The viscous soluble fibre found in oats ((1 → 3),(1 → 4) β-d-glucan) has been demonstrated to have cholesterol-lowering effects(Reference Ripsin, Keenan and Jacobs1, Reference Brown, Rosner and Willett2). However, despite the majority of trials showing a cholesterol-lowering effect in hypercholesterolaemic subjects, no clear dose–response relationship has been demonstrated.
In addition, not all oat products show similar effects(Reference Poppitt3–Reference Naumann, van Rees and Onning5). The cholesterol-lowering properties appear to be linked to the physico-chemical properties of the soluble β-glucan fraction, rather than the total soluble fibre content per se (Reference Wolever, Tosh and Gibbs6). Putative effects have been attributed to an influence on the sequestering of bile acids in the gut, reducing re-absorption and return to the liver for further synthesis(Reference Andersson, Ellegard and Andersson7–Reference Ellegard and Andersson9). The direct effects of oat bran on cholesterol levels might also be better seen in the immediate postprandial period through a dramatic effect on decreased chylomicron cholesterol(Reference Lia, Andersson and Mekki10).
The cholesterol-lowering effects of β-glucan may be influenced by a number of factors, primarily the molecular weight, solubility and viscosity in the product (as consumed) and these are dependent on the food microstructure, dosage and the type of food processing that has been undertaken. For example, the process of enrichment may affect efficacy(Reference Poppitt3). Possible unfavourable structural changes that occur to β-glucan during commercial extraction include depolymerisation of the linear structure which decreases molecular weight and viscosity(Reference Wursch and Pi-Sunyer11). Under mild extraction conditions, endogenous β-glucanase enzymes may not be deactivated and thereby further increase depolymerisation(Reference McLeary12) which could lower efficacy. Endogenous β-glucanase enzymes are also active during food preparation(Reference Aman, Rimsten and Andersson13), causing degradation of β-glucan in food products. In addition, freezing and storage may reduce the extractability of β-glucan in the intestine(Reference Beer, Wood and Weisz14). On the other hand, processes in which oats are heated in the presence of water, such as baking, boiling and extrusion(Reference Regand, Tosh and Wolever15, Reference Beck, Tosh and Batterham16), increase the solubility of β-glucan and viscosity which has been associated with an increase in bioactivity(Reference Wolever, Tosh and Gibbs6, Reference Lan-Pidhainy, Brummer and Tosh17). Variation is in part explained by the physico-chemical properties of the β-glucan in the food and the diet overall(Reference Wood18). Thus, the level of β-glucan administered is not the only factor to consider when evaluating the science.
In 1997, the US Food and Drug Administration(19) approved health claims for oat fibre, based on the relationship demonstrated between dietary soluble β-glucan fibre and a decrease in serum cholesterol concentrations. The Food and Drug Administration has concluded that fibre is efficacious in lowering total cholesterol and LDL-cholesterol (LDL-C) by about 5–10 %, at doses of 3 g/d from either oat bran or rolled oats. This amount is provided by approximately 55 g oat bran (minimum 5·5 % β-glucan) or 75 g rolled oats (4 % β-glucan). More recently, the European Food Safety Authority(20), French Agency for Food, Environment and Occupational Health & Safety(21), the Joint Health Claims Initiative in the UK and the Food Directorate, Health Products and Food Branch of Health Canada have permitted claims for cholesterol-lowering effects at this level of β-glucan intake(22).
The present study aimed to assess the ability of two different doses of oat β-glucan delivered in different food formats (rolled oat porridge, a cereal bar and ready-to-eat (RTE) oat flake breakfast cereal) to lower cholesterol in mildly hypercholesterolaemic Australian adults.
Methods
Participants
The present study was a 6-week parallel, randomised, controlled, single-blind trial conducted with three arms. The two intervention groups were provided with oat porridge and oat-based cereal bars (group oats high (OH), higher β-glucan, 3·2 g/d) and RTE oat flakes and puffed rice and wheat bars (group oats low (OL), lower β-glucan, 1·5 g/d), respectively. The control group (minimal β-glucan) received cornflakes, puffed rice and wheat bars in plain packs.
Volunteers for the study were recruited via advertisements in the local media, including newspaper, television and radio. Approaches were also made to local medical practices, ambulance and fire services and via university staff emails. Inclusion criteria were as follows: men 25–75 years and premenopausal women >25 years or 5 years postmenopause (but not on hormone replacement therapy) with total serum cholesterol ≥ 5 and < 7·5 mmol/l; BMI >20 and < 32 kg/m2; regular breakfast cereal consumer (four to five times/week); stable weight (within 3 kg over past 3–6 months); and of general good health. Exclusion criteria include the following: major illnesses (including diabetes mellitus, known CHD and chronic renal failure); lipid-lowering medication; familial hypercholesterolaemia; fasting glucose >5·6 mmol/l; total cholesterol >7·5 mmol/l; taking dietary supplements that could influence cholesterol (e.g. fish oil); pregnancy/lactation; food allergies or habits inhibiting compliance with the study design; illiteracy and inadequate conversational English.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Human Research Ethics Committee of the University of Wollongong (HE08/036). Written informed consent was obtained from all participants.
Baseline period
After eligibility had been determined on the basis of a telephonic interview and a fasting screening blood test to assess total cholesterol concentrations, eligible participants completed a computerised 7 d diet-history interview (DietAdvice, Xyris Software, 2009; Highgate Hill, QLD, Australia) to assess habitual nutrient intake. Dietary data were analysed using the FoodWorks software system (Xyris Software, version 5, 2007). Nutrient intake data were analysed in terms of energy and macronutrients using the AusNut (Allfoods) Revision 18 database. At 1 week later, eligible participants were randomly assigned to one of three diet groups and attended the university clinic for a dietary counselling session with a dietitian.
Randomisation and concealment
Randomisation was performed by a researcher independent of the subject interface using the RALLOC command in STATA (version 10.1; College Station, TX, USA). The procedure was stratified by sex and used permuted blocks, with the size and order of the blocks being random. All test foods were packaged in plain, opaque coded wrapping at the point of manufacturing and factory packaging. Since dietary advice was given in terms of food groups, it was not possible to blind the dietitians, but the participants were not informed as to their diet group allocation.
Interventions
Test foods
The between-group dietary difference occurred with the provision of alternative cereal foods in plain individual portion packs, batch packaged and provided by Cereal Partners Worldwide Limited (Rutherglen, VIC, Australia). Participants were required to consume one packet of cereal and one cereal bar per d with a minimum of five of each per week. β-Glucan analyses (AOAC (Association of official Analytical Chemists) 995.16) of the products were provided by Medallion Labs, Minneapolis, MN, USA. Cereal bars contained 1·99 % β-glucan; RTE oat flakes, 2·42 % β-glucan; and oat porridge, 4·17 % β-glucan. Control foods (RTE cornflakes and puffed rice bars with no β-glucan) were not analysed as maize and rice do not contain significant quantities of β-glucan. The amount of β-glucan provided in the product servings is shown in Table 1. The OH group received 3·24 g β-glucan/d and the OL group received 1·45 g β-glucan/d. The viscosity, solubility and molecular weight of all test foods were analysed by the Guelph Food Research Centre, Agriculture and Agri-Food, Canada. β-Glucan was extracted from the food samples (10 g dry weight basis) using conditions similar to those found in the upper gastrointestinal system. The extraction was done on 10 g of food at 37°C with digestive enzymes. The physiological extraction and characterisation of soluble β-glucans were carried out as described previously(Reference Tosh, Brummer and Miller23). The viscosity of the extract was measured using a rheometer with a cone-and-plate configuration. The solubility of β-glucan was calculated from the total β-glucan and the amount solubilised during the extraction process. The molecular weight of β-glucan in solution was analysed using size-exclusion HPLC. Measurements were done on duplicate extractions.
Table 1 Physico-chemical properties of test foods
(Mean values and standard deviations)
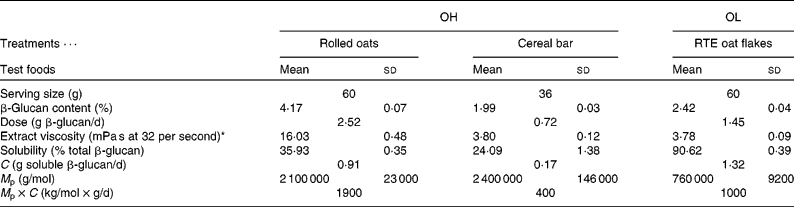
OH, oats high; OL, oats low; RTE, ready-to-eat; C, daily dose of soluble β-glucan; M p, peak molecular weight.
* Viscosity of physiological extract from 10 g cereal.
Dietary prescription
Each group was advised by Accredited Practising Dietitians on a healthy low-fat diet utilising the core food groups outlined in the Australian Guide to Healthy Eating(24). Energy intakes were calculated to equate to individuals' estimated energy requirements for weight maintenance using the Mifflin equation(Reference Mifflin, St Jeor and Hill25) and applying a physical activity factor of 1·25. Cereals and cereal bars provided were modelled into the dietary prescription to achieve macronutrient intakes of approximately 45–50 % carbohydrates, 20–25 % energy (E%) protein and 25–30 E% fat (Table 2). Participants were provided with educational material and eating plans. Participants were instructed not to eat products which would otherwise alter cholesterol such as those containing plant sterols and fish oil/n-3 supplements while in the study and were given details of such foods. Dietitians monitored participants throughout the study at follow-up appointments at 3 and 6 weeks.
Table 2 Baseline characteristics of the study subjects
(Mean values and standard deviations)

OH, oats high; OL, oats low; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Participants were issued with a diet compliance booklet in which they noted how many serves from each food group were consumed daily and completed a checklist of the number of packets of cereal or study bars consumed. Diet compliance booklets were returned at completion of the study.
Procedures
Each participant attended the University clinic to receive their 3-week supply of study foods and for dietary assessment and dietary counselling at commencement of the study and after 3 weeks. Measurements were taken at the 3-week follow-up visit and at the final 6-week visit. Blood samples were collected by qualified phlebotomists and sent for analysis at a quality-assured pathology laboratory (Southern IML Pathology, Wollongong, NSW, Australia). Insulin resistance was assessed using the homeostasis model assessment (HOMA) calculator (version 2.2.2, 12 December 2007; www.dtu.ox.ac.uk). Anthropometric measurements and blood pressure readings were taken using a Dinamap XL Vital Signs Monitor (GE Healthcare, Chalfont St Giles, Buckinghamshire, UK). Body weight and percentage of body fat were measured while standing using bioelectrical impedance scales (Tanita BF-622W; Tanita Corporation of America, Arlington Heights, IL, USA). These scales have been validated and are thought to be a reasonable comparison with dual-energy X-ray absorptiometry as a reference method(Reference Batterham, Tapsell and Jenkins26).
Activity and physical discomfort questionnaires were completed at the 3- and 6-week visits. Symptoms were scored on a scale from 1 to 6, with 1 being no symptoms and 2 to 6 being symptoms of increasing frequency/severity.
Sample size
Sample size was determined using an ANOVA approach, based on outcomes reported in a 6-week trial that used a similar dosage of oat-derived β-glucan(Reference Gerhardt and Gallo27). It was assumed that no change would occur in cholesterol in the control group, with the two intervention groups expected to show a change of 10 % from baseline concentrations, with standard deviations of 10 %. Using α values of significance of 0·05–0·01 and 90 % power, a sample size of between nineteen and twenty-seven participants per group was required. To account for dropout, thirty participants per group were targeted for recruitment.
Statistical analysis
The primary outcome measures were fasting (10 h) total and LDL-serum cholesterol. Secondary outcomes were changes in fasting serum HDL, TAG, glucose, insulin and systolic blood pressure. Statistical analysis was performed using SPSS (version 17.0; SPSS, Inc., Chicago, IL, USA). Model assumptions were checked before analysis. The analysis of primary and secondary variables was conducted using a linear mixed model for repeated measures or a general linear model for repeated measures as stated in the text. As weight loss is known to improve lipid, blood pressure, glucose and insulin measures, and weight decreased significantly during the study period in all groups, the analysis of all biochemical variables was adjusted for weight, as a continuous time-varying covariate. Adjusted and unadjusted analyses are presented. (The advantage of the use of the linear mixed model in this analysis is that partial datasets were incorporated and in this way, data from all subjects who commenced the study were included in the analysis.) An exploratory post hoc analysis was also conducted to investigate the effects in the responders. Responders were defined as those subjects who experienced a decrease of any magnitude ( < 0·000 mmol/l) in LDL-C (n 60). Non-responders were those who experienced no change or an increase ( ≥ 0·000 mmol/l) in LDL-C (n 27). The analysis was also conducted in the form of a percentage change from baseline, defined as the difference between the values for week 6 and baseline, expressed as a percentage of the baseline measure. Factors that predicted LDL-responders were investigated using logistic regression analyses. Both univariate and multivariate analyses were conducted in the model building procedure. The following variables were considered in the logistic regression: study group, sex, age, baseline BMI, baseline percentage of body fat, waist circumference, baseline cholesterol, TAG and HDL. Multivariate analysis was conducted using an entry model (where all variables were included) and a backward elimination procedure with exclusion based on the likelihood ratio test. One-way ANOVA or t tests were conducted for the responder analysis with post hoc follow-up of significant results as indicated. Proportions were compared using Pearson's χ2 analysis. Results are presented as means and standard deviations unless otherwise indicated.
Results
Participants
Recruitment was conducted continuously over a 13-month period. A total of 328 people expressed interest in the present study and 127 were eligible for the confirmatory blood test, of whom ninety-five (fifty-one females, forty-four males) met the study criteria. Among these, five participants withdrew (four females, one male) before receiving the study foods. Randomisation was conducted for the remaining ninety participants by a researcher independent of the clinical interface (M. J. B.). The male:female composition of each group was 15:15 in the OH group, 11:17 in the OL group and 15:17 in the control group. Withdrawals after the first counselling visit left 15:15 in the OH group (n 30), 11:15 in the OL group (n 26) and 15:16 in the control group (n 31; Fig. 1).

Fig. 1 Flow chart for the study.
The study sample was middle-aged (51 (sd 10·22) years), overweight (BMI 27·26 (sd 4·10) kg/m2) men and women with mildly elevated cholesterol levels but otherwise healthy (Table 2). There were no differences between the groups at baseline in the variables measured (P = 0·423–0·954).
Clinical outcomes
All three diet groups produced a reduction in total cholesterol levels (time effect P < 0·001), but there was no difference in reduction between the groups (Table 3 and Fig. 2; group effect: P = 0·563; interaction effect: P = 0·663). Similarly, all groups showed reductions in LDL-C (time effect P < 0·001) and HDL-cholesterol (time effect: P < 0·001), but between-group differences were not evident (Fig. 2). No change in TAG was evident (time effect: P = 0·279). Since there was no difference in response according to β-glucan dosage, the OH and OL groups were combined and a two-group exploratory analysis was conducted. Percentage reduction from baseline in LDL-C was − 8·42 (sd 17·41) v. − 5·48 (sd 12·36) % for the OH+OL and control groups, respectively (P = 0·363), resulting in a mean difference from baseline between the two groups of − 2·94 (se 3·22) %.
Table 3 Changes in clinical parameters at 0, 3 and 6 weeks
(Mean values and standard deviations)

OH, oats high; OL, oats low; SBP, systolic blood pressure.
* Adjusted for weight change.

Fig. 2 Mean change at 6 weeks, expressed as a percentage of baseline values, in total cholesterol and LDL-cholesterol (LDL-C) by diet group, with standard errors represented by vertical bars.
Subgroup responder analysis
Subgroup analyses of responders (n 60) identified a trend towards a greater reduction in LDL-C from baseline at 6 weeks in the OH and OL groups (P = 0·086, one-way ANOVA; Table 4). This difference was significant (P = 0·044, one-way ANOVA) when LDL-C reduction was expressed as % change from baseline ( − 18·3 (sd 11·1) %, − 18·1 (sd 9·2) % and − 11·7 (sd 7·9) % in the OH, OL and control groups, respectively), although the post hoc analysis adjusted for multiple comparisons showed that these between-group differences were of borderline significance for the two oat groups v. controls (P = 0·067 and P = 0·097 for the OH and OL groups, respectively; Tukey's honestly significant difference test). Post hoc analyses found no difference in response between the OH and OL groups; therefore, a two-group analysis (OH and OL groups combined) was performed. Between-group differences from baseline were significant for LDL-C ( − 18·2 (sd 10·1) % v. − 11·7 (sd 7·9) %; mean difference − 2·8 (se 2·3) for the OH+OL v. control groups, respectively; P = 0·008).
Table 4 Change in LDL-cholesterol (LDL-C), according to the total group and LDL-C responders only
(Baseline measures, standard deviations and number of subjects)
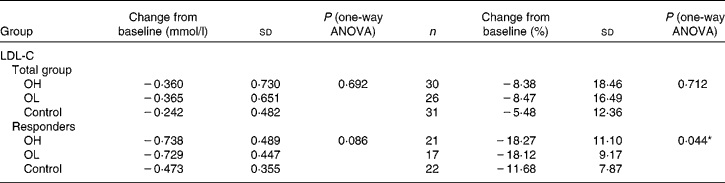
OH, oats high; OL, oats low.
* Post hoc analysis showed that the reduction in LDL-C was less in the control group than in the intervention groups; however, the differences were not significant when adjusted for multiple comparisons (P = 0·067 and 0·097 for the OH and OL groups, respectively; Tukey's honestly significant difference test).
Predictors of LDL-responders
Baseline total cholesterol was the only significant predictor of LDL-responder, both in univariate and multivariate (backward stepwise) logistic regression models; the other variables entered (age, sex, BMI, body fat, TAG and HDL-cholesterol) were not significant. For every one unit increase in total cholesterol, there was a 2·850 times increase in the odds of being a responder (P = 0·026). That is, increasing cholesterol is more likely to result in LDL response.
Other outcomes
There were no significant changes in glucose, insulin or blood pressure measurements. Homeostatic model assessment (HOMA) scores showed no significant difference for time, group or interaction (P>0·05, linear mixed model). All groups lost weight (time effect: P = 0·005; linear mixed model), with no difference between the groups (Table 3). After 3 weeks, all groups lost an average of 1·0 (sd 1·32) kg and the two intervention groups continued to lose about 50 % more in the second 3-week period (0·64 (sd 0·78) and 0·62 (sd 0·78) kg, respectively). All groups decreased their percentage of body fat ( − 0·45 (sd 2·30), − 1·28 (sd 1·84) and − 0·57 (sd 1·32) in the OH, OL and control groups, respectively; P = 0·263; linear mixed model).
Dietary compliance
There were no significant interaction effects between diet groups for energy and macronutrient intakes (P>0·05). All groups reported reductions in energy intakes (time effect: P < 0·001), and in percentage of energy from total fat and saturated fat (time effect: P < 0·001). All groups reported increases in percentage of energy from protein and in polyunsaturated:saturated (P:S) ratio (time effect: P < 0·001; Table 5). Even though the participants were modelled for weight maintenance, by providing low-fat healthy eating advice, the oral intake of participants changed. Participants reduced, limited or completely removed high-energy, low-nutrient snack foods (e.g. potato crisps, chocolate, pastries) and alcohol from their regular eating patterns. A shift in energy consumption may have occurred by removing and limiting these foods and beverages from their diets, even when they were replaced with other foods.
Table 5 Reported energy and macronutrient intakes at 0, 3 and 6 weeks*
(Mean values and standard deviations)

OH, oats high; OL, oats low; E%, percentage of energy; CHO, carbohydrate; P:S, polyunsaturated:saturated.
* Not all participants completed dietary assessments at the follow-up visits.
Compliance with consumption of the test and control foods was high. All groups consumed both the breakfast cereal and cereal bar products equally. Of the forty-two serves of each pack provided, the control group consumed a mean of 38 (sd 4) cereals and 37 (sd 5) bars, comparing well with the 39 (sd 3) and 39 (sd 2) in the OL group and 37 (sd 5) and 36 (sd 8) in the OH group. Based on returned test product packets, only three subjects had compliance of less than 50 %, but these participants were not excluded as intention-to-treat analyses were performed. No linear relationship between compliance and LDL-C change was demonstrated (r − 0·149, P = 0·277). Participants were able to consume the 60 g serving sizes of oats-containing cereals with no reported difficulty.
Due to the low number of reported symptoms of rating 3 or higher, data were recorded as no symptoms or symptoms and compared between the groups using χ2 analysis. There was no significant difference between the groups in the frequency of reported gastrointestinal symptoms on flatulence, bloating and diarrhoea (P = 0·442, 0·201 and 0·467, respectively). There were no reported adverse events. There were no significant changes in the levels of physical activity between or within the groups during the intervention (Table 3).
Physico-chemical properties of test foods
The laboratory results for the physico-chemical properties of each of the test foods are shown in Table 1. The control samples contained insignificant levels of β-glucan. The physiological extraction procedure was performed on these products and the viscosity of the extract from the puffed rice bar was 1·23 mPa s and the cornflake extract had a viscosity of 0·93 mPa s (measured at 32 per second), which was close to the viscosity of water (0·72 mPa s at 37°C). The β-glucan-containing foods had higher extract viscosities. The viscosities of the extracts from the cereal bar and the RTE oat flakes were quite similar despite having different molecular weights and solubilities. The cereal bar had low β-glucan solubility (24·09 % of total), probably because of low water availability during processing, and the molecular weight was similar to the molecular weight in oat bran(Reference Tosh, Brummer and Miller23). The RTE oat flakes had much higher solubility (90·62 % of total) because of the high temperatures and water availability during processing. The increase in β-glucan in solution was offset by a reduction in molecular weight which can be caused by very high shear. The viscosity of the extract from the rolled oats was the highest, with the solubility and molecular weight of β-glucan typical of oatmeal and oat bran.
The development of viscosity in the gut has been shown to be related to the physiological functioning of oat β-glucan(Reference Wolever, Tosh and Gibbs6). Since only the soluble portion of β-glucan contributes to viscosity development, it is more physiologically active than the insoluble portion. Taking into account the solubility of β-glucan in each test food, the dose of soluble β-glucan was calculated. The OL group consumed 1·32 g soluble β-glucan provided by the RTE oat flakes, whereas the OH group consumed 1·08 g soluble β-glucan from the combination of rolled oat porridge and cereal bar. So, although the total dose was higher for the OH group, they received slightly less of the physiologically active soluble β-glucan.
Discussion
For this sample of otherwise healthy overweight adults with mild hypercholesterolaemia, we found significant cholesterol-lowering effects with a low-fat and low-saturated-fat diet, with and without 1·5 or 3·0 g oat β-glucan/d delivered in three different food forms. Despite the effects of the healthy low-fat diet in all groups, it is possible that other factors, such as differences in metabolism, may have influenced our ability to differentiate between the groups. The secondary analysis of responder data suggests that this may be the case, showing a higher reduction in LDL-C with oat β-glucan compared with the control diet (18 % v. 11 %; P = 0·04).
Meta-analyses have demonstrated that a daily intake of 3 g β-glucan/d can lower LDL-C concentrations by about 4 % or 0·13 mmol/l(Reference Ripsin, Keenan and Jacobs1, Reference Brown, Rosner and Willett28). It is generally accepted that for every 1 % lowering of serum cholesterol levels, the risk of development of CHD is reduced by 2–3 %(Reference Wood18). In the present study, the oat groups had reductions in LDL-C from baseline in the region of approximately 8·4 %, at β-glucan doses of either 1·5 or 3 g/d. The magnitude of this effect is similar to that reported in a recent 12-week weight loss study in which the 3 g/d β-glucan group reduced LDL-C by 8·7 %, compared with the control group ( − 4·3 %)(Reference Wood18, Reference Maki, Beiseigel and Jonnalagadda29). The present study also reported higher reductions in total cholesterol over 6 weeks ( − 7·8 %) compared with that study ( − 5·4 %); however, the lack of between-group differences compared with the control group in the present study is related to a higher-than-expected cholesterol-lowering effect in the control group which adopted a low-fat eating pattern for 6 weeks. It should be noted that the Maki et al. (Reference Maki, Beiseigel and Jonnalagadda29) study reported a significant between-group difference for total cholesterol only in per-protocol analyses which included participants who were at least 80 % compliant with study product consumption and had no major protocol violations. Their intention-to-treat analysis failed to demonstrate significant differences between the groups, even with a large sample size of 174 participants; so again, it may be worth pursuing the characteristics of responders in future studies that aim to expose the effects of β-glucan delivered in various food forms and dosages.
A recent systematic review of the consistent association between oat consumption and cardiovascular risk factors(Reference Ruxton and Derbyshire30) also found one study that showed cholesterol reduction in all groups including the low-fat control diet(Reference Denmark-Wahnefried, Bowering and Cohen31). In our case, the healthy background diet appeared to influence weight, bearing in mind that weight loss alone can improve lipid profiles(Reference Tapsell, Batterham and Teuss32). In addition, concurrent changes in dietary fat occurred in the sample, including the displacement of saturated fat, which may confound the relationship between increased fibre intake and blood cholesterol levels(Reference Butt, Tahir-Nadeem and Khan33). In the study reported here, we did not show effects on TAG, but the lowered HDL-cholesterol produced by the sample was consistent with the lower reported intake of dietary fat ( < 30 E%). A recent meta-analysis of thirty controlled feeding studies(Reference Cao, Mauger and Pelkman34) showed that both modified-fat (30–50 E%) and low-fat (18–30 %) diets lower LDL-C, but modified-fat diets tend to lower HDL-cholesterol less, and may produce greater reductions in TAG. Finally, in the present study, the lack of effects on the secondary outcomes of blood pressure, glucose, insulin and homeostasis model assessment (HOMA) indices probably reflected the relatively short period of study and the normal baseline levels recorded.
The present results may also have been influenced by the food matrix of the intervention foods. When oat-derived β-glucan was incorporated into bread and cookies and provided at a level of 6 g/d for 4 weeks, no cholesterol-lowering effect was observed(Reference Kerchoffs, Hornstra and Mensink35). The authors concluded that the food matrix or the food processing, or both, may be influential in limiting the cholesterol-lowering properties of oat bran. In particular, a high-molecular-weight β-glucan molecule can entangle to form viscous solutions(Reference Anttila, Sontag-Strohm and Salovaara36) and high solubility must be maintained to ensure this effect. Reduction in molecular weight or solubility will therefore effect viscosity and have follow-on effects on physiological functionality. It has previously been shown that the viscosity of the in vitro extract of oat-containing foods positively correlates with cholesterol reduction(Reference Wolever, Tosh and Gibbs6, Reference Tosh, Brummer and Miller23) and also glycaemic response(Reference Lan-Pidhainy, Brummer and Tosh17, Reference Tosh, Brummer and Wolever37) and the satiety biomarkers cholecystokinin(Reference Beck, Tosh and Batterham16) and peptide YY(Reference Beck, Tapsell and Batterham38). The viscosity of β-glucan solutions is dependent mainly on the concentration of β-glucan in solution and its molecular weight.
In the present study, the OH and OL (3 g β-glucan and 1·5 g β-glucan, respectively) test foods had near-equal cholesterol-lowering effects; yet molecular weights, solubility and viscosities were variable. Using viscosity as a measure of bioactivity, it could be expected that in the present study, cholesterol reduction should have been greater in OH v. OL as the viscosity of rolled oats was higher (16·03) than the extruded oat flakes. Similarly, the molecular weight of β-glucan was also higher in both the rolled oats and cereal bar (OH group; 2 100 000 v. 2 400 000 g/mol, respectively) than in the extruded oat flakes (OL group; 760 000 g/mol). However, previous studies have shown that high and medium molecular weights of β-glucan such as those of the two groups in the present study can significantly reduce LDL-C(Reference Wolever, Tosh and Gibbs6) and modulate the blood glucose response(Reference Tosh, Brummer and Wolever37). It was also shown in the present study that the dose of solubilised β-glucan was comparable (1·32 in OL v. 1·08 g in OH) across the two intervention groups, which may also explain the similar results observed. Nevertheless, the present findings in the study reported here are consistent with other studies demonstrating that extrusion to produce an oats-containing breakfast cereal may only slightly decrease molecular weight yet increase solubility of β-glucan, compared with regular oats that have higher molecular weight with poorer solubility(Reference Beck, Tosh and Batterham16).
To take into account the drop in viscosity caused by partial depolymerisation in the RTE oat flakes during processing, the parameter M p × C was calculated, where M p is the molecular weight of the β-glucan in solution and C is the daily dose of soluble β-glucan. Wolever et al. (Reference Wolever, Tosh and Gibbs6) suggest that M p × C may be a more robust measure of β-glucan bioactivity than the viscosity of extracted β-glucan, because molecular weight and C are relatively insensitive to extrinsic factors(Reference Wolever, Tosh and Gibbs6). The M p × C value for the OH treatment was 1600, whereas it was 1000 in the OL treatment. The similarity in these values may explain the similar reduction in cholesterol achieved with the two treatments in the present study. For comparison, these values are not that dissimilar to the values found for treatments at concentrations of 3 g β-glucan/d of medium molecular weight (M p = 528 000) (3M) and 4 g β-glucan/d of low molecular weight (M p = 211 000) (4L) for extruded oat bran cereals studied previously(Reference Wolever, Tosh and Gibbs6). In that study, the 3M cereal had an M p × C value of 1500 and significantly lowered LDL-C by 4·7 % (P = 0·012; n 64). However, the 4L cereal produced a non-significant LDL-C lowering of 2·3 % (P = 0·205; n 63), with an M p × C value of 840. This may be due to the low M p × C value reflecting a potentially lower bioactivity of the β-glucan. It should also be considered that the larger number of participants in the oat bran cereal study may have increased the ability to distinguish differences between the treatment and control groups compared with the present study in which less than half that number of participants (n 30) were randomised to the OH (3 g β-glucan) treatment group.
There are a number of potential limitations to the present study. The study may have been under-powered, since sample size was determined assuming that no change in serum cholesterol from baseline would occur in the control group and that weight would remain stable in all groups. Further, the expected magnitude of reduction in LDL-C of 10 % in the two intervention groups(Reference Mifflin, St Jeor and Hill25) appears to have been too optimistic. The finding from secondary analyses that a higher baseline total cholesterol was predictive of LDL response to β-glucan consumption in responders is consistent with previous studies(Reference Ripsin, Keenan and Jacobs1), but regression to the mean(Reference Bland and Altman39) may have been a confounder in this analysis.
In conclusion, favourable reductions in total and LDL-C in healthy diets with oat β-glucan are supported by strong mechanistic evidence of oat β-glucan on cholesterol levels. However, in the present study, the incorporation of β-glucan from oat foods into a healthy low-fat diet for 6 weeks did not lead to a further significant decrease in serum cholesterol compared with a low-fat diet alone. No effect of the intervention diets was observed regarding the secondary outcomes (glucose, insulin resistance and blood pressure). The findings from the present study suggest that a smaller quantity (1·5 g/d) of medium-molecular-weight oat β-glucan with high solubility may be equally as effective as a higher quantity (3 g/d) of high-molecular-weight β-glucan. Further examination of these factors is warranted to assist in determining the lowest effective doses of oat β-glucan within different food matrices that may be influential in effectively reducing cholesterol in moderately hypercholesterolaemic populations.
Acknowledgements
Financial support for the present study was provided by a research grant from Cereal Partners Worldwide Limited. Dr Yasmine Probst is thanked for her assistance with the analysis of dietary intake, Ms Holley Jones for initial inputs into project management and Mr Kiefer Zhang for some data collection. None of the authors has any conflicts of interest to declare. K. E. C. was involved in the conceptualisation of the study design, overall management of the project and primarily responsible for manuscript writing. L. C. T. was responsible for the conceptualisation of the study design and editing of the manuscript. M. J. B. contributed to the sampling and randomisation, statistical data analysis, data interpretation and editing of the manuscript. J. O. was involved in the data collection, data entry and overall data management, and editing of the manuscript. R. T. was responsible for the data collection, data entry and editing of the manuscript. E. B. contributed to the methodology and editing of the manuscript. S. M. T. conducted the physico-chemical analyses of the test foods, and was involved in the interpretation of the data analyses and editing of the manuscript.









