More than three decades ago, the American Academy of Pediatrics recommended starting the primary prevention of atherosclerosis as early as 2 years of age1. Since then, autopsy studies have demonstrated that early coronary atherosclerosis or precursors originate in childhood and adolescence, and are correlated with high serum total cholesterol (TC) and LDL-cholesterol (LDL-C) levels, and low HDL-cholesterol (HDL-C) levels. Monogenic factors that cause high cholesterol levels include familial hypercholesterolaemia (FH). Polygenic hypercholesterolaemia (PH) results from the expression of multiple genes combined with environmental effectors such as diet high in saturated fat and cholesterol levels as early as during childhoodReference Goldstein, Schrott, Hazzard, Bierman and Motulsky2. There are documented data that in adolescents with FH functional and morphological signs of premature atherosclerosis can already be endetected and it is assumed that these deleterious sequelae may be postponed by an early reduction in atherogenic lipoproteinsReference Hoffmann, Dirisamer, Heher, Kostner, Widhalm and Neunteufl3. Moreover, serum cholesterol levels above the 95th percentile (i.e. > 1300 mg/l) due to PH should also be encountered4. Thus, it is generally accepted within the paediatric field that children and adolescents affected by hypercholesterolaemia should be treated as soon as possible in order to reduce their elevated cholesterol levels. The cornerstone of all therapeutic regimens is the dietary approach. By means of a classical diet (low-fat, high-MUFA and -PUFA) a decrease of LDL-C in the range of 10–15 % can be achievedReference Keys, Anderson and Grande5–Reference Mensink and Katan7. Moreover, soya protein has well-documented beneficial effects on serum lipid levels in adults, the changes being related to the level and duration of intake and initial serum lipid concentrationsReference Zhan and Ho8. The American Heart Association Nutrition Committee has recently summarised available adult data, indicating that the beneficial LDL-C-lowering effect is within the range of a few percentage pointsReference Sacks, Lichtenstein, Van Horn, Harris, Kris-Etherton and Winston9. Also, diets with substitution of animal protein by soya protein have an additional lowering effect in some but not in all patientsReference Glueck, Tsang, Fallat and Mellies10–Reference Widhalm, Brazda, Schneider and Kohl12.
Acknowledging this body of information in the adult population, the lack of studies assessing the potential beneficial effect of soya protein in children and adolescents affected by FH appears surprising, especially in the face of the cited need for early preventive measures. This is particularly true when it comes to the evaluation of the effect and feasibility of such an intervention over a prolonged period of time, thus depicting a ‘real-life scenario’ v. a mere short-term intervention. In this regard, acceptance of soya products is of great importance. Information on whether or not soya products are accepted by the patient is key to the concept of long-term therapy.
The present study aimed to examine whether a sustained cholesterol-lowering effect of a soya protein diet can be achieved in children and adolescents with proven FH and PH during a period of at least 3 months. In addition, acceptance and preference of soya products was assessed.
Patients and methods
Subjects
Initially, twenty-three patients (twelve males; eleven females), eight with FH (four males; four females), fifteen with PH (eight males; seven females) who presented sequentially to the Paediatric Out-patient Department of Clinical Nutrition and Metabolism at Vienna Medical University Hospital for treatment were included in the programme. Mean age was 8·8 (sd 4·2) years (range 4–18 years). All patients were otherwise healthy with BMI being 16·7 (sd 2·6) kg/m2 at baseline (Table 1). FH was diagnosed either by LDL-receptor gene analysis or if the following criteria were fulfilled: LDL-C > 1550 mg/l, TC > 2700 mg/l, TAG < 1000 mg/l and at least one family member diagnosed with FHReference Williams, Schumacher, Barlow, Hunt, Ware, Pratt and Latham13. Patients were considered to have PH if LDL-C exceeded 1300 mg/l without MEDPED criteria being fulfilled. Patients on treatment with lipid-lowering drugs or medication known to influence lipid metabolism were excluded. Finally, data of sixteen patients (ten male and six females; eight patients with FH (four male and four female) and eight patients with PH (six male and two female)) were available for evaluation. Thus, seven patients had to be excluded, two of them having been diagnosed with soya protein allergy, and five not adhering to either phase 1 or phase 2 diet as documented in repeated dietary records. Patients with FH and PH had significantly different baseline TC and LDL-C levels (351·4 (sd 91·9) v. 257·6 (sd 30·4) mg/l and 253·4 (sd 56·4) v. 178·3 (sd 33·3) mg/l). The patients excluded did not differ from those included in regard to age, BMI and baseline serum levels of the measured lipoproteins. All procedures were performed according to the Helsinki Declaration of 1975 as revised in 1996 and according to guidelines of Good Clinical Practice.
Table 1 Patient characteristics (n 16)
(Mean values and standard deviations)

Methods
All subjects underwent a thorough physical examination. Blood samples were taken for determination of fasting serum lipoproteins (TC, LDL-C, HDL-C and TAG) at baseline, after 3 months (end of phase 1) and after 6 months (end of phase 2) following an overnight fast. Cholesterol and TAG levels were measured enzymically by using test kits from Boehringer Mannheim GmbH (Mannheim, Germany): CHOD-PAP and GPO-PAP (enzymic colorimetric methods), respectively. VLDL were removed by ultracentrifugationReference Widhalm, Maxa and Zyman14. Apo A1, B and lipoprotein(a) were measured by nephelometry with test kits from Boehringer Mannheim.
Diet
After inclusion into the programme patients were to follow a diet reduced in SFA, modified in fatty acid pattern (increase in MUFA and PUFA) and rich in complex carbohydrates and phytonutrients for 3 months (phase 1 diet). Thereafter, patients and their parents were instructed to include soya protein of at least 0·25 g/kg body weight into their diet for a period of 3 further months, thereby substituting soya protein for animal protein (Fig. 1). This was achieved by the introduction of soya foods (Alpro soya dairy-free milk alternatives), which were consumed substituting dairy protein and/or a mixture of other animal proteins. Each patient was instructed to consume a minimum amount of soya products per d. Adherence to both types of diet was assessed by means of multiple 1-week dietary records, which a registered dietitian discussed with the patient and parents in detail whereby written material containing personalised dietary recommendations was provided. Patients and their families were seen weekly throughout the first month, thereafter after 2 and 3 months of each phase. If a patient did not show up to an appointment, an alternative appointment was sought by the dietitian, thereby maximising adherence. Quantitative fatty acid intake was calculated by analyses of the dietary records. The content of isoflavones in the soya products used was on average 27 mg isoflavones per 10 g soya.
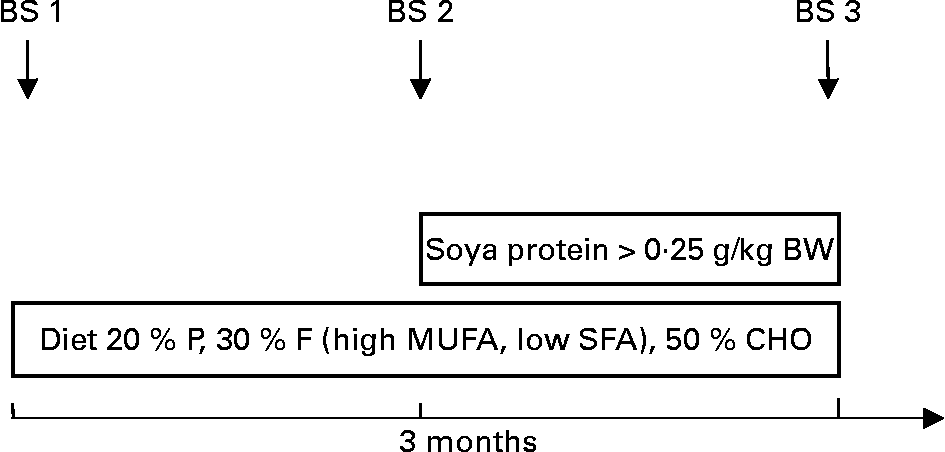
Fig. 1 Study protocol. BS, blood sample; BW, body weight; P, protein; F, fat; CHO, carbohydrates.
Assessment of acceptance and preference of soya products
A questionnaire was handed out to patients, all of which were returned. Patients had to judge the overall taste of the consumed products as ‘very good’, ‘fair’ and ‘bad’. In addition, patients were asked to list their three favourite products, from which a ranking was made.
Statistical analysis
Comparisons between baseline and post-intervention data were tested by an unpaired t test with a significance level of 5 % and two-sided 95 % confidence limits. Calculations were conducted using Statistical Analysis Software (version 8.2; SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics are presented in Table 1. BMI did not change significantly during both phases (data not shown).
Adherence to diet
Fatty acid intake, presented as percentage of total dietary fat intake, is shown in Fig. 2. SFA intake decreased significantly by 20 % from baseline to the end of phase 1 (46·1 (sd 5·1) v. 36·9 (sd 0·9) %, respectively; P < 0·0001) and by 18 % from the end of phase 1 to the end of phase 2 (36·9 v. 30·3 %, respectively; P < 0·0001). At the same time, intake of PUFA significantly increased by 44·1 % during phase 1 (17·9 (sd 5·1) v. 25·8 (sd 0·9) %, respectively; P < 0·0001) and by 32·9 % during phase 2 (25·8 (sd 0·9) v. 34·3 (sd 4·7) %, respectively; P < 0·001). The SFA:PUFA ratio decreased from 2·6 to 1·4 to 0·9, respectively (P < 0·0001). TC intake was 242 (sd 56) mg at baseline, being significantly lower after phase 1 (132 (sd 23) mg; P < 0·0001) and phase 2 (110 (sd 11) mg; P < 0·01). Dietary intake of MUFA did not change significantly during the phases. Average soya protein intake during phase 2 was 0·50 (sd 0·20) g/kg body weight, with no statistically significant difference between patients with PH and FH.
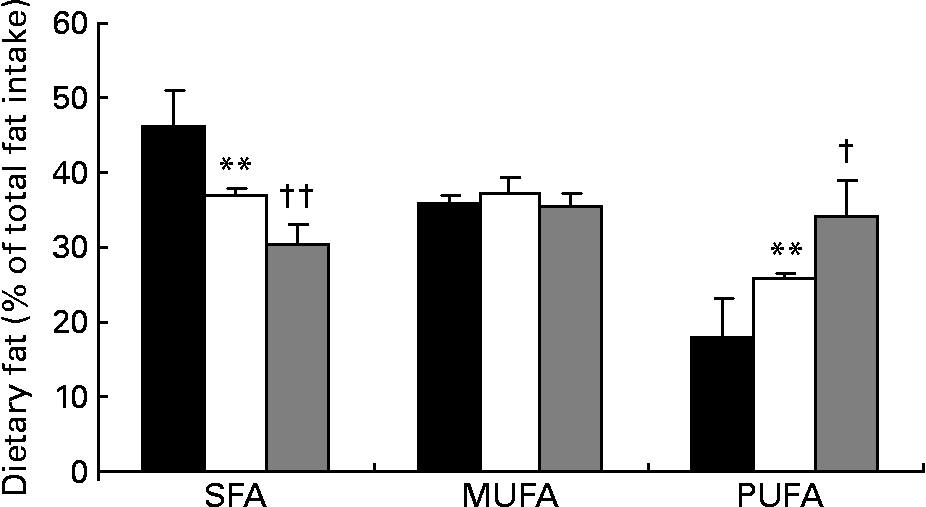
Fig. 2 Intake of dietary fat in percentage of total fat intake at baseline (■), after phase 1 (□) and after phase 2 (▒). Values are means, with their standard deviations represented by vertical bars.*Mean value is significantly different from that at baseline (P < 0·0001). Mean value is significantly different from that at the end of phase 1: † P < 0·001, †† P < 0·0001.
Serum cholesterol levels
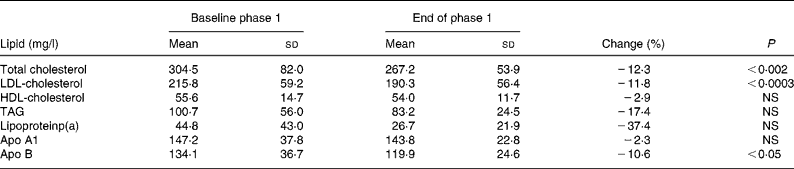
The phase 1 diet resulted in a significant reduction of serum concentrations of TC, LDL-C and apo B by 12·3, 11·8 and 10·6 %, respectively, serum concentrations of HDL-C, TAG, apo A1 and lipoprotein(a) not yielding statistical differences (Table 2).
Table 2 Serum lipid concentrations at baseline and after the phase 1 diet
(Mean values and standard deviations for sixteen subjects)
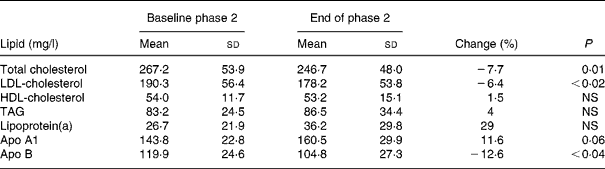
Dietary intake of soya protein during phase 2 resulted in a significant decrease of serum concentrations of TC, LDL-C and apo B by 7·7, 6·4 and 12·6 %, respectively. Serum concentrations of TAG, HDL-C, apo A1 and lipoprotein(a) did not change significantly (Table 3).
Table 3 Serum lipid concentrations before and after the phase 2 (soya-substituted) diet
(Mean values and standard deviations for sixteen subjects)
‘Non-responders’ v. ‘responders’ to soya protein
While twelve of the sixteen patients presented with lower TC levels, for four patients no decrease of TC was seen at the end of phase 2. The former subjects were considered to be ‘responders’, the latter ‘non-responders’. There was no significant difference between the groups in regard to age, BMI, change in BMI, relative amount of SFA, MUFA and PUFA intake during the phases, and baseline serum levels of the measured lipoproteins (data not shown). Table 4 presents laboratory data of the groups. Serum levels of HDL-C and apo A1 of responders v. non-responders were comparable at the end of phase 1, but significantly differed at the end of phase 2 (Table 4).
Table 4 Serum lipid concentrations at baseline, at the end of the phase 1 diet and at the end of the phase 2 diet for ‘responders’ (R; n 12) and ‘non-responders’ (NR; n 4) to soya protein
(Mean values and standard deviations)
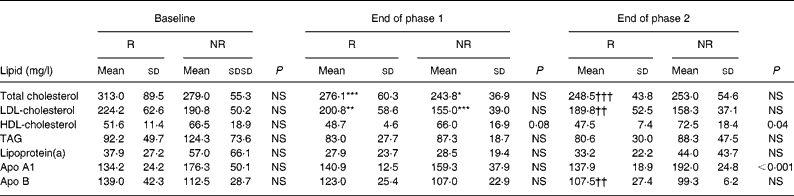
Mean value is significantly different from that of the same group at baseline: * P < 0·05, ** P = 0·02, *** P = 0·01.
Mean value is significantly different from that of the same group at the end of phase 1: †† P = 0·02, ††† P < 0·01.
Level of acceptance and preference
Six patients qualified the consumed soya products as ‘very good’, nine as ‘fair’ and none as ‘bad’. Alpro chocolate soya drink and dessert were ranked top before vanilla soya drink and dessert, plain yoghurt alternative and strawberry drink.
Discussion
To the best of our knowledge the present study is the first to show that prolonged substitution of soya protein for animal protein in a low-fat, fat-modified diet is of additional benefit when aiming to lower serum concentrations of TC and LDL-C in children and adolescents with FH and PH. Moreover, the present study underscores the importance of diet as the cornerstone of lipid-lowering therapy.
The effect of a diet low in SFA and high in unsaturated fatty acids (phase 1 diet) on reduction in TC and LDL-C achieved by our children is in accordance with other studies in children, adolescents and adultsReference Keys, Anderson and Grande5, Reference Grundy6. While a recent meta-analysis on the effect of soya protein on the lipid profile in adults identified thirteen studies with a duration of 12 and more weeks (maximum 26 weeks)Reference Zhan and Ho8, previous studies in children and adolescents have demonstrated a beneficial effect of a soyabean-substituted diet with hypercholesterolaemia only for short-term interventionsReference Widhalm, Brazda, Schneider and Kohl12, Reference Laurin, Jacques, Moorjani, Steinke, Gagne, Brun and Lupien15. The present study participants achieved a significant reduction of TC and LDL-C in an extended design of 3 months. The extent of the reduction in TC and LDL-C is comparable with those presented in the meta-analysis of Andersen et al. with baseline TC between 2600 and 3350 mg/l in adultsReference Anderson, Johnstone and Cook-Newell16, yet more than reported by Sacks et al. Reference Sacks, Lichtenstein, Van Horn, Harris, Kris-Etherton and Winston9. These authors stated in their American Heart Association Science advisory that a very large amount of soya protein, more than half the daily protein intake, may lower LDL-C by a few percentage points when it replaces dairy protein or a mixture of animal proteinsReference Sacks, Lichtenstein, Van Horn, Harris, Kris-Etherton and Winston9. Two previous studies investigating the effect of a soya protein-substituted diet in children in a shorter design found a significant larger reduction of TC and LDL-CReference Gaddi, Descovich, Noseda, Fragiacomo, Nicolini, Montanari, Vanetti, Sirtori, Gatti and Sirtori11, Reference Widhalm, Brazda, Schneider and Kohl12. The difference may be explained by the greater hypocholesterolaemic effect of the run-in (phase 1) diet in the present study, and possibly by a negative dose–response relationship between length of intervention and the decline in LDL-C, as reported in adults by Zhan & HoReference Zhan and Ho8. However, it is necessary to acknowledge that not all of the patients achieved a reduction in TC and LDL-C in the present study. Our data demonstrate that serum levels of TC and LDL-C were not decreased by the addition of soya protein in four of the sixteen patients, even though the ‘non-responders’ had lower levels of TC and LDL-C after introduction of the phase 1 diet. In comparison, Rose et al. reported a reduction in only half of the children with FHReference Rose, Allen, Pearse and Chapell17. Introduction of a soya protein-substituted diet did not result in a significant change in serum levels of HDL-C in the present study group. This is in accordance with someReference Teixeira, Potter, Weigel, Hannum, Erdman and Hasler18–Reference Hoie, Graubaum, Harde, Gruenwald and Wernecke20 but in contrast to other adult studies showing moderate increases in HDL-CReference Anderson, Johnstone and Cook-Newell16. Yet, apo A1 levels were increased by trend, which is in agreement with studies demonstrating increased apo A1 secretion and gene expression in liver cellsReference Lamon-Fava21. On the other hand, it must be mentioned that while there was no significant difference regarding age, BMI, change in BMI, relative amount of SFA, MUFA and PUFA intake during the phases, and baseline serum levels of the measured lipoproteins, the group of ‘non-responders’ tended to have higher levels of HDL-C and apo A1 at the end of phase 1. Moreover, patients regarded as ‘non-responders’ had significantly higher concentrations of serum HDL-C and apo A1 than their TC- and LDL-C-responsive counterparts after inclusion of soya protein into their diet. Thus, ‘responders’ and ‘non-responders’ yielded beneficial effects of soya protein substitution, although on different serum lipoproteins. This variable lipaemic response has also been shown to be existent in healthy, normolipaemic menReference Nilausen and Meinertz22. We can only speculate on the reason of this variable response, differing genetic background, i.e. underlying mutations of the LDL-receptor, being a possible cause. However, interpretation of these data demands precaution since serum lipoprotein levels can change substantially from day to day. Also, the small number of patients in the ‘non-responder’ group needs to be taken into consideration. Therefore, further studies specifically looking at the responder v. non-responder issue are warranted.
As far as the mechanisms underlying the cholesterol-lowering effect of soya protein are concerned, multiple explanations have been presented. There is evidence that soya protein enhances LDL-receptor activity in FHReference Lovati, Manzoni, Canavesi, Sirtori, Vaccarino, Marchi, Gaddi and Sirtori23. In this regard, LDL-receptor expression in the liver has been shown to be stimulated by the 7S-globulin of soya proteinReference Gianazza, Eberini, Arnoldi, Wait and Sirtori24. This isolated protein component and its active subfractions might stimulate LDL-receptors by direct interaction with gene promotersReference Manzoni, Duranti, Eberini, Scharnag, März, Castiglioni and Lovati25. Other studies suggest an association with factors involved in the digestion of the proteins, absorption of their constituent amino acids, and with mechanisms of both catabolism and excretion of cholesterolReference Huff and Carroll26, Reference West, Beynen, Terpstra, Scholz, Carroll and Woodward27. The approximate 13 % decrease in apo B paralleling levels of TC and LDL-C in our patients is in accordance with data showing that the soya phyto-oestrogens genistein and daidzein decrease apo B secretion from HepG2 cells through multiple mechanisms by lowering cholesterol synthesis, cholesterol esterification, microsomal TAG transfer protein, and enhancing LDL-receptor activityReference Borradaile, de Dreu, Wilcox, Edwards and Huff28. Moreover, soya protein has been suggested to act as a strong antioxidantReference Castiglioni, Manzoni, D'Uva, Spiezie, Monteggia, Chiesa, Sirtori and Lovati29.
It needs to be taken into consideration that even though we had a 3-month run-in phase, we could not test the effect of a soya-substituted diet against a control group. This was due to the fact that almost all parents of the children eligible for the study insisted on their child receiving any possible nutritional therapy, thus being in the intervention group.
In addition, it is of note that the present study allows us to positively answer the question whether it is feasible to introduce soya products into the diet of children and adolescents over a period of at least 3 months. Evaluation of dietary protocols and specifically designed questionnaires revealed high levels of acceptance, which is a precondition for this type of dietary therapy. Importantly, in order to achieve high compliance, adherence to diet must be monitored closely by the dietitian (for practical reasons at least monthly during the first couple of months) in cooperation with the paediatrician following a family approach.
Although several studies have documented the safety of exposure to soya-based formula in infancy on endocrinological and reproductive outcome in young adulthoodReference Strom, Schinnar, Ziegler, Barnhart, Sammel, Macones, Stallings, Drulis, Nelson and Hanson30 there is hardly any data on the safety of prolonged soya intake in children. It is thus not surprising that the scientific community has seen a recent discussion on potential dangers of regular soya intake in the long runReference Siegel-Itzkovich31.
In conclusion, diet is indisputably the cornerstone in the treatment of paediatric patients with elevated serum cholesterol concentrations when aiming at lowering cholesterol levels and thereby postponing the use of cholesterol-lowering agents. Inclusion of soya products may offer an additional long-term dietary option in hypercholesterolaemic paediatric patients.
Acknowledgements
We gratefully acknowledge the excellent support provided by the staff of the Paediatric Out-patient Department at Vienna Medical University Hospital. We thank C. Franz, A. Fekete and S. Daemon for their expert support with blood sampling and evaluation of the dietary records.








