Age-related macular degeneration (AMD) is one of the main causes of irreversible blindness in older people( Reference Friedman, O'Colmain and Muñoz 1 ). It is a complex disease triggered by factors that are fundamentally associated with old age and genetic and environmental alterations( Reference Ambati, Ambati and Yoo 2 ). Histopathological studies have shown that retinal pigment epithelium (RPE), Bruch's membrane and choriocapillaris are primarily involved in this pathological process( Reference Pauleikhoff, Harper and Marshall 3 ). It has been suggested that a gradual anomalous deposition of lipids in Bruch's membrane( Reference Pauleikhoff, Harper and Marshall 3 ), originating from the dysfunction of RPE cells( Reference Ruberti, Curcio and Millican 4 ), leads to an increase in its thickness( Reference Ramrattan, van der Schaft and Mooy 5 ), directly interfering with the metabolism of sensory retina, RPE and choriocapillaris( Reference Pauleikhoff, Harper and Marshall 3 , Reference Ramrattan, van der Schaft and Mooy 5 – Reference Korte, Reppucci and Henkind 8 ). It is known that oxidised LDL represent an important stimulus for the increase in the concentrations of chemotactic and adhesion molecules that attract macrophages. These cells produce inflammatory cytokines, tissue factors, vascular endothelial growth factor and other angiogenic factors( Reference Sakurai, Anand and Ambati 9 – Reference Higgins, Wang and Dockery 13 ), triggering or worsening the macular degenerative process( Reference Penfold, Provis and Billson 14 ).
It has already been documented experimentally that a cholesterol-enriched diet induces an increase in the concentrations of chemotactic molecules and adhesion molecules and a consequent increase in the accumulation of macrophages in the choroid and sclera( Reference Torres, Noronha and Casella 15 – Reference Torres, Muccioli and Maia 17 ). It has also been demonstrated that this diet induces a retinal suffering( Reference Torres, Maia and Précoma 18 ) and a consequent increase in the expression of NO synthase (NOS) 2( Reference Yücel, Akar and Yücel 19 ). Both the increase in macrophage accumulation and ischaemia may be responsible for the increase in vascular endothelial growth factor expression observed in the choroid–sclera complex of hypercholesterolaemic rabbits( Reference Torres, de Noronha and Casella 20 ). Consequently, a hypercholesterolaemic diet can experimentally simulate the alterations observed in AMD.
Flaxseed is a functional food rich in α-linolenic acid that exhibits anti-inflammatory, antithrombotic and antihypertensive effects( Reference Simopoulos 21 , Reference Uauy and Valenzuela 22 ). Epidemiological studies have shown that there is an association between the consumption of n-3 fatty acids and the prevention of AMD, as it slows the progression of the disease at its early stages( Reference Seddon, Rosner and Sperduto 23 – Reference SanGiovanni, Chew and Clemons 25 ). Lignans, another component of flaxseed, inhibit the proliferation of vascular endothelial cells and reduce the oxidation of LDL( Reference Prasad 26 , Reference Touré and Xueming 27 ). Due to the significant involvement of oxidative stress in the pathogenesis of AMD( Reference Beatty, Koh and Henson 28 ), flaxseed can potentially inhibit the progression of macular degenerative disease. In addition, its fibres decrease cholesterol concentrations in the blood and liver( Reference Camire and Dougherty 29 , Reference Savaiano 30 ), playing a beneficial role in the evolution of AMD( Reference Dashti, McGwin and Owsley 31 – Reference Friedman 33 ).
The aim of the present study was to evaluate the effect of flaxseed on choroid–sclera complex thickness and on LDL oxidation in the sclera, choroid and retina of diet-induced hypercholesterolaemic rabbits.
Methods
The study protocol was approved by the Animal Experimentation Ethics Committee of the Pontificia Universidade Catolica do Parana and was implemented in compliance with the guidelines established by the Association for Research in Vision and Ophthalmology.
Experiment environment
The experimental procedures were performed at the Surgical Technique Laboratory at PUC-PR and the Study Center of the Hospital Angelina Caron. The animals were kept in the bioterium (macroenvironment) under a 12 h light–12 h dark cycle, with air changes and room temperature maintained between 19 and 23°C. The animals were given water and standard Nuvilab® (Nuvital) rabbit chow (Table 1) ad libitum 2 weeks before the start of the experiment.
Table 1 Composition of Nuvilab® (Nuvital) rabbit feed*

* The feed contained the following constituents: whole ground maize; wheat bran; soyabean bran; leguminous hay; limestone; bicalcium phosphate; NaCl; vitamin and mineral premix; amino acids.
Animals used and experiment outline
A total of twenty-one 4-month-old New Zealand male albino rabbits (Oryctolagus cuniculus), weighing about 1·5 kg, were used in the study. The animals were divided into two groups: group 1 (G1; eleven animals), fed the standard rabbit diet Nuvilab® (Nuvital) enriched with 0·5 % cholesterol freeze-dried egg for 8 weeks, and group 2 (G2; ten animals), fed the same diet provided to G1 rabbits plus 8 g/kg ground brown flaxseed of Brazilian origin. Each G2 rabbit was fed an average amount of 30 g of flaxseed for 8 weeks. The 0·5 % cholesterol-enriched diet, used throughout the experiment, was obtained by diluting 600 g of powdered egg in 500 ml of water.
The serum concentrations of total cholesterol (TC), LDL-cholesterol (LDL-C), TAG and fasting glucose of each rabbit were measured at the start of the experiment and at the time of killing. These concentrations were determined with an automated enzymatic method, using the Bayer ADVIA 1200® Clinical Chemistry System (Siemens). Blood samples were collected by marginal ear vein puncture under general anaesthesia with 15 mg/kg of ketamine and 35 mg/kg of xylazine. The animals were killed under endovenous anaesthesia with 5 ml of pentobarbital, and their eyes were immediately placed in 4 % paraformaldehyde (Merck) in 0·1 m-phosphate (pH 7·4) for 4 h for immunohistochemical analysis.
Histomorphometric analysis (quantitative)
The eyes of each rabbit were removed and fixed for analysis. However, only one eye was randomly selected. After fixation, the samples were evaluated macroscopically using a coronal section at the optic nerve level, dividing the eye globes into two equal halves (lower and upper). The lower half was stored for future studies. The upper half was dehydrated, diaphanised and embedded in paraffin using a Leica® histotechnique system (Leica), model TP 1020. The Leica® EG 1160 Embedding Station (Leica) was used to prepare the paraffin blocks. These blocks were sectioned at 5 μm using a Leica® RM2145 microtome (Leica) to obtain histological sections, which were then placed on albumin-smeared glass slides, stained with haematoxylin–eosin and mounted on 24 × 90 mm cover slips, with Entellan Mounting Media (Merck).
The haematoxylin–eosin-stained slides were evaluated with the aid of a 4 × objective lens and a blue overhead projector marker for quantitative analysis. The posterior portion of the hemi-sectioned ocular globe was manually divided into ten equal segments (from the pars plana to the contralateral pars plana). Images of the segments were obtained using an Olympus BX50 microscope (Olympus) coupled to a Sony camera (Sony Corporation). The choroid–sclera complex thickness of the ten segments was determined by performing four linear morphometric measurements in each captured image using the Image Pro Plus® software (Media Cybernetics, Inc.). Later, the mean of the four measurements of each of the ten segments was obtained. The thickness is expressed in μm.
Tissue preparation and immunohistochemical analysis
The histological slides were deparaffinised and rehydrated and then treated to block the endogenous peroxidase. The slides were washed with deionised water and incubated in a wet chamber at 95°C for 20 min for antigen recovery. After this step, the endogenous peroxidase was blocked again. The slides were stained with Abcam® anti-oxidised LDL antibody of mouse origin, at a 1:600 dilution, for the analysis of choroid and sclera samples. The mouse Santa Cruz® (Santa Cruz Biotechnology) primary monoclonal nitrotyrosine, at a 1:50 dilution, was used for the analysis of sensory retina samples. The slides were then stained with a secondary antibody, Polymer HRP anti-mouse/rat Detection System (DakoCytomation, Inc.), and incubated at room temperature for 30 min. They were then stained by dripping a freshly prepared diaminobenzidine-mixed substrate (DakoCytomation, Inc.) and once again incubated for 3–5 min. The slides were counterstained with Mayer haematoxylin and mounted.
Positive and negative controls were used in all evaluations, and the slides were initially analysed by a masked observer. In this analysis, positive and negative results were recorded for the oxidised LDL and nitrotyrosine markers. The positive areas acquired a brownish hue and were studied by colour morphometry. This procedure was performed by capturing images of five consecutive fields, from the pars plana to the contralateral pars plana, with a 40 × objective lens coupled to a Olympus BX50 microscope (Olympus), which was coupled to a Sony DXC-107A camera (Sony Corporation) and the Image Pro Plus® software (Media Cybernetics, Inc.). This software enabled the observer to select and colour the positive areas and automatically calculate the immunoreactive area, expressed in μm2. The data obtained were compiled into a Microsoft Excel spreadsheet (Microsoft Corporation) for statistical analysis. The variable immunoreactive area represents the sum of all positive areas in each of the five studied fields. This colour morphometry method has already been used in other studies( Reference Torres, Noronha and Casella 15 – Reference Torres, Maia and Précoma 18 , Reference Torres, de Noronha and Casella 20 ).
Statistical analysis
Student's t test for independent samples was used to compare the treatment groups in relation to quantitative variables. The evaluations performed at the start of the experiment and at the time of killing were compared using Student's t test for paired samples. The normality condition was evaluated using the Shapiro–Wilk test. The variables that did not present a symmetric condition were submitted to a logarithmic transformation. P values < 0·05 indicated statistical significance. STATISTICA version 8.0 (StatSoft) was used for data processing.
Results
Biochemical variables
The average daily hypercholesterolaemic diet intake was approximately 200 g in both groups. At the start of the experiment, there was no significant difference in the biochemical variables and weight between G1 and G2 rabbits. At the time of killing, a significant increase was observed in TC concentrations in both groups, which was greater in G1 rabbits than in G2 rabbits. Nevertheless, the increase in G2 rabbits was approximately five times the initial level, whereas that in G1 rabbits was eight times the initial level. Similar results were recorded for LDL-C concentrations, with G1 rabbits exhibiting a significant increase when compared with G2 rabbits at the end of the experiment.
No significant changes were observed in the remaining variables. Values of the biochemical variables and weight at the start and end of the experiment (at the time of killing) are given in Table 2. P values for the differences in the biochemical variables and weight at the start and end of the experiment are given in Table 3.
Table 2 Values of the biochemical variables and weight at the start and end of the experiment (Mean values and standard deviations; median, minimum and maximum values)

G1, cholesterol-enriched diet group; G2, cholesterol-enriched diet plus flaxseed supplementation group; Dif, difference.
* Student's t test for independent samples (P< 0·05).
Table 3 P values* for the differences in the biochemical variables and weight at the start and end of the experiment

G1, cholesterol-enriched diet group; G2, cholesterol-enriched diet plus flaxseed supplementation group.
* Student's t test for paired samples (P< 0·05).
Histomorphometric analysis with haematoxylin–eosin
There was an increase in the concentrations of macrophages in G1 rabbits when compared with G2 rabbits (Fig. 1), which induced a significant increase in choroid–sclera complex thickness in G1 rabbits when compared with G2 rabbits (P< 0·001; Table 4).

Fig. 1 Histomorphometric analysis of choroid (C) and sclera (S) samples with haematoxylin–eosin. Magnification 200 × . (a) C–S complex of the cholesterol-enriched diet group (G1). A large number of histiocytes (H), responsible for C–S complex thickness, were observed. (b) C–S complex of the cholesterol-enriched diet plus flaxseed supplementation group. Few H and a thinner layer were observed when compared with the samples of G1 rabbits.
Table 4 Choroid and sclera morphometry (in μm) (Mean values and standard deviations; median, minimum and maximum values)
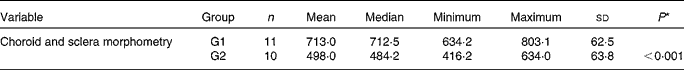
G1, cholesterol-enriched diet group; G2, cholesterol-enriched diet plus flaxseed supplementation group.
* Student's t test for independent samples (P< 0·05).
Immunohistochemical analysis of the choroid and sclera with the anti-oxidised LDL marker
A significant increase was observed in the expression of the anti-oxidised LDL marker in the choroid and sclera of G1 rabbits when compared with G2 rabbits (P< 0·001; Table 5). A brownish hue was predominant in the choroid–sclera complex of G1 rabbits, revealing a high immunoreactivity to this marker (Fig. 2). Conversely, a bluish hue was predominant in the choroid–sclera complex of G2 rabbits, revealing a low immunoreactivity to this marker.
Table 5 Total area immunoreactive to the anti-oxidised LDL marker in the choroid and sclera calculated by means of colour morphometry (μm2) (Mean values and standard deviations; median, minimum and maximum values)

G1, cholesterol-enriched diet group; G2, cholesterol-enriched diet plus flaxseed supplementation group.
* Student t test for independent samples (P< 0·05).

Fig. 2 Immunohistochemical analysis of choroid (C) and sclera (S) samples with the anti-oxidised LDL marker. Magnification 200 × . (a) C–S complex of the cholesterol-enriched diet group. The C and S exhibited a high immunoreactivity to the anti-oxidised LDL marker, characterised by the predominance of a brownish hue. (b) C–S complex of the cholesterol-enriched diet plus flaxseed supplementation group. The C and S exhibited a low immunoreactivity to the anti-oxidised LDL marker, characterised by the predominance of a bluish hue.
Immunohistochemical analysis of the retina with the anti-nitrotyrosine marker
A significant increase was observed in the expression of the anti-nitrotyrosine marker in G1 rabbits when compared with G2 rabbits (P= 0·002; Table 6). A brownish hue was predominant in the sensory retina of G1 rabbits, revealing a high immunoreactivity to this marker (Fig. 3). The internal plexiform layer exhibited a higher immunoreactivity to this marker when compared with the other layers. Conversely, a bluish hue was predominant in the retina of G2 rabbits, revealing a low immunoreactivity to this marker.
Table 6 Total area immunoreactive to the anti-nitrotyrosine marker in the retina calculated by means of colour morphometry (μm2) (Mean values and standard deviations; median, minimum and maximum values)
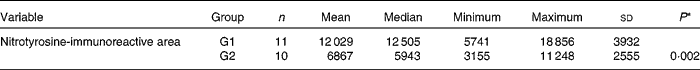
G1, cholesterol-enriched diet group; G2, cholesterol-enriched diet plus flaxseed supplementation group.
* Student t test for independent samples (P< 0·05).

Fig. 3 Immunohistochemical analysis of retina samples with the anti-nitrotyrosine marker. (a) Retina of the cholesterol-enriched diet group. The retinal layers, mainly the inner plexiform layer, exhibited a high immunoreactivity to the anti-nitrotyrosine marker, characterised by the predominance of a brownish hue. (b) Retina of the cholesterol-enriched diet plus flaxseed supplementation group. The retinal layers exhibited a low immunoreactivity to the anti-nitrotyrosine marker, characterised by the predominance of a bluish hue. PRL, photoreceptor layer; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Magnification 200 × .
Discussion
In the present study, diet-induced hypercholesterolaemic rabbits were supplemented with flaxseed to investigate its effect on choroid–sclera complex thickness and on LDL oxidation in the sclera, choroid and retina. It is known that in the great vessels, such as the coronary and carotid arteries, dyslipidaemia induces endothelial dysfunction, permitting the passive entry of LDL into the vascular intima, where the LDL become oxidised by the action of reactive oxygen species. Oxidised LDL stimulate the endothelial cells of the vessels to increase the expression of vascular adhesion molecules (P-selectin, intercellular adhesion molecule and vascular cell adhesion molecule 1), which, along with monocyte chemoattractant protein-1 ligands and their C-C chemokine receptor type 2 receptors, facilitate the activation of monocytes and their adhesion to the endothelium. The monocytes, by means of the scavenger receptors (CD36 and SR-A), absorb the oxidised LDL and form histiocytes. These histiocytes, in turn, express inflammatory cytokines, enzymes and growth factors, which, along with the cytokines released by the activated T cells, promote the inflammatory process and the proliferation and migration of the smooth muscle and endothelial cells into the vessel intima, thus forming the atherosclerotic plaque( Reference Libby 34 – Reference Nicolletti, Caligiuri and Hansson 36 ).
One of the beneficial effects of flaxseed is its role in the reduction of serum TC and LDL-C concentrations. Adequate cholesterol concentrations are important for the prevention of CVD. Studies have reported decreases in plasma TC and LDL-C concentrations ranging from 5 to 15 %, depending on the amount of n-3 consumed( Reference Kris-Entherton, Harris and Appel 37 , Reference Jenkis, Kendall and Marchie 38 ). A study carried out using 50 g/d flaxseed supplementation for 4 weeks has reported reductions of 9 and 18 % in serum TC and LDL-C concentrations, respectively( Reference Cunnane, Ganguli and Menard 39 ). Similarly, another study carried out using 20 and 30 % flaxseed supplementation has reported reductions of 21 and 33 % in plasma TC concentrations and of 33 and 67 % in plasma LDL-C concentrations, respectively( Reference Ratnayake, Behrens and Fischer 40 ). The inclusion of 15 % flaxseed in the diet of hypercholesterolaemic rabbits has been found to prevent the progression of hypercholesterolaemia and to significantly reduce serum TC (13 %) and LDL-C (44 %) concentrations( Reference Shakir and Madhusudhan 41 ). Another study has reported a reduction of 33 % in TC concentrations and of 35 % in LDL-C concentrations( Reference Prasad 42 ). In the present study, both groups exhibited a similar increase in serum TC and LDL-C concentrations. Nevertheless, G1 rabbits exhibited a final cholesterol concentration that was eight times the initial level. On the other hand, G2 rabbits exhibited final plasma TC and LDL-C concentrations that were significantly lower than those of G1 rabbits. These results show the beneficial effects of flaxseed on TC and LDL-C concentrations in G2 rabbits, which did not exhibit an increase in concentrations as G1 rabbits did. Similar findings were recorded in an 8-week study that analysed the effect of lignan in rabbits( Reference Prasad 43 , Reference Lucas, Lightfoot and Hammond 44 ).
It has been experimentally demonstrated that a cholesterol-enriched diet induces an increase in the concentrations of macrophages in the choroid and sclera( Reference Torres, Muccioli and Maia 17 , Reference Salazar, Ramírez and de Hoz 45 ). These cells, considered essential for the origin of atherosclerotic plaques( Reference Libby 34 ), have also been reported to be associated with AMD( Reference Sakurai, Anand and Ambati 9 , Reference Oh, Takagi and Takagi 46 , Reference Cousins, Espinosa-Heidmann and Csaky 47 ). In addition, it has been suggested that AMD and atherosclerosis share similar physiopathogenic mechanisms( Reference Friedman 33 ). The present study corroborates the findings of earlier studies that hypercholesterolaemia may induce the same events in the choroid–sclera complex. The exacerbated expression of oxidised LDL in the choroid and sclera observed in G1 rabbits could account for the increase in the expression of chemotactic molecules( Reference Torres, Noronha and Casella 15 ), such as monocyte chemoattractant protein-1, adhesion molecules( Reference Torres, Luchini and Torres 16 ) and intercellular adhesion molecule-1, which would induce an increase in macrophage concentrations in the choroid–sclera complex and, consequently, an increase in the thickness of the choroid and sclera as observed in the present study and in other studies( Reference Torres, Luchini and Torres 16 , Reference Torres, Muccioli and Maia 17 , Reference Torres, de Noronha and Casella 20 , Reference Torres, de Noronha and Casella 48 ). From the ocular point of view, it is important to note that oxidised LDL, chemotactic molecules, adhesion molecules and macrophages are associated with the physiopathogenesis of AMD and may trigger or worsen this disease( Reference Ambati, Ambati and Yoo 2 , Reference Sakurai, Anand and Ambati 9 , Reference Grossniklaus, Ling and Wallace 10 , Reference Oh, Takagi and Takagi 46 – Reference Sakurai, Taguchi and Anand 49 ).
As in the great vessels( Reference Prasad 26 , Reference Touré and Xueming 27 ), flaxseed flour supplemented to diet-induced hypercholesterolaemic rabbits (G2) reduced oxidised LDL expression in the choroid and sclera significantly. It is known that flaxseed is composed of PUFA, which, among other vascular effects, have anti-inflammatory properties and reduce leucocyte chemotaxis( Reference Simopoulos 21 , Reference Uauy and Valenzuela 22 ). Similarly, flaxseed contains lignans, which inhibit the oxidation of PUFA, reducing LDL oxidation( Reference Prasad 26 , Reference Westcott and Muir 50 ). Hence, these factors may account for the significant reduction of oxidised LDL expression in the choroid–sclera complex of G2 rabbits when compared with G1 rabbits, contributing to a lower concentration of histiocytes in the choroid and sclera. It is important to point out that lignans also reduce the absorption of cholesterol in the intestine, reducing its serum concentrations( Reference Prasad 26 , Reference Westcott and Muir 50 , Reference Prim, Baroncini and Précoma 51 ). This effect was observed in the present study; i.e. TC concentrations were significantly lower in G2 rabbits than in G1 rabbits, contributing directly or indirectly to the histomorphometric and immunohistochemical findings in the choroid–sclera complex of the animals treated with flaxseed flour.
The antioxidant action of flaxseed flour in the retina was evaluated using an anti-nitrotyrosine marker, considered to be a marker of NO and peroxynitrite( Reference Ischiropoulos, Zhu and Beckman 52 ). NO exerts beneficial effects by promoting vasodilation and antiproliferation and may be considered antithrombotic. On the other hand, NO may exert damaging effects, such as cell lesion and apoptosis. Some of the damaging effects of NO are caused by the presence of peroxynitrite, a powerful oxidant that is formed by the reaction of NO and superoxide anion radical (O·– 2)( Reference Lefer, Scalia and Campbell 53 ). Peroxynitrite mediates tyrosine nitration and nitrotyrosine formation, easily detected by anti-nitrotyrosine antibodies( Reference Ischiropoulos, Zhu and Beckman 52 ). Conversely, cell apoptosis induced by an excessive increase in NO concentrations is caused by the constant elevation of intracellular Ca concentrations( Reference Suenobu, Shichiri and Iwashina 54 – Reference Boullerne, Nedelkoska and Benjamins 56 ). On the other hand, NOS, the enzyme responsible for the production of NO free radicals, has three established isoforms, NOS I, NOS II and NOS III, found in different parts of the eye( Reference Behar-Cohen, Goureau and D'Hermies 57 , Reference Park, Pardhasaradhi and Gianotti 58 ). As has been demonstrated earlier, hypercholesterolaemia induces an increase in Ca concentrations in the sensory retina( Reference Torres, Maia and Précoma 18 ) and induces NOS-2 expression, increasing the oxidising injury of the retinal tissue( Reference Yücel, Akar and Yücel 19 ). It is known that NO is a major stimulator of choroidal neovascularisation in AMD( Reference Ando, Yang and Nambu 59 ). In the present study, the retina of G1 rabbits (hypercholesterolaemic) exhibited a high immunoreactivity to the anti-nitrotyrosine marker, as documented in previous studies( Reference Yücel, Akar and Yücel 19 ). Flaxseed supplementation reduced nitrotyrosine expression significantly in G2 rabbits, and the decrease in serum cholesterol concentrations that it promoted may have contributed to this effect. On the other hand, we re-state that flaxseed also inhibits the oxidation of PUFA and reduces the oxidation of LDL( Reference Prasad 26 , Reference Touré and Xueming 27 ), factors that may have preserved the sensory retina of G2 animals.
In the present study, an immunohistochemical technique was used to analyse the sclera, choroid and retina of diet-induced hypercholesterolaemic rabbits. Immunohistochemistry, used to study paraffin-embedded material, enables the researcher to locate and identify the protein in the analysed tissue. We report that the Western blotting technique offers high-sensitivity detection and would improve the analysis of the studied protein. However, this technique is used to study fresh or frozen tissues. As the ocular globes were fixed in paraformaldehyde and then embedded in paraffin, it was not possible to complement the study with the Western blotting technique.
In the present study, it was possible to observe the beneficial effects of flaxseed on the biochemical variables such as TC and LDL-C concentrations, as well as on the choroid and sclera, of diet-induced hypercholesterolaemic rabbits. Nevertheless, future experiments should include an analysis of the plasma fatty acid composition and the plasma lignan content, so that the difference between the two groups regarding the incorporation of the active component of flaxseed can be demonstrated.
Macrophages, oxidised LDL and reactive oxygen species, such as peroxynitrite, play an important role in the genesis of AMD. In the present study, flaxseed was found to preserve the choroid–sclera complex of rabbits fed a cholesterol-enriched diet by inhibiting the migration of macrophages into the choroid and sclera. Flaxseed was also found to reduce LDL oxidation in the sclera, choroid and retina of these animals. Further studies are required to demonstrate whether, apart from the cardioprotective effect, flaxseed can also exert other beneficial effects in AMD.
Acknowledgements
The authors are grateful to the staff of the Graduate Department of the Pontificia Universidade Catolica do Parana for their time and assistance and the Hospital Angelina Caron for allowing the use of the laboratories and equipment for the present experiment. They also thank Professor Marcia Olandoski for helping with the statistical analysis.
The present study received no specific grant from any funding agency or any commercial or not-for-profit sector.
The authors’ contributions are as follows: R. J. d. A. T. made substantial contribution to the study conception and design, analysed and interpreted the data, drafted the manuscript, critically revised the manuscript, approved the final version of the manuscript to be submitted, and was responsible for administrative, technical and material support supervision; A. L. made substantial contribution to the study conception and design, drafted the manuscript, approved the final version of the manuscript to be submitted, and was in charge of the financial and management issues; A. S. B. made substantial contribution to the study conception and design, drafted the manuscript, approved the final version of the manuscript to be submitted, and was responsible for administrative, technical and material support supervision; L. B. P. and M. L. S. collected the data, critically revised the manuscript, approved the final version of the manuscript to be submitted, and carried out the statistical analysis; A. F. C. analysed and interpreted the data, drafted the manuscript, approved the final version of the manuscript to be submitted, and was responsible for technical and material support supervision; R. P. d. A. T. and N. F. d. F. S. collected the data, critically revised the manuscript, approved the final version of the manuscript to be submitted, and was responsible for administrative, technical and material support supervision; L. N. analysed and interpreted the data, critically revised the manuscript, approved the final version of the manuscript to be submitted, and was in charge of the financial and management issues; B. M. P. collected the data, critically revised the manuscript, approved the final version of the manuscript to be submitted, and was in charge of the financial and management issues; L. A. d. A. T. made substantial contribution to the study conception and design, critically revised the manuscript, approved the final version of the manuscript to be submitted, and was responsible for administrative, technical and material support supervision; D. B. P. analysed and interpreted the data, drafted the manuscript, approved the final version of the manuscript to be submitted, and was responsible for research group leadership.
None of the authors has any conflicts of interest to declare.











