Prebiotics have been defined as non-digestible food compounds that selectively stimulate the intestinal flora and metabolic activity after colonic fermentation. The human colon is believed to be the predominant site of action and represents the most heavily populated intestinal region with up to 1012 colony-forming units/ml(Reference Cummings, Gibson and Macfarlane1). Lactobacilli and bifidobacteria are considered indicator organisms of prebiotic stimulation; in terms of gut health, bifidobacteria are currently considered to be the most significant organisms(Reference Gibson and Roberfroid2). Dietary supplements of a prebiotic such as fructo-oligosaccharides (FOS) increase the content and proportion of bifidobacteria(Reference Gibson and Roberfroid2–Reference Brunser, Figueroa, Gotteland, Haschke-Becher, Magliola, Rochat, Cruchet, Palframan, Gibson, Chauffard and Haschke5), which exert a positive influence on gut health. Proliferation of these bacteria allows a more predominant role for the intestinal flora as they have been shown to decrease the quantity of potentially hazardous bacteria by their metabolites and protect from intestinal infections(Reference Ito, Deguchi, Matsumoto, Kimura, Onodera and Yajima6, Reference Gibson and Wang7). Previous studies have documented positive effects on absorption of nutrients and minerals(Reference Coudray, Bellanger, Castiglia-Delavaud, Remesy, Vermorel and Rayssignuier8), stimulation of the immune system and synthesis of vitamins(Reference Roberfroid9). Moreover, prevention of constipation(Reference Bouhnik, Coffin and Rambaud10), colon cancer(Reference Reddy11) and improvement of blood sugar and lipid profile(Reference Jackson, Taylor, Clohessy and Williams12) are also attributed to prebiotics.
Prebiotics are also employed as fat-replacers and to improve food texture. For both health and technical reasons food products are increasingly supplemented with prebiotics. In Europe, a large range of prebiotic-supplemented food products is already available. The most commonly used prebiotics in Europe are FOS. The range of supplemented products includes (powdered) milk and fermented milk products, cereals and clinical supplements for pregnant women. FOS are naturally found in a variety of vegetables such as asparagus, leeks, artichokes, onions and garlic and have been classified as a food ingredient in the European Union, Canada and Japan (‘generally recognised as safe’ status in the USA) and are an acceptable food additive in Australia and New Zealand(13). By-products of bacterial fermentation of prebiotics are gases including H2, CO2, H2S and CH4, which may cause intestinal discomfort, i.e. flatulence, bloating and abdominal pain(Reference Grimble14, Reference Marteau and Flourie15). Recently, the prebiotic properties of acacia gum (Fibregum® (Colloïdes Naturels International CNI, Rouen, France), a natural soluble fibre derived from acacia gum) have been described and a synergy for the bifidogenicity has been observed with the combination of FOS and acacia gum(Reference Rochat, Baumgartner, Jann, Rochat and Ballevre16). Due to the growing range of supplemented products, consumers are exposed to increasing amounts of prebiotics and may ingest daily doses above the threshold for induction of side effects. In vitro fermentation time for acacia gum is significantly longer than that for FOS and studies have suggested a more favourable abdominal side-effect profile(Reference Cherbut, Michel, Raison, Kravtchenko and Severine17).
Replacement of a proportion of FOS by acacia gum may thus attenuate the side effects of prebiotics with the additional advantage of a synergistic effect on the growth of intestinal bifidobacteria. Hence, the objectives of the present study were primarily to compare changes in intestinal comfort and secondarily to compare visceral sensitivity during rectal barostat distension following long-term ingestion of a single prebiotic (FOS), a mixture of FOS and acacia gum (BLEND) or a rapidly fermented carbohydrate (maltodextrin) in healthy volunteers. In addition, independent of treatment phases, determinants of wellbeing were analysed.
Methods
Subjects and study design
A total of eight male and twelve female healthy volunteers completed this controlled, double-blind, cross-over, randomised trial. They were recruited from the University Hospital and Universities in Zürich by in-house advertisement. Candidates with anal pathology, previous gastrointestinal surgery, pregnancy, bowel dysfunction and drug abuse were excluded from the study. Only candidates with normal findings and ingesting a similar habitual diet, as assessed by a dietary questionnaire at the screening visit, were included in the study. During a screening visit before acceptance for the study the candidates underwent haematology, blood chemistry, urine measurements, and a complete physical examination. Women of childbearing potential were required to have a negative urine pregnancy test. With the exception of birth control pills and hormone replacement therapy no regular medication was allowed during the study. Approval of the study was granted by the Ethical Committee of the University Hospital Zürich (EK-926) and all participants gave their written informed consent.
The study lasted a total of 18 weeks and was composed of five testing periods:
(1) a first 2-week run-in period during which the volunteers ingested maltodextrin, and answered a diary and a questionnaire;
(2) a 5-week ingestion period of the first product (FOS or BLEND, diary, questionnaire);
(3) a 4-week wash-out period (no product intake, diary or questionnaire);
(4) a second 2-week run-in period (maltodextrin, diary, questionnaire);
(5) a 5-week ingestion period of the second product (BLEND or FOS, diary, questionnaire).
The volunteers had to visit the study centre (visit 1, visit 2) at the end of both 5-week treatment periods. The subjects came in after a 6 h fasting period for stool specimen collection and barostat testing of anorectal sensory function and rectal compliance.
Products
The products used were as follows:
(1) FOS: food-grade Raftilose® P95, produced by partial hydrolysis of chicory root inulin, containing oligofructose, fructose, glucose and sucrose (ORAFTI, Tienen, Belgium).
(2) BLEND: 1:1 mixture of FOS and acacia gum, a food-grade purified alimentary fibre derived from acacia gum (Fibregum®) consisting of branched arabino-galactans polymers, as described previously(Reference Cherbut, Michel, Raison, Kravtchenko and Severine17).
(3) Maltodextrin, an easily digested, neutral, slightly sweet-tasting carbohydrate obtained by hydrolysis of natural maize starch (Glucidex; Roquette, Lestrem, France). The starches are cooked and than acid and/or enzyme treated to break them down into smaller polymers.
Doses and packaging
The subjects were asked to ingest daily, except for the wash-out period, one sachet containing 10 g product 2 h postprandially in a drink, for example, tea, coffee, mineral water, soft drink or fruit juice. All products were packed by Nestlé (Konolfingen, Switzerland). Volunteers, support stuff and investigators were blinded regarding the ingested product. The run-in periods were not blinded since maltodextrin is sweet and easily recognised as compared with FOS or BLEND.
The subjects received the first set containing the products, questionnaires and diary for the first two periods at the screening visit (visit 0) and the second set for the last two periods at visit 1.
Questionnaire
During the run-in and product intake periods, the volunteers answered twice weekly to a questionnaire by cellular phone or Internet. The volunteers were contacted by an automated telemedical patient data-capturing system (Medcontrol AG, 6314 Unterägeri, Switzerland). The volunteers entered information on gastrointestinal complaints and symptoms of general wellbeing into the data-capturing system. The questionnaire inquired about the occurrence and intensity (none (0), slight (1), moderate (2), strong (3)) of:
(1) pain at stomach or abdomen during last 3 d (‘pain’);
(2) feeling bloated during last 3 d (‘bloat’);
(3) being disturbed by passing wind (‘wind’);
(4) being disturbed by belching or burping (‘belch’);
(5) being disturbed by frequent bowel movements during last 3 d (‘DiaFreq’);
(6) being disturbed by urgent need to defecate (‘DiaUrg’);
(7) being disturbed by constipation (‘Obstip’);
(8) being bothered by frequent rumbling noise from stomach or gut (‘borborygmi’);
(9) being adversely affected by nausea (‘nausea’);
(10) having suffered from heart burn or acid reflux (‘reflux’);
(11) being negatively influenced by ingestion of the products (‘general’);
(12) being forced to reduce daily activity, i.e. job, university, household (‘work’).
In the figures, the sequence of the questionnaire items is reordered by the frequency of reported symptoms, with ‘wind’ as most frequent first, and ‘reflux’ as least frequent last. For quality control, a daily written diary for corresponding evaluation of bloating, pain and nausea on a four-point grading scale (none, slight, moderate, strong) and additional evaluation of stool consistency (hard, normal, unformed, watery), frequency of defecations as well as the time of product intake was filled in by the volunteers. Since corresponding questionnaire and diary outcomes correlated well in preliminary analyses only the questionnaire data are reported.
Rectal barostat
The barostat procedure was performed in a left lateral 15° Trendelenburg position (the body is laid flat on the back (supine position) with the feet higher than the head). A 10 cm-long polyethylene balloon was inserted into the rectum such that the proximal end was 5 cm inside the anal verge. Minimal distending pressure was determined by 1 mmHg/min stepwise rectal expansion until changes in respiratory excursion were apparent (at about 30 ml balloon volume). Then a conditioning distension was performed, inflating the balloon by 2 mmHg increments every 30 s in the range of 0–40 mmHg to avoid changes in compliance and sensation in subsequent inflations.
Rectal compliance was assessed twice by 2 mmHg stepwise increments of intra-balloon pressure beginning at 0 mmHg pressure up to 40 mmHg or until subjects reported pain. During the last 10 s of each pressure increase, the volunteers were asked to score their perception of lower abdominal sensation using a six-point graphic-rating scale ranging from ‘no feeling’ to ‘painful’. After a 15 min break, phasic rectal distensions at 12, 24, 36 and 42 mmHg pressure above minimal distending pressure were performed in randomised order. During the last 15 s of each random distension, subjects were asked to rate their abdominal symptoms, i.e. the urgency to defecate (‘urgency’), bloating (‘wind’), discomfort (‘discomfort’) and pain (‘pain’) using a 100 mm visual analogue scale (VAS) with ‘none’ at 0 and ‘worst ever’ at 100. The equilibration interval between distensions was 2 min at minimal distending pressure. The method of measuring anorectal sensation and compliance was described in detail in previous studies of our laboratory(Reference Thumshirn, Coulie, Camilleri, Zinsmeister, Burton and Van Dyke18, Reference Fox, Stutz, Menne, Fried, Schwizer and Thumshirn19).
Stool microflora
Freshly passed faecal samples were placed into cryotubes and frozen in liquid N2 and kept at − 70°C until analysis at the Nestlé Research Centre using fluorescent in situ hybridisation. The probe used in the study was Bif164, specific for Bifidobacterium (Reference Brunser, Figueroa, Gotteland, Haschke-Becher, Magliola, Rochat, Cruchet, Palframan, Gibson, Chauffard and Haschke5).
Data analysis and statistics
The comparison of the questionnaire between FOS and BLEND was the primary outcome of the present study. Secondary outcomes were:
(1) comparison of questionnaire scores between treatment phase (FOS or BLEND) and run-in;
(2) intra-subject correlation of intestinal symptoms with general and work wellbeing to identify the dominant physiological correlate of intestinal comfort;
(3) comparison of visceral sensitivity under FOS and BLEND, as measured by VAS scores during rectal distension;
(4) composition of the faecal microflora under FOS and BLEND.
For the primary endpoint, questionnaire outcomes were dichotomised into 0 and 1, where 0 means ‘no symptoms reported’. The binary data were analysed with a generalised linear mixed model (GLMM), logistic link, and quasi-binomial family. Subject was the random term, sex and treatment (FOS or BLEND) and their interaction term were fixed terms; the subject's run-in means pooled over both phases were used as a covariable. The GLMM model is considered robust against unbalanced sex distributions. Results were reported as odds and OR with CI. All CI and P values for the questionnaire analysis were computed as contrasts of the GLMM analysis. No adjustments for multiple testing were applied to P values and CI. For questionnaire item 10 (‘reflux’), which was reported by four subjects in only twenty out of 582 calls, the GLMM procedure did not converge, and therefore was removed from primary endpoint analysis. For questionnaire item 12 (‘work’), the fitted probability for the male subgroup is close to zero, which can result in a high false-alarm rate; this item was also analysed by the Markov-Chain Monte-Carlo (MCMC) GLMM procedure to check for robustness.
Symptoms in the run-in phase were not significantly different from each other, and were pooled per subject. For comparison against the run-in phase as secondary outcome, a GLMM analysis was done without sex breakdown to avoid convergence problems when sex was used as a factor.
The influence of intestinal symptoms on general wellbeing (‘general’) and on being forced to reduce daily activity (‘work’) were analysed by multiple regression analysis and best predictors were reported as adjusted squared correlation coefficients.
Rectal functional parameters and threshold pressures as assessed by rectal barostat were analysed by exact Wilcoxon matched pair tests. VAS scores were transformed by an offset logarithmic transformation (log (VAS +5)) in order to stabilise variances and expressed as CI of VAS-equivalent balloon stimulation pressures. The slopes of VAS and graphic ratings, computed with pressure as the independent variable, served as a measure of rectal sensitivity. Slopes were determined separately for each sensation (wind, urgency, pain, discomfort), and for the sensation sum score by a linear mixed model, with subject as a random variable and treatment as fixed. For analysis of continuous data, a linear mixed model was used, using power weighting if required based on inspection of residual distribution. The logarithms of the total stool bacteria count and the bifidobacteria count were analysed by paired t tests.
Data were considered significant at a level of α ≤ 0·05. All statistics were evaluated and plotted using the open-source computing environment ‘R’ (version 1.9.0; R Foundation for Statistical Computing, Vienna, Austria). Mixed models were estimated with package nlme under R(Reference Pinheiro and Bates20) and function glmmPQL in MASS(Reference Venables and Ripley21); the MCMC model was computed with DPpackage(Reference Jara22).
Results
Eight male and twelve female healthy volunteers (median age 28 (range 20–37) years) of normal BMI (median 22·5 (range 20·1–25·3) kg/m2) and weight (median 67 (range 53–84) kg) completed the study without any complications or adverse events. Valid questionnaire responses were obtained in 154 of 160 possible responses of the run-in phase (96 %) and in 426 of 440 possible responses (97 %) of the treatment phases, indicating very high compliance rates.
Fructo-oligosaccharide v. prebiotic mixture of fructo-oligosaccharide and acacia gum
The most frequently reported symptoms for both FOS and BLEND were ‘bloating’ and ‘wind’, with an odds over 1, corresponding to more than 50 % of the calls (Fig. 1). The most prominent difference between FOS and BLEND was found for item 11 (‘general wellbeing’) of the male group, which was larger under FOS treatment (Fig. 2 (a)) with an OR of 9·6 (P = 0·002). To check whether this was a statistical artifact of the GLMM method(Reference Hauck and Donner23), a Bayesian estimate(Reference Jara22) was computed for this item giving an even larger OR for the sex difference of 27 (95 % CI 4·3, 121; P values commonly not given for Bayesian methods).

Fig. 1 Odds of reported ‘symptoms present’ to ‘symptoms absent’ for once-daily ingestion for 5 weeks of a 10 g prebiotic mixture of food-grade fructo-oligosaccharide (FOS; Raftilose® P95) and acacia gum (1:1; BLEND) (a) and for once-daily ingestion for 5 weeks of 10 g FOS (b). Horizontal bars represent 95 % CI from generalised linear mixed model analysis of questionnaire data. CI are between-subjects and it is not valid to visualise within-subject tests against run-in. Odds are on a logarithmic scale; the dotted vertical line shows the value of 50 % reported calls with non-zero symptoms. Items are ordered by overall frequency of reporting. (△), Females, baseline values; (▲), females, treatment values; (○), males, baseline values; (●), males, treatment values; Obstip, being disturbed by constipation; Borbor, borborygmi; DiaUrg, being disturbed by urgent need to defecate; DiaFreq, being disturbed by frequent bowel movements during last 3 d. For further details of the questionnaire items of the automated telemedical data-capturing system, see Methods.
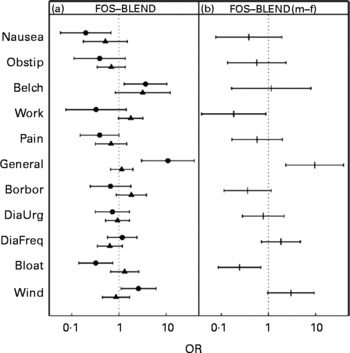
Fig. 2 (a) Symptom score OR on a logarithmic scale of once-daily ingestion for 5 weeks of 10 g food-grade fructo-oligosaccharide (FOS; Raftilose® P95) v. once-daily ingestion for 5 weeks of a 10 g prebiotic mixture of food-grade FOS and acacia gum (1:1; BLEND) for females (▲) and males (●), with 95 % CI represented by horizontal bars. (b) Between-sexes FOS v. BLEND difference from interaction term in generalised linear mixed model. The dotted vertical line at the OR of 1 corresponds to ‘no difference in reported frequency’. m, Males; f, females; Obstip, being disturbed by constipation; Borbor, borborygmi; DiaUrg, being disturbed by urgent need to defecate; DiaFreq, being disturbed by frequent bowel movements during last 3 d. For further details of the questionnaire items of the automated telemedical data-capturing system, see Methods.
The higher incidence of ‘belching’ under FOS (P = 0·09 for females; P = 0·01 for males) had the same tendency for both sexes. ‘Bloating’ under FOS and BLEND was expressed differently for sex, as computed from the interaction term in the GLMM (Fig. 2 (b), and last column in Table 1; P = 0·008) with an OR of 1·3 ( = higher under FOS) for females and 0·3 ( = lower under FOS) for males. Most symptom scores increased during the treatment intervals and the run-in period (Fig. 3).
Table 1 Fructo-oligosaccharide (FOS) v. prebiotic mixture of FOS and acacia gum for primary endpoint questionnaire items, by sex*
(Odds ratios)

* For abbreviations of the questionnaire items of the automated telemedical data-capturing system, see Methods.
† OR >1 indicate more frequent symptoms under FOS. The P values test against the null hypothesis of an OR = 1·0; no correction for multiple testing has been applied.

Fig. 3 Smoothed time series of symptom scores by treatment. (—), Pooled score results of the two 2-week baseline phases with 10 g maltodextrin once daily (run-in); (…), score results of the 5-week ingestion period of 10 g food-grade fructo-oligosaccharide (FOS; Raftilose® P95) once daily; (–-), score results of the 5-week ingestion period of a 10 g mixture (1:1) of FOS and acacia gum once daily (BLEND). The symptoms are (a) wind, (b) bloating, (c) being disturbed by frequent bowel movements during last 3 d, (d) being disturbed by urgent need to defecate, (e) borborygmi, (f) general wellbeing, (g) pain, (h) reducing daily activity (work), (i) belching, (j) being disturbed by constipation, (k) nausea, (l) reflux. For further details of the questionnaire items of the automated telemedical data-capturing system, see Methods.
Fructo-oligosaccharide and prebiotic mixture of fructo-oligosaccharide and acacia gum v. run-in
The response scores for ‘bloating’ and ‘wind’ were significantly higher during FOS and BLEND treatment as compared with the pooled run-in period (P < 10− 10 for both items and sexes). Occurrence of ‘borborygmi’ was significantly increased in both phases (P < 10− 5). ‘General’ wellbeing under FOS was reduced compared with run-in (OR 5·4; P < 10− 7), but less under BLEND (OR 2·9; P = 0·001), an effect that was overlaid by the extreme sex differences for this item. ‘Nausea’ and ‘reflux’ were almost never reported. During run-in only 14 % of all responses to the questionnaire were larger than 0 indicating slight (in most of the cases), moderate, or strong effects. Of the responses under FOS and BLEND, 30 % were larger than 0.
Determinants of gut comfort
Irrespective of the treatments, in both sexes changes in gut symptoms affected ‘general’ wellbeing more strongly (R 2 >0·35) than wellbeing during ‘work’ (R 2 >0·20; Fig. 4). In men, ‘borborygmi’ were the strongest determinant of gut comfort (R 2 >0·26). Stool-related symptoms were secondary for males. In priority sequence, ‘bloating’, ‘wind’ and ‘stool frequency’ were main determinants of wellbeing in women. ‘Belching’, which was significantly higher under FOS, is a major determinant for general wellbeing, but is not a relevant factor influencing work.

Fig. 4 Main determinants of intestinal symptoms for items ‘General’ (reduced general wellbeing) (a and b) and ‘Work’ (influence at work) (c and d) for males (a and c) and females (b and d). Initially all ten other questionnaire item scores were used to predict ‘General’ and ‘Work’ score respectively, and an Akaike information criterion-controlled subsets regression reduced predictors to the four most dominant ones. The vertical scale shows adjusted squared correlation coefficient; for example, for n 5/13 %, five of the eight male subjects included in the study reported non-zero symptom score values, and overall 13 % of the responses were >0. borbor, Borborygmi; obstip, being disturbed by constipation; DiaFreq, being disturbed by frequent bowel movements during last 3 d; DiaUrg, being disturbed by urgent need to defecate. For further details of the questionnaire items of the automated telemedical data-capturing system, see Methods.
Rectal barostat
No significant effects of treatment type on rectal compliance and asymptotic compliance were detected. Threshold pressures and rectal sensations (urgency, wind, discomfort and pain) were also similar for all treatments during phasic distension (Table 2). In the GLMM analysis of dichotomised sensory responses the scores for urgency were higher under FOS compared with BLEND (OR 2·2; P = 0·01), with discomfort and pain showing the same tendency (Fig. 5).
Table 2 Rectal function and sensations after a 5-week period of fructo-oligosaccharide (FOS) or prebiotic mixture of FOS and acacia gum (BLEND) ingestion as assessed by barostat*
(Medians and interquartile ranges (IQR) for twenty subjects)
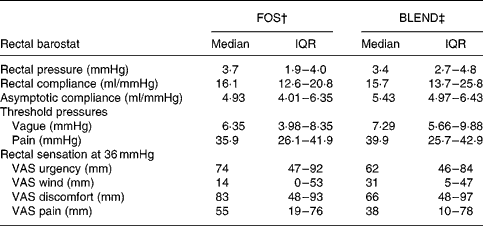
VAS, visual analogue scale.
* No significant differences in sensory scores were found using a mixed model analysis, and there were no differences in rectal functional parameters and threshold pressures by exact Wilcoxon matched pair tests.
† Functional parameters after a 5-week ingestion period of 10 g food-grade Raftilose® P95 once daily.
‡ Functional parameters after a 5-week ingestion period of a 10 g mixture (1:1) of FOS and acacia gum once daily.

Fig. 5 Generalised linear mixed model estimates of OR and 95 % CI (represented by horizontal bars) of rectal sensations during phasic rectal barostat distensions. Values above 1 indicate that higher symptoms were reported under fructo-oligosaccharide (FOS) compared with the prebiotic mixture of FOS and acacia gum (BLEND).
Stool analysis
No differences in faecal bifidobacteria concentration and the number of total bacteria as well as bifidobacteria:total bacteria ratio were observed between FOS and BLEND (bifidobacteria: 9·8 (sd 0·3) v. 9·7 (sd 0·4) log colony-forming units/g; total bacteria: 10·4 (sd 0·3) v. 10·4 (sd 0·3) log colony-forming units/g).
Discussion
The main results of the present study were: (i) evidence to suggest that FOS generates slightly more abdominal side effects than acacia gum; (ii) indication of sex differences of determinants in gut wellbeing; (iii) confirmation that self-observation induces a sensitisation or higher level of alertness to gut signals; (iv) that patient monitoring using an automated telemedical data-capturing system assures excellent questionnaire response rates.
The differences between scores for FOS and BLEND were small, i.e. smaller than those between both product periods and the run-in period; only belching was significantly stronger under FOS in both sexes. It remains to be elucidated whether the daily 10 g supplement was simply below the threshold for the induction of more pronounced effects, and to what extent sexes differ in their response to fibre supplementation.
Sex differences in transit time, faecal weight and biochemistry and in dietary fibre intake have been reported(Reference Lampe, Fredstrom, Slavin and Potter24) and either of these differences may explain the weaker sex-specific responses. The results of the present study support the occurrence of sex differences in response to prebiotic supplementation as has been observed in studies describing sex differences in response to dietary fibre supplements(Reference Martinez, McPherson, Annegers and Levin25–Reference Mathew, Peters, Chatterjee, Kulldorff and Sinha27). These effects surpass a purely symptomatic response: combined data from two large intervention trials(Reference Jacobs, Lanza, Alberts, Hsu, Jiang, Schatzkin, Thompson and Martinez28) revealed that dietary fibre both as a supplement or in a high-fibre diets protect men but not women against colorectal adenoma recurrence(Reference Fuchs, Giovannucci, Colditz, Hunter, Stampfer, Rosner, Speizer and Willett26, Reference Jacobs, Lanza, Alberts, Hsu, Jiang, Schatzkin, Thompson and Martinez28). Sex differences in brain responses to rectal distention have also been reported, suggesting different neural processing or behavioural changes to the perception of gut stimuli(Reference Berman, Munakata, Naliboff, Chang, Mandelkern, Silverman, Kovalik and Mayer29–Reference Lawal, Kern, Sanjeevi, Hofmann and Shaker31). Not surprisingly, in the present study sexes differed also in their perception of behavioural changes induced by ingestion of dietary prebiotics. These sex differences need to be taken in account when targeting populations of both sexes with dietary fibre supplements, as has already been done for recommended daily fibre intake. Further experiments are needed to confirm and characterise the sex differences in response to fibre ingestion. The inclusion of larger doses of fibre supplements would certainly answer the questions about threshold doses in both sexes, and could also confirm sex differences.
Both products significantly enhanced scores for bloating and wind. ‘Borborygmi’ were significantly enhanced under treatment with FOS. Moreover, there was a tendency for enhanced scores for belching and ‘general’ impairment under FOS only. Only 30 % of all responses to the questionnaire during FOS or BLEND were larger than zero, suggesting that the supplemented dose had generally small effects on abdominal symptoms. Nonetheless, both FOS and BLEND showed significant effects as compared with the run-in phase with maltodextrin. It remains unclear whether carrying out the run-in period in an unblinded way may have contributed to the size of the effects between FOS or BLEND and run-in. The increase in some of the symptoms during the treatment periods was rather surprising. In fact, adaptation to the 10 g supplement was to be expected; however, symptoms generally tended to increase over time (Fig. 3). The increase was also observed during the run-in (maltodextrin) periods, suggesting that it is rather a higher level of alertness in self-observance that leads to the ‘sensitisation’ than the product itself.
The sensory score for ‘urgency’ during the barostat test was significantly higher under FOS than under BLEND, with ‘discomfort’ and ‘pain’ having the same tendency. The differences between FOS and BLEND point in the same direction as the questionnaire results. An increase in urgency in the questionnaire, with no additional stimulus applied, was not observed under FOS.
To evaluate further differences between FOS and BLEND, concentrations of bifidobacteria as indicators of gut health were quantified. There were no significant differences in gut bifidobacteria concentration or total bacterial content between FOS and BLEND, probably due to the low dose of prebiotic supplemented in the present study. Possibly, the replacement of a large proportion of the fibres ingested with the regular diet by FOS or BLEND would have helped to reveal differences between the supplements as well as between the run-in periods and the supplements. This had not been addressed in the present study to avoid increasing the cumbersome stool collections to a total of four collections.
In general, prebiotics are safe and mainly well-tolerated food supplements with only slight differences as observed between the products FOS and BLEND. However, BLEND did show a slightly more favourable side-effect profile in comparison with FOS, supporting the concept of slower fermentation in the genesis of abdominal symptoms. Further experiments are needed to confirm and characterise the sex differences in response to fibre ingestion. The inclusion of larger doses of fibre supplements would clarify the role of sex-specific threshold doses.
Acknowledgements
All authors contributed equally for intellectual input and writing of the manuscript. D. M. and O. G. performed the statistical analysis. M. G., F. R. and K. O. are employed by the Nestlé Research Centre. All authors declare no conflict of interest. The work was funded by a grant from the Nestlé Research Centre.
Marianna Giarrè passed away before submission of the manuscript. Because of her friendly, warm and constructive attitude it was always a pleasure to interact with her.
The excellent technical assistance of Dr U. Sahrbacher and Bernadette Stutz is greatly acknowledged.









