results were quite unexpected. The obese mice fed the high-fat bean diet lost weight throughout the 12 d feeding study. Recognising the potential importance of the observation, body length was measured at necropsy and the Lee Index, a measure of adiposity, was computed(Reference Cox, Laughton and Powley47). As shown in Table 4, body weight and Lee Index were reduced by bean feeding. Total plasma cholesterol and LDL-C were reduced in bean-fed mice in comparison to the high-fat control group. As anticipated, treatment effects were not observed on either HDL-cholesterol or TAG, a finding consistent with the widely reported resistance of these measures to change in the mouse, an aspect of cholesterol metabolism that distinguishes mice from humans(Reference Turk and Laughlin38, Reference Moghadasian, Frohlich and McManus39). While recognising that the mouse is resistant to atherogenesis and that mice differ from humans in their metabolism of cholesterol, developing an assessment model using the mouse is attractive. The availability of genetically engineered mouse models affords an unprecedented opportunity to evaluate causal relationships and C57Bl/6 are commonly used to create these engineered mouse models(Reference Moghadasian, Frohlich and McManus39).

Fig. 1 Growth curves of mice fed either high-fat control (HF CTRL, ![]() ) or high-fat 60 % (w/w) small red dry bean (HF 60 % SR,
) or high-fat 60 % (w/w) small red dry bean (HF 60 % SR, ![]() ) for 12 d. Values are means, with their standard errors for each point represented by vertical bars (n 20). *The growth curves were statistically different (P < 0·05; repeated-measures ANOVA). **From 5 d post experimental diet until the end of the experiment, significant differences between HF CTRL and HF 60 % SR were observed (P < 0·05).
) for 12 d. Values are means, with their standard errors for each point represented by vertical bars (n 20). *The growth curves were statistically different (P < 0·05; repeated-measures ANOVA). **From 5 d post experimental diet until the end of the experiment, significant differences between HF CTRL and HF 60 % SR were observed (P < 0·05).
Table 4 Effects of dietary dry bean (12 d) on body weight and plasma lipid profile in the mouse
(Mean values with their standard errors)
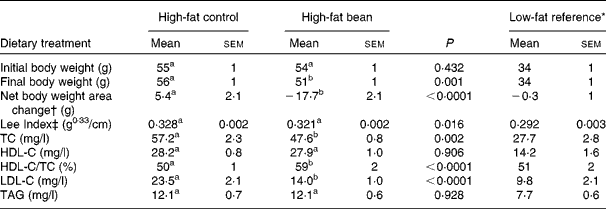
TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol.
a,b Mean values within a row with unlike superscript letters were significantly different between high-fat control and high-fat bean (P < 0·05; ANOVA).
* Mice in this group were fed a low-fat diet (Research Diets: D12923) from weaning. They were age-matched to the mice fed a high-fat diet and were maintained in the same animal holding room. These mice were not included in the statistical analyses presented in this table.
† Net body weight area changes were generated from area under body weight curves after comparing the body weight on different days of the experimental diet treatment using GraphPad Prism 5 software.
‡ Lee Index was calculated according to the formula, Lee Index = (body weight (g))0·33/(nose-to-anus length (cm))(Reference Cox, Laughton and Powley47).
Experiment 4
This study was undertaken to confirm the findings in Expt 3 that bean had effects on body weight regulation and plasma cholesterol. In this experiment, bean dose was reduced to 30 % w/w. Fig. 2 confirms that bean feeding resulted in initial weight loss in obese mice. In order to determine if the effect persisted over time, the impact on body weight was assessed for 7 weeks. While there was plateauing of the weight loss effect and some degree of recovery, a difference in body weights was maintained. Included in this experiment was a negative control group. These animals were switched to a low-fat diet at the same time that bean feeding was initiated. This change has been reported to result in weight loss, a finding confirmed in this experiment(Reference Burrage, Baskin-Hill and Sinasac41–Reference Ley, Backhed and Turnbaugh46). As shown in Fig. 2, the bean-fed and negative control groups had a similar pattern of weight change. The negative control provides an example of the growth pattern for which dry bean varieties can be screened. Table 4 quantifies these effects. Bean feeding reduced weight relative to the high-fat control group over the course of the experiment, but the 4 g difference in final body weights between groups was not statistically significant. Nonetheless, the body composition of the bean-fed mice was shifted to a less obese state as indicated by the reduction in the Lee Index and lower plasma leptin, an indicator of adipocyte mass(Reference Cox, Laughton and Powley47, Reference Hajer, van Haeften and Visseren48). As in Expts 1–3, plasma total cholesterol (P = 0·032) and LDL-C (trend P = 0·067) were also reduced in the bean-fed obese mice.

Fig. 2 Growth curves of mice fed high-fat (HF, ![]() ) diet, HF diet including 46·5 % (w/w) small red bean (SR,
) diet, HF diet including 46·5 % (w/w) small red bean (SR, ![]() ), or low-fat diet switched from HF diet (HF–LF,
), or low-fat diet switched from HF diet (HF–LF, ![]() ) for 7 weeks. Values are means, with their standard errors for each point represented by vertical bars (n 8). The growth curves among groups were significantly different (P < 0·05; repeated-measures ANOVA). HF, SR or HF–LF growth curves were significantly different from each other (P < 0·05 for each paired comparison; post hoc comparisons by the method of Bonferroni).
) for 7 weeks. Values are means, with their standard errors for each point represented by vertical bars (n 8). The growth curves among groups were significantly different (P < 0·05; repeated-measures ANOVA). HF, SR or HF–LF growth curves were significantly different from each other (P < 0·05 for each paired comparison; post hoc comparisons by the method of Bonferroni).
Discussion
The traditional approach in experimental nutrition has been to conduct 28 d animal feeding studies in order to assess the effects of a dietary intervention. This approach has served the field well; however, the use of shorter time frames, frequently 7–14 d in duration, is increasing and has been reported to have greater sensitivity to detect cellular and molecular responses that might otherwise go unobserved(Reference Narasaka, Endo and Fu49). Other than this enhanced sensitivity, short-term feeding studies have the inherent benefit of reducing the amount of material needed for evaluation as well as the time and cost of an experiment. These considerations led to the design of the short-duration experiments reported in this investigation.
In the present study, two rodent models involving rats or mice were used to investigate the effect of consumption of cooked dry bean incorporated into a purified diet formulation on aspects of lipid metabolism associated with CVD risk. Clear evidence was obtained that dietary bean reduced circulating levels of total cholesterol and LDL-C in rats and mice. An initial screening of rat liver for the effects of dry bean on regulatory enzymes involved in fatty acid or cholesterol biosynthesis was negative, indicating that a focus on cholesterol and bile acid secretion and degradation in the gut should receive priority in future studies of mechanism. Our results indicate that a very simple 7 d feeding study with plasma total cholesterol as an endpoint can be used for screening bean varieties. To illustrate the intent of screening for cardioprotective activity, the objective would be to identify cultivars that give the same effect on plasma cholesterol at 7·5 % w/w in the diet as the 60 %w/w concentrations of bean reported in Table 2.
The effect of dry bean feeding was also assessed in a mouse model of diet-induced obesity that is being used by many laboratories to gain insights into disease mechanisms associated with obesity. The finding that bean feeding not only lowered total cholesterol (P = 0·032) and LDL-C (P = 0·067), but also had anti-obesogenic activity is of potential interest. The observed effects on change in body weight and body composition were unexpected because we have not observed dry bean to affect weight gain in non-obese rats(Reference Thompson, Thompson and Brick31). Despite the fact that no previous reports of such an effect were found in the literature, the observed changes in body weight and body fat as reflected in the Lee Index and plasma leptin data are plausible, given the evidence that bean feeding can alter the gut microbiome(Reference Bennink and Rondini14) and that such changes have been reported to influence body weight regulation and obesity(Reference Rezzi, Martin and Shanmuganayagam44–Reference Ley, Backhed and Turnbaugh46, Reference Backhed, Ding and Wang50) (Table 5).
Table 5 Effects of dietary beans (7-week) on body weight and plasma lipid profile in the mouse
(Mean values with their standard errors, n 6)
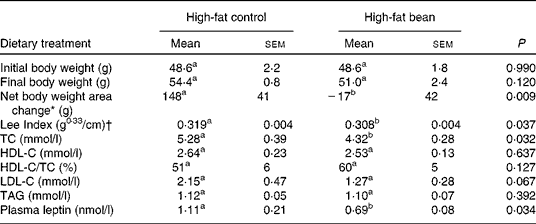
TC, cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol.
a,b Mean values within a row with unlike superscript letters were significantly different between high-fat control and high-fat bean (P < 0·05; one-tailed t test).
* Net body weight area changes were generated from area under body weight curves after comparing the body weight on different days of the experimental diet treatment using GraphPad Prism 5 software.
† Lee Index was calculated according to the formula, Lee Index = (body weight (g))0·33/(nose-to-anus length (cm))(Reference Cox, Laughton and Powley47).
Summary and conclusions
While the effect of dry bean consumption on whole-body energetics requires additional scrutiny, these observations have the potential to draw together two seemingly unrelated global issues, the obesity pandemic that if unabated will wreak social and economic havoc globally because of the associated increase in chronic diseases that accompany obesity, and the emerging crisis in global food security that will undoubtedly refocus attention on the role of pulses as a staple food crop.
Acknowledgements
The present study was supported in part by USAID grant no. REE-A-00-03-00094-00, the Bean Health Alliance, and United States Department of Agriculture National Institute of Food and Agricultural Agriculture and Food Research Initiative Project no.: 2009-01929. The authors thank Amanda Blasingame, Erica Danielle, John McGinley, Angie Neil, Elizabeth Neil and Denise Rush for their excellent technical assistance. Z. Z. and W. J. conducted the analyses of plasma and tissue and participated in data evaluation. H. J. T. designed the experiments and assisted with data evaluation; and also provided oversight of all aspects of the experiment. All authors participated in the writing of the manuscript. The authors declare that there are no conflicts of interest.









