Bread is one of the most common food items consumed all around the world. In the UK the industrial sector represents 80 % of total production, with a market worth £3·4 billion( 1 ). The most commonly consumed bread in western countries is wheat bread, of which gluten is a crucial functional component. Gluten is a protein contained in flour that is key to the bread-making process. Mixing with water causes the gluten to swell and develop a network that gives a viscoelastic dough with the ability to retain gas, a property vital to allow bread to rise( Reference Wieser 2 ). Gluten is largely responsible for the unique texture of wheat bread. In the last few years gluten has gained much more attention because of the increasing phenomenon of people complaining of gastrointestinal (GI) symptoms (altered bowel habit, abdominal pain, bloating and nausea) when they eat wheat, despite not having coeliac disease (CD)( Reference Carroccio, Mansueto and Iacono 3 ). However, little is known about the ‘in vivo’ effects of bread gluten content on GI physiology. MRI has unique capabilities when it comes to imaging complex food materials during their processing within the GI tract. In a recent study( Reference Marciani, Pritchard and Hellier-Woods 4 ), we compared the upper GI processing of a wholemeal bread (WMB) meal with an equienergetic rice pudding (RP) meal. The MRI appearance of the two study meals inside the stomach was markedly different, reflecting very different physico-chemical environments and mobility of water molecules in the different meals. The WMB meal formed a large dark bolus with brighter signal around it in the stomach. The RP meal showed instead a darker layer at the bottom of the stomach consistent with sedimented particulate with a brighter (greater water content) layer above. Despite the higher volume of the RP meal, we demonstrated that the WMB emptied more slowly than did the RP meal.
Building on the previous study, we planned an open-label mechanistic crossover trial in healthy volunteers to explore the effects of breads with increasing gluten content on GI volumes evaluated with MRI. On the basis of previous studies, our first hypothesis was that increasing gluten content will increase gastric content viscosity leading to less rapid breakdown of bread structure and hence greater heterogeneity and delayed gastric emptying, possibly explaining the bloating and other symptoms that some people report. Our secondary end points were also to evaluate GI symptoms, the small bowel water content (SBWC), colonic volumes and colonic gas content.
Methods
Test meals
We analysed the three study breads for total energy and macronutrient composition (Table 1). The bread was spread with margarine and raspberry jam and was consumed with 100 ml orange juice.
Table 1 Macronutrient composition of the test meals per 100 g of bread
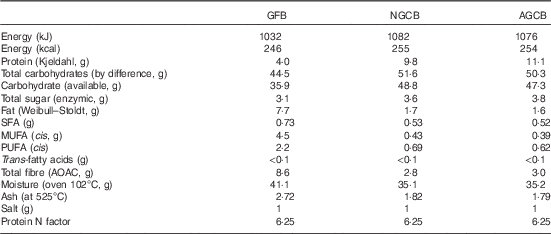
GFB, gluten-free bread; NGCB, normal gluten content bread; AGCB, added gluten content bread; AOAC, Association of Analytical Communities.
Gluten-free bread (GFB) was purchased from Warburtons Limited. Normal gluten content bread (NGCB) and added gluten content bread (AGCB) were manufactured by Campden BRI using the Chorleywood bread process. NGCB was produced using a commercial UK bread flour (Centurian); AGCB was supplemented with 3 % (flour basis) dried vital wheat gluten (Roquette UK Ltd); the amount of water was adjusted to produce loaves that are comparable to commercial UK plant bread. The bread was presented as a sliced loaf. Each slice was 37·5 g in weight. NGCB and AGCB breads were coloured with small amounts of food-grade yellow-orange carotene (E160a) dye to clearly label the bread as experimental bread and to provide a potential marker for compliance as the dye can be detected in stool with a strong absorption spectrum at 400–500 nm. The other components of the meal were purchased from a main supermarket (all Sainsbury’s own brand, Sainsbury’s) as follows: pure orange juice from concentrate, margarine, seedless raspberry jam. The three meals were designed to be the same weight and volume. Also appearance and temperature were similar between meals. Single portions of the meal were given to the subjects to be eaten at home twice a day (at breakfast and with the evening meal) starting from 2 d before the study day.
The amount of bread to be eaten for every meal was 150 g (four slices), accompanied with 24 g of margarine and 34 g of seedless raspberry jam. Also 100 ml of concentrated orange juice was drunk. The participants were instructed to consume the bread at room temperature, to not toast the bread and to spread the jam evenly on the bread and to drink the juice with the meal. The maximum time to consume the meal was 15 min. Each test meal weighed 308 g mass, providing 2698–2757 kJ (645–659 kcal) energy, with only a small difference between the three meals.
Subjects
Totally, twenty subjects (ten females and ten males, 26·1 (SD 1·3) and 27·6 (SD 2·1) years old, respectively, BMI 21·8 (SD 1·6) and 22·6 (SD 1·8) kg/m2, respectively) were screened. A total of twelve subjects completed the study with a full data set for analysis. They were six females and six males, aged 28·3 (SD 9·9) and 25·8 (SD 6·9) years, respectively, and with a BMI of 22·7 (SD 2·1) and 22·8 (SD 1·6) kg/m2, respectively. Seven subjects were excluded, because they failed to attend the first study day, and one subject was excluded because of a diagnosis of irritable bowel syndrome. Inclusion criteria were subjects with age between 18 and 55 years and able to give informed consent.
Exclusion criteria were known allergy to carotene, inability to abstain from smoking for the duration of the study, pregnancy declared by candidate, history of pre-existing GI disorder, history of previous resection of any part of the GI tract other than the appendix or gall bladder, contraindications for MRI scanning, taking any drug known to alter GI motility in the 2 weeks before the test, antibiotic or probiotic treatment in the past 4 weeks, inability to lie flat or exceeding the scanner limits of weight <120 kg, participation in night shift works (between midnight and 06.00 hours) the week before the study day, strenuous exercise >10 h/week and participation in any medical trials for the past 3 months.
All subjects completed also a Rome III Bowel Symptom Questionnaire to exclude a functional bowel disorder and an MRI safety questionnaire. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Nottingham University’s Medical School Research Ethics Committee. All volunteers gave informed written consents before experiments. The trial registration name was ‘Effects of Bread Gluten Content on Gastrointestinal Volumes (EGG)’; the registration identification number was NCT02104115, and the URL for the registry is https://clinicaltrials.gov/ct2/show/NCT02104115?term=bread+gluten+content&rank=1
Study design
This study was a randomised, mechanistic, open-label, crossover investigation. Volunteers were studied on three separate days, 1 week apart, consuming one of the three different breads each week. Each study meal (four slices of the study breads, margarine, raspberry jam and orange juice) was consumed twice a day starting from 2 d before the study day. The subjects were asked to fast from 20.00 hours the previous evening and to avoid alcohol, caffeine, strenuous exercise and any medication that could affect GI function for 18 h before the experiment. They were only allowed a small glass of water on waking. They filled a questionnaire to ensure adherence to the study day restrictions. They underwent a baseline fasted scan from 08.30 hours (defined at t=−45 min time point). At 09.00 hours, they were asked to eat their study meal within a maximum time of 15 min, and at 09.15 hours the subjects underwent the first immediate postprandial scan (defined as t=0). This was followed by a scan every 60 up to 360 min. On completion of each scan, they filled a 100 mm visual analogue scale symptom questionnaire scoring their feeling of fullness, hunger, desire to eat, bloating, flatulence, abdominal pain and diarrhoea.
MRI
Images were acquired on a whole-body 1·5 T scanner (Achieva; Philips Medical System). Volunteers were positioned supine with a sixteen-element SENSE receiver coil wrapped around the abdomen, and each period of imaging lasted between 5 and 10 min, after which volunteers were removed from the scanner and allowed to sit upright.
Images of the stomach were acquired using a balanced turbo field echo (BTFE or TrueFISP) sequence. A total of twenty five transverse images were acquired with the following sequence parameters: field of view (FOV) 400×320 mm, acquired resolution 2·01×1·76 mm2, slice thickness 10 mm, no slice gap, repetition time (TR) 2·8 ms, echo time (TE) 1·4 ms, flip angle (FA)=80°, matrix size 256×256, SENSE factor 2 images of fluid in the abdomen 0, and 1 excitation acquired in a 9 s breath-hold.
Images of fluid in the abdomen were acquired using a single-shot, fast-spin echo sequence (rapid acquisition with relaxation enhancement). A total of twenty-four coronal images were acquired using the following sequence parameters: FOV 400×400 mm, acquired resolution 1·56×2·9 mm, slice thickness 7 mm, no slice gap, TR=8000 ms, TE=320 ms, fat saturation SPIR, matrix size 512×512, and 1 excitation acquired in a 24 s breath-hold. These images were used to measure the SBWC.
The abdomen was also imaged using a dual echo fast-field echo sequence. A total of twenty four coronal images were acquired using the following sequence parameters: FOV 450×362 mm, acquired resolution 2·01×2·87, slice thickness 7 mm, no slice gap, TR=158 ms, TE1:TE2·2·3:4·6 ms, FA=80°, matrix 256×256, SENSE factor 1·7, phase over sampling 2·0, matrix size 256×256, 1 excitation acquired in a 13 s breath-hold. These images were used to measure colonic volumes and to estimate colonic gas volumes. For the gas volume analysis, an additional set of images with the radiofrequency excitation switched off were acquired at baseline only to evaluate the noise distribution.
Data analysis
Gastric volumes were measured by manually tracing a region of interest around the meal and around the gas within the stomach on each image slice using Analyze9™ software (Mayo Foundation) and then summing across slices. At each time point, the sum of solid meal and gas volume gave the corresponding total gastric volume. Gastric half-emptying times T 50 % were calculated using a three-parameter fit allowing a lag time before an exponential decay. T 50 % was then calculated as the time when half the volume had emptied. The initial volume was also a fitted parameter.
The apparent heterogeneity score of the stomach fundus was recorded by analysing MRI images of the stomach using the gastric volume sequence to develop a scorecard. The images were graded on the basis of the heterogeneity of the chyme present, with 5 representing chyme that had an overall sharp edge with visibly sharp-edged lumps within the mass. Image 3 represented a heterogeneous single mass with unidentifiable lumps present, and a score of 0 represented homogenised chyme with no evidence of undigested bread (Fig. 1). Using the scorecard, the images from each visit were then scored by a single operator who was blind to the bread type.

Fig. 1 Fundal heterogeneity scoring card. The apparent heterogeneity of the chyme was graded developing a score card 0–5. Five represents chyme that had an overall sharp edge with visibly sharp edged lumps within the mass. Three represents a heterogeneous mass with some unidentifiable lumps present. Images were taken at t=60 min.
The SBWC was measured using in-house software and methods previously validated( Reference Hoad, Marciani and Foley 5 ). Briefly, bright water signal from organs other than the small bowel is segmented manually, leaving only pixels containing water signal above a calculated threshold to integrate.
Individual regional colon volumes were manually segmented from the coronal data on each image slice using Analyze9™ software, as previously described( Reference Pritchard, Marciani and Garsed 6 ). Regional boundaries commenced at caecum (ascending) and were fixed in a coronal plane at the superior point of the hepatic flexure (ascending to transverse) and splenic flexure (transverse to descending) and terminated at the sagittal plane of the commencement of sigmoid colon where the descending colon deviates posteriorly or medially. Each colon region was identified within each coronal image slice, building a 3D representation of the morphology from which the volume of each region was measured.
Colonic gas volumes were estimated after measuring colonic volumes. The regions of the positions of the colonic segments (defined above), original dual echo data, and baseline noise data were loaded into custom-written software (IDL® 6.4; Research Systems Inc.). Regions of gas were determined automatically on a slice-by-slice basis (due to the variation in noise across the slices). For each slice, the noise distribution of a colonic region (ascending colon (AC), transverse colon (TC) and descending colon (DC)) was defined (means and standard deviations, and then voxels from the colonic image data that were below a threshold of mean plus three standard deviations for both echo 1 and echo 2 and were connected to at least four other pixels were estimated as colonic gas. These gas volumes were then summed across the slices for each colonic region, and regional volumes were summed to estimate a total gas volume.
Power and statistical analysis
We have published previously data( Reference Marciani, Pritchard and Hellier-Woods 4 ) on gastric emptying of WMB, which contained approximately 1·5 % gluten. In that study the half gastric emptying time T 50 % (gastric half emptying time) was 132 (sd 26) min, n 12 healthy volunteers. On the basis of that, in a paired study using n 15 subjects and power of 90 % we calculated we could detect with P<0·05 a 15 % difference in T 50 % between the different breads, which we consider the minimal clinically relevant difference. We planned to recruit n 18 to allow for dropouts.
Data were assessed for normality using the Shapiro–Wilk’s test. Normally distributed data were assessed using parametric methods, and non-parametric analyses were applied to non-normally distributed data. Differences in postprandial gastric volume V0 and gastric emptying T 50 % were assessed using one-way ANOVA followed by Tukey’s post hoc test.
Results
Appearance of the breads in the stomach
Fig. 2 shows a significant difference in heterogeneity score between the three different types of bread. Using Friedman’s ANOVA, the heterogeneity of each bread was shown to be significantly different (P=0·0030) with the heterogeneity scores for GFB and AGCB also showing a significant difference; bread with added gluten showed almost double the mean heterogeneity score AUC 1–3 h (5·5 (interquartile range (IQR) 0·5)) than did the GFB (3·32 (IQR 0·5)).

Fig. 2 Heterogeneity scores of the gastric fundus showing the AUC 1–3 h after feeding with the three study meals. GFB, gluten free bread; NGCB, normal gluten content bread; AGCB, added gluten content bread. Values are medians (n 12), with interquartile ranges represented by vertical bars. Friedman’s ANOVA P = 0·003. * P<0·05 (Dunn’s multiple comparisons test).
Gastric emptying
The postprandial gastric volumes at T=0 are reported in Table 2. They did not differ between the three bread meals (P=0·2242) in keeping with the similar weight and volume of the three kinds of bread given to the participants. The MRI appearance of the GFB differed when compared with the other two study meals inside the stomach (Fig. 3). The GFB meal formed a more homogeneous bolus occupying the whole stomach, whereas the NGCB and AGCB meals showed darker boluses separated by brighter liquid phase.
Table 2 MRI parameters measured from n 12 healthy adult participants who were fed three different gluten content study meals (Mean values with their standard errors)
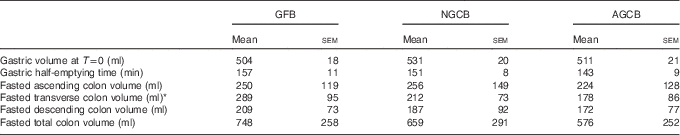
GFB, gluten-free bread; NGCB, normal gluten content bread; AGCB, added gluten content bread.
* P=0·02 with Kruskal–Wallis analysis and Dunn’s for multiple comparison test.

Fig. 3 Representative example of axial MRI images of the abdomen of a healthy volunteer fed with the gluten free bread (GFB), normal gluten content bread (NGCB) and added gluten content bread (AGCB) on three different occasions. Images were taken at t=0 min.
The plots of gastric volumes against time were similar for the three different meals (Fig. 4). There was no difference in the half emptying time T 50 % between the three meals (Table 2; P=0·1332). The gastric volume had mostly returned to baseline for all meals by T=360 min (n 4 for GFB, n 4 for NGCB and n 3 for AGCB had not yet returned to baseline by 360 min). The volumes of intragastric gas measured were variable ranging from 10 to 93 ml with no differences between meals at the different time points (P>0·05).

Fig. 4 Plot of the volume of the gastric contents for the healthy volunteers after they consumed the three different study breads. ![]() , Gluten-free bread (GFB);
, Gluten-free bread (GFB); ![]() , normal gluten content bread (NGCB);
, normal gluten content bread (NGCB); ![]() , added gluten content bread (AGCB). Values are means (n 12), with standard deviations represented by vertical bars.
, added gluten content bread (AGCB). Values are means (n 12), with standard deviations represented by vertical bars.
Small bowel water content
The SBWC data are shown in Fig. 5. The mean fasted SBWC was 54 (SD 41) ml for the three study breads. The three study meals induced an initial drop in SBWC after feeding. After 240 min (4 h after feeding) there was a similar rise in SBWC for all three meals. The curves were similar for GFB compared with NGCB and AGCB meals with no significant differences.

Fig. 5 Plot of the volume of the small bowel water content for the healthy volunteers after they consumed the three different study meals. ![]() , Gluten-free bread (GFB);
, Gluten-free bread (GFB); ![]() , normal gluten content bread (NGCB);
, normal gluten content bread (NGCB); ![]() , added gluten content bread (AGCB). Values are means (n 12), with standard deviations represented by vertical bars.
, added gluten content bread (AGCB). Values are means (n 12), with standard deviations represented by vertical bars.
Colonic volumes
Fasted total and regional colonic volumes are shown in Table 2. Fasted transverse colonic volume was significantly higher after GFB compared with NGCB and AGCB (P=0·02; Fig. 6). Total and regional colonic volumes after feeding were similar for the different study meals (two-way log-transformed ANOVA effect of bread type not significant). Similarly, the amount of colonic gas was low (median value between 2 and 4 ml for the three breads with an interquartile range between 1 and 6 ml) and similar at the different time points with the three study meals.

Fig. 6 Fasted colonic transverse volume (t=–45 min) after 2 d preload with the three study meals. ![]() , Gluten-free bread (GFB);
, Gluten-free bread (GFB); ![]() , normal gluten content bread (NGCB);
, normal gluten content bread (NGCB); ![]() , added gluten content bread (AGCB). Values are medians (n 12), with interquartile ranges represented by vertical bars. Kruskall–Wallis analysis for non-parametric data P=0·02. * P<0·05 (Dunn’s multiple comparisons test).
, added gluten content bread (AGCB). Values are medians (n 12), with interquartile ranges represented by vertical bars. Kruskall–Wallis analysis for non-parametric data P=0·02. * P<0·05 (Dunn’s multiple comparisons test).
Symptoms
The fullness scores showed the expected pattern with an immediate rise after eating the meal and declining with time, reaching again baseline values towards the end of the experiment at T=360 min, with no significant differences between meals (P>0·05). As predicted, these healthy subjects scored zero or very low symptoms with no postprandial differences between meals for fullness, hunger, desire to eat, flatulence, bloating, abdominal pain and diarrhoea (P>0·05). Bloating scores after GFB were slightly higher compared with NGCB and AGCB, but there was no correlation between bloating scores and total colonic volumes with the three study meals (P=0·62, r −0·16). Moreover, there was no correlation between gastric volume at 60 min after feeding (time point 3) and fullness with the three study breads (P>0·05).
Discussion
On the basis of our previous study( Reference Marciani, Pritchard and Hellier-Woods 4 ), our first hypothesis was that increasing bread gluten content will increase gastric content heterogeneity and delay gastric emptying. Our results only partially confirmed this hypothesis.
The three study meals had rather different appearances in the stomach. Confirming our previous study( Reference Marciani, Pritchard and Hellier-Woods 4 ), the MRI signal intensity of the GFB was higher (brighter images) compared with the WMB, indicating higher content of mobile water. We demonstrated using our scoring system that increasing the gluten content of the study meal increased the fundal chyme heterogeneity with AGCB>NGCB>GFB. This may be related to the physical and chemical properties of the different kind of breads. GFB is essentially starch with polysaccharide gums acting to stabilise the crumb structure, whereas wheat bread consists of a protein (gluten) and starch matrix that is inherently more coherent and likely to remain as discrete lumps after chewing. The scoring of the apparent heterogeneity of the luminal contents was carried out subjectively. Automated computational image analysis methods could carry out this task objectively in future work. Our second hypothesis was that GFB empties faster from the stomach compared with the same volume of NGCB and AGCB. Despite the different appearance and physical properties of the three study meals, gastric emptying times were similar showing that the stomach can compensate for differing physical properties to minimise their influence on gastric emptying. Previously we have demonstrated that a RP meal has a faster gastric emptying compared with WMB, which we interpreted as being due to the greater viscosity of the bread meal( Reference Marciani, Pritchard and Hellier-Woods 4 ). Moreover, according to the intragastric appearance of the three study meals, it was possible that sieving( Reference Meyer, Thomson and Cohen 7 – Reference Marciani, Gowland and Fillery-Travis 9 ) would allow faster gastric emptying of liquids and small fragments of the GFB compared with solids and large fragments of the other two kinds of bread, which were retained by the stomach to undergo antral grinding. Higher mobile water content shown by the GFB in the stomach should have lowered viscosity( Reference Brown, Schulze-Delrieu and Schrier 10 , Reference Marciani, Gowland and Spiller 11 ), but despite this gastric emptying did not differ. It appears therefore, as we have previously shown with more artificial meals, that gastric emptying rate is more dependent on meal energy content than intragastric viscosity( Reference Marciani, Gowland and Spiller 11 ).
The appearance of the mean SBWC time course resembles previous reports( Reference Hoad, Marciani and Foley 5 , Reference Marciani, Cox and Hoad 12 ). The subjects’ mean fasted baseline SBWC was less than the mean values demonstrated in previous studies probably because of the characteristics of the study meal and the 2-d preload with an amount of bread (8 slices/d, 300 g/d) greater than that is normally consumed. Postprandially, the SBWC signal fell to the same extent with each meal, with the minimum values 120 min after feeding and rising thereafter. The early drop in SBWC is likely related to the absorption of glucose and sucrose in the liquid phase (jam and orange juice) of the meal( Reference Hinder and Kelly 8 , Reference Collins, Houghton and Read 13 ) and to the gastro-ileal reflex after feeding, which empties the ileal contents into the ascending colon. The later rise in SBWC is likely to be dependent on pancreaticobiliary and enterocyte secretions after the arrival of protein and fat into the small bowel. We have previously shown that the bran increases the SBWC( Reference Hebden, Blackshaw and D’Amato 14 ). The lack of effect of gluten suggests that this effect requires a particulate rather than just increased viscosity.
The significantly higher volume of the fasting transverse colon after GFB compared with breads containing gluten was unexpected. It is likely because of the 2-d preload with the GFB in which wheat is substituted by tapioca and potato flours. The GFB fibre content at 8·6/100 g was higher than that of the NGCB and AGCB at 2·8 and 3·0/100 g. This increase in fibre would be expected to increase bacterial mass and metabolism that may have contributed to the larger colonic volumes .Moreover, the bread was made and frozen to allow use of a single batch. The freezing is known to cause the development of partially retrograded starch that is resistant to amylase and hence enters the colon where it acts as a prebiotic, providing substrate for bacterial metabolism. The presence of gums may also have influenced digestibility.
We did not find any correlation between bloating scores and colonic volumes in this group. Also fundal chyme heterogeneity scores did not correlate with any symptom. To determine whether these MRI parameters correlate with the time course of the symptoms that many patients experience will require studies on patients, as healthy volunteers rarely develop symptoms with these test meals.
Our analysis showed similar energy content for the three study breads; however, GFB had a higher fibre and fat content (vegetable oils), which helps to soften the bread crumb and reduce the rate of staling. GFB also contained egg, which is added to help the bread structure and as a source of protein.
In conclusion, in healthy subjects intragastric behaviours of breads with different gluten content are different but gastric emptying times are similar. The 2-d preload with GFB increases the volume of the fasting colon, possibly because of the increased resistant starch from the potato and tapioca flours in GFB. Our findings suggest that when evaluating the effect of GF diets in patients it will be important to consider the effect of added components as well as just the removal of gluten. Our data support the idea that GF diets do not exert their effect neither by altering gastric emptying nor by reducing colonic volumes. It leaves open whether the apparent intolerance of gluten products in those without the evidence of CD is in fact because of a nocebo effect based on belief rather than objective changes in GI function as has recently been clearly demonstrated in a randomised placebo-controlled trial( Reference Biesiekierski, Peters and Newnham 15 ).
Acknowledgements
The authors would like to thank the National Institute for Health Research (NIHR) Biomedical Research Unit in Gastrointestinal and Liver Diseases at Nottingham University Hospitals NHS Trust and The University of Nottingham for help with preparing test meal. This is a summary of an independent research funded by the NIHR Biomedical Research Unit. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
This work was supported by an internal academic funding.
The authors’ responsibilities were as follows: F. K. G., L. M., P. A. G. and R. C. S. designed research; F. K. G. designed and produced the gluten-containing breads; M. C., L. M., H. S., G. M., C. L. H. and G. C. conducted research; M. C., L. M., H. S., C. L. H. and G. C. analysed data and samples; M. C., F. K. G., L. M., H. S., C. L. H., P. A. G. and R. C. S. wrote paper; R. C. S. had the primary responsibility for final content. All authors read and approved the final manuscript.
There are no conflicts of interest.











