The prevalence of obesity is increasing rapidly worldwide and the importance of developing dietary strategies to tackle the problem is being widely acknowledged( 1 , 2 ). Although it is well known that excess energy intake is the primary dietary cause, alterations in food patterns or nutrients must be considered( Reference Slavin 3 ). Consumption of diets high in dietary fibres has been reported to result in a number of beneficial health effects, including a reduced risk of CVD, diabetes, hypertension, obesity and gastrointestinal disorders( Reference Anderson, Baird and Davis 4 ). High dietary fibre intake may prevent weight gain by reducing appetite and energy intake( Reference King, Craig and Pepper 5 , Reference Wanders, van den Borne and de Graaf 6 ). Dietary fibres may decrease intestinal passage time, leading to a more gradual nutrient absorption and prolonged feelings of satiety. The slower nutrient uptake may result in reduced postprandial glucose and insulin responses( Reference Biorklund, van Rees and Mensink 7 – Reference Kendall, Esfahani and Hoffman 11 ). The lower glucose and insulin concentrations may, in turn, result in reduced inhibition of lipolysis, higher circulating NEFA concentrations and consequently increased fat oxidation( Reference Saris 12 , Reference Brand-Miller, McMillan-Price and Steinbeck 13 ). Additionally, dietary fibres can be fermented in the colon, with the production of SCFA (acetate, butyrate and propionate) and gases (H2, CH4 and CO2), which may enhance satiety and affect energy metabolism via various mechanisms( Reference Chinda, Nakaji and Fukuda 14 ). Epidemiological evidence of these health-protective effects of dietary fibres has led to industrial interest in developing novel dietary fibres that can be incorporated into a variety of food products to increase their fibre content. The question remains as to whether these functional soluble fibres provide the same health benefits associated with ‘traditional’ dietary fibres. Only a few soluble fibres can be used for incorporation into food products and beverages. The use of polydextrose (PDX) and soluble maize fibre (SCF), also known as Soluble Gluco Fiber in Europe, seems to be a good option since they have a higher digestive tolerance compared with other soluble fibres, similar to, for example, inulin( Reference Flood, Auerbach and Craig 15 – Reference Bruhwyler, Carreer and Demanet 17 ). PDX has randomly polymerised branched chains and various types of glycosidic bonds( Reference Raninen, Lappi and Mykkanen 18 ). SCF is a fibre that is obtained from maize starch, comprising glucose oligosaccharides with digestion-resistant glycosidic linkages, and contains minor amounts of monosaccharides and disaccharides( 19 ). Both fibres are poorly digested in the small intestine, but are partially fermented by the gut microbiota( Reference Vester Boler, Rossoni Serao and Bauer 20 ). We hypothesised that the replacement of available carbohydrates with PDX or SCF may result in a reduced inhibition of postprandial fat oxidation rate and increased satiety mediated through differences in glycaemic and insulinaemic and NEFA responses. A higher postprandial fat oxidation rate may have an impact on fat storage and satiety and may thereby translate into a negative energy balance in the long term( Reference Ellis, Hyatt and Hunter 21 – Reference Langhans 23 ). It may also result in less lipid accumulation in non-adipose tissues, thereby improving insulin sensitivity and metabolic profile in the longer term( Reference Ravussin and Smith 24 , Reference Holloway, Bonen and Spriet 25 ). In the present study, we investigated the effect of replacement of 30 % of the available carbohydrates at breakfast and lunch with PDX or SCF on 24 h fat oxidation. Furthermore, we also investigated differences in postprandial, daytime and night-time substrate oxidation, energy expenditure (EE), hydrogen concentration, plasma profiles of glucose, insulin, FFA and TAG, and appetite sensation after the dietary treatments.
Research design and methods
Subjects
A total of eighteen overweight subjects (nine men and nine women) aged between 20 and 50 years participated in a single-blind, randomised cross-over study. The men and women were matched for age and BMI (Table 1). Additional inclusion criteria were fasting plasma glucose concentration < 6·1 mmol/l and a stable body weight in the last 3 months (weight gain or loss < 3 kg). None of the subjects was on medication (except contraception) or was engaged in sports activities for more than 3 h/week. Body composition was determined by bioimpedance (Bodystat Quadscan 4000; Bodystat Limited). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Medical Ethical Committee of Maastricht University Medical Centre (MUMC+). Written informed consent was obtained from all the subjects before participation in the study. The present trial was registered at ClinicalTrials.gov (identifier: NCT01163006).
Table 1 Subject characteristics* (Mean values with their standard errors)
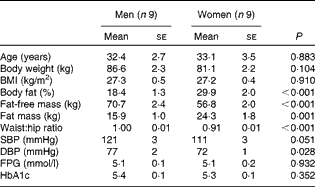
SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycated Hb A1c.
* Men and women were compared using two-tailed Student's t test for unpaired samples.
Study design
The design of the study was randomised, single-blind and cross-over with four different dietary interventions, including two fibre diets with PDX or SCF, also known as Soluble Gluco Fiber in Europe, and two control diets (full energetic (FULL) and isoenergetic), with a 1-week washout period between the dietary interventions (see the ‘Diet composition’ section). The subjects were instructed not to change their lifestyle and dietary habits during the washout period. Before each test period, the subjects were given a standardised meal (2385 kJ, 18·3 % of energy (En%) protein, 33·3 En% carbohydrate and 48·4 En% fat) at 19.00 hours the evening before they entered the respiration chamber. Each respiration chamber visit started after the continuous glucose monitoring system (Gold Medtronic; MiniMed, Inc.) was inserted into the periumbilical region of the abdomen of the subjects at 20.00 hours and ended 36 h later at 08.00 hours. The continuous glucose monitoring system was calibrated four times per d against finger-stick glucose determinations. In the first 12 h, the subjects got accustomed to the chamber, and during the first night, the sleeping metabolic rate (SMR) was assessed to estimate total energy intake. The SMR was calculated as the lowest EE (measured in 30 min intervals) over three consecutive sleeping hours( Reference Schoffelen, Westerterp and Saris 26 ). Based on the SMR (multiplied with a physical activity index of 1·55( Reference Schrauwen, van Marken Lichtenbelt and Westerterp 27 )), total energy intake was estimated. The subjects were fed in energy balance on the FULL diet, since the difference between 24 h EE and energy intake was less than 10 % deviation( Reference Schrauwen, van Marken Lichtenbelt and Westerterp 27 ). For standardisation of the measurements, the humidity in the respiration chamber was fixed, clothing was standardised and physical activity was prescribed by means of a standardised physical activity protocol( Reference Westerterp-Plantenga, van Marken Lichtenbelt and Strobbe 28 ). The standardised physical activity protocol consisted of 30 min stepping exercises (three sets of 5 min stepping followed by 5 min rest), which were performed three times during the day at 09.00, 13.00 and 20.00 hours.
In the morning at 07.30 hours, an indwelling cannula was inserted into an antecubital vein for the withdrawal of the first fasting blood samples. Thereafter, the subjects were given one of the four different diets in a randomised order. They were given a breakfast at 08.00 hours, a lunch at 12.00 hours and a dinner at 17.30 hours and three snacks at 10.00, 15.30 and 20.30 hours. Water and tea (except green tea) were available ad libitum. The profiles of glucose, NEFA, TAG and insulin were determined over a 14 h period during daytime from 08.00 until 21.30 hours. Blood sampling was performed immediately before ingestion of the three meals (breakfast, lunch and dinner) at t= 0 min and 30, 60, 120 and 240 min postprandially. Hydrogen concentration was measured in end-expiration (alveolar) breath samples every 2 h from 08.00 until 22.00 hours and again at 08.00 hours the next morning using a gastrolyser (Bedfont Scientific Limited), as a marker for colonic fermentation. Visual analogue scales and well-being questionnaires were completed nineteen times throughout the test day as described in detail below.
Diet composition
The four different dietary interventions consisted of two fibre diets (Sta-lite® PDX and Promitor™ SCF, manufactured by Tate and Lyle) and two control diets (FULL and isoenergetic). The PDX and SCF diets were prepared by replacing 30% of the available carbohydrates of the FULL control diet with, respectively, PDX (56·7 (sem 0·9) g/d) and SCF (54·6 (sem 0·9) g/d). Logically, both fibre diets had a lower energetic value than the FULL control diet. To correct for the lower energetic value, we also compared the PDX diet with a second control diet with the same energetic value as the PDX diet (isoenergetic control). The FULL control diet and PDX and SCF diets consisted of approximately the same macronutrient composition in grams. The isoenergetic control diet and PDX diet had approximately the same macronutrient composition in En% (Table 2). The food products consisted of test products provided by Tate and Lyle (Innovation Centre, France) and fresh and packed products bought at a convenience store in the Netherlands. The test products provided by Tate and Lyle included breakfast cereals, pudding and two instant drinks (a powder mix for a cocoa instant drink and a powder mix for an orange drink), all containing PDX or SCF. In the FULL and isoenergetic diets, placebo test products consisting of full available carbohydrates with no PDX or SCF added were added. The test products were supplemented with fresh and packed products to have a normal dietary pattern. The breakfast consisted of breakfast cereals with semi-skimmed milk and a cocoa instant drink (test powder and semi-skimmed milk). The lunch consisted of wheat bread with margarine, Gouda cheese 48+ and salami, vanilla pudding (test powder and full milk) and an instant orange drink (test powder and water). The subjects were given spaghetti bolognese with cheese for dinner. The three snacks that the subjects were given were high-fat curd with sugar and a piece of cake in the morning, pieces of unpeeled apple in the afternoon and salted peanuts and Gouda cheese 48+ in the evening. An overview of the diet composition is given in Table 2. Besides the fibre test products, which were only given in the PDX and SCF conditions, the remainder of the diet consisted of 19·1 (sem 0·3) g fibre in the full energetic condition, 16·0 (sem 0·3) g fibre in the isoenergetic condition and 14·6 (sem 0·2) g fibre in both the PDX and SCF conditions.
Table 2 Diet composition (Mean values with their standard errors)
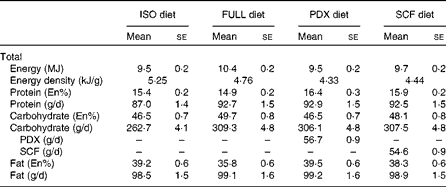
ISO, isoenergetic; FULL, full energetic; En%, percentage of energy; PDX, polydextrose; SCF, soluble maize fibre.
24 h energy expenditure
The 24 h EE and substrate oxidation as well as the pattern of these parameters during the day and night were calculated from 07.30 to 07.30 hours. Substrate oxidation was calculated from VO2 (litres/min) and VCO2 (litres/min) according to the equations of Frayn( Reference Frayn 29 ). EE was calculated using the formula of Weir( Reference Weir 30 ). During the stay in the respiration chamber, 24 h urine samples were collected from 08.00 to 08.00 hours. The subjects emptied their bladder at 08.00 hours before starting the collection. Urine volume and N concentration were measured, the latter using a nitrogen analyser (type CHN-O-Rapid; Heraeus). The SMR during the second night was defined as the lowest mean EE measured over three consecutive hours between 00.00 and 07.00 hours and was used in further calculations. Diet-induced thermogenesis (DIT) and activity-induced EE were calculated as described previously( Reference Westerterp, Wilson and Rolland 31 ).
Questionnaires
The visual analogue scale questionnaire (on a 100 mm scale) contains questions about the desire to eat and feelings of hunger, fullness and satiety( Reference Flint, Raben and Blundell 32 ). Opposing extremes were described at either end of a 100 mm horizontal line, and the subjects marked the line on a paper to indicate how they felt at that moment. Well-being was determined using a questionnaire on the occurrence of complaints (nausea, abdominal bloating, stomach–intestinal cramps, diarrhoea and other symptoms). Symptoms were graded on a ten-point scale ranging from grade 1, representing ‘not present’, to grade 10, representing ‘strongly present’. The subjects were asked to mark how they felt at the moment. For the analysis of the well-being questionnaire, a symptom was considered present if the grade scored was >5 (data not shown).
Biochemical analysis
Blood was collected in pre-cooled tubes containing 0·2 m-EDTA (Sigma). After collection, blood samples were centrifuged immediately at 4°C for 10 min at 1000 g and frozen at − 80°C until analysis. Plasma glucose, NEFA and TAG concentrations were determined using standard enzymatic techniques automated on a Cobas Fara centrifugal spectrophotometer (Roche Diagnostics). Plasma insulin concentration was measured using a double-antibody radioimmunoassay (Linco Research).
Statistical analyses
Differences between the diets were examined using repeated-measures ANOVA. First, the PDX diet was compared with the FULL and isoenergetic diets, and thereafter the SCF diet was compared with the PDX and FULL diets. When a significant diet × time interaction was observed, least square difference post hoc testing was carried out. Sex was included as a between-subject factor in the statistical model for all the analyses since it may affect the intervention response. When the interaction sex × diet and the factor sex were not statistically significant, the factor sex was excluded from the statistical model for the final analysis. Postprandial AUC was calculated using the trapezoidal rule for all the plasma metabolites, energy and substrate metabolism and appetite scores. For the analysis of EE, substrate oxidation and appetite scores, we only had complete data for sixteen subjects. Data are reported as means with their standard errors. A P value < 0·05 was considered statistically significant. Statistical analyses were performed using the statistical program SPSS 16.0 for Mac OS X (SPSS, Inc.).
Results
Interstitial and plasma glucose and plasma insulin responses
There were no differences in integrated interstitial glucose responses between the four dietary interventions over 24 h (Fig. 1(a)). This was also reflected in the plasma, since there were no differences in plasma glucose responses over the day (Fig. 1(b)). The peak glucose response after breakfast was lower after consumption of both fibre diets than after consumption of the FULL diet (FULL v. PDX P= 0·06, FULL v. SCF P= 0·03). After lunch, the peak glucose response was lower after consumption of the SCF diet than after consumption of the FULL diet (FULL v. SCF P= 0·03) (Fig. 1(b)). During the night, consumption of the PDX diet resulted in the highest glucose response (AUC night P= 0·02 and P= 0·03, post hoc FULL v. PDX P= 0·007, ISO v. PDX P= 0·08, SCF v. PDX P= 0·05) (Fig. 1(a)). After breakfast and lunch, the insulin response was significantly lower after consumption of the PDX and SCF diets than after consumption of the FULL diet (AUC breakfast (8–10 h) P= 0·04 and P= 0·001, post hoc FULL v. PDX P= 0·02, FULL v. SCF P= 0·003; AUC lunch (12–14 h) P= 0·04 and P= 0·01, post hoc FULL v. PDX P= 0·03, FULL v. SCF P= 0·01). Over the whole day, the reduced insulin response was only significant for the SCF diet (Fig. 2).
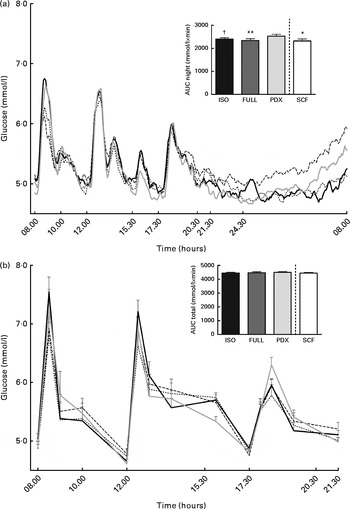
Fig. 1 Postprandial glucose responses. (a) Interstitial glucose response measured with a continuous glucose monitoring system over 24 h and AUC measured during night-time. (b) Plasma glucose response and AUC measured during daytime. Repeated-measures ANOVA was carried out with least square difference post hoc testing using the integrated responses (AUC). Values are means (n 18), with their standard errors represented by vertical bars. Mean value was significantly different from that of the PDX diet: * P< 0·05, ** P< 0·01. † Mean value was marginally significantly different from that of the PDX diet (P< 0·1). ISO, isoenergetic (![]() ); FULL, full energetic (
); FULL, full energetic (![]() ); PDX, polydextrose (
); PDX, polydextrose (![]() ); SCF, soluble maize fibre (
); SCF, soluble maize fibre (![]() ).
).
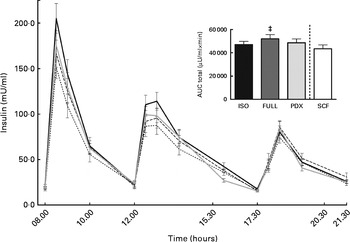
Fig. 2 Postprandial insulin response. Insulin response and AUC measured during daytime. Repeated-measures ANOVA was carried out with least square difference post hoc testing using the integrated responses (AUC). Values are means (n 18), with their standard errors represented by vertical bars. ‡ Mean value was significantly different from that of the SCF diet (P< 0·05). ISO, isoenergetic (![]() ); FULL, full energetic (
); FULL, full energetic (![]() ); PDX, polydextrose (
); PDX, polydextrose (![]() ); SCF, soluble maize fibre (
); SCF, soluble maize fibre (![]() ).
).
NEFA and TAG responses
During the day, the integrated NEFA response was significantly higher after consumption of the PDX, SCF and isoenergetic diets than after consumption of the FULL diet, indicating that the higher NEFA response was due to the lower amount of energy in the diets rather than due to a specific response to PDX or SCF (Fig. 3(a) and (b)). For both fibre diets, the lower concentrations of insulin in the postprandial state may reduce the inhibition of lipolysis and may explain the higher NEFA response observed. There were no differences in TAG responses between all the diets (Fig. 3(c) and (d)).
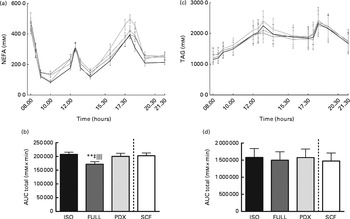
Fig. 3 Postprandial NEFA and TAG responses. (a) NEFA response and (b) AUC NEFA measured during daytime. (c) TAG response and (d) AUC TAG measured during daytime. Repeated-measures ANOVA was carried out with least square difference post hoc testing using the integrated responses (AUC). Values are means (n 18), with their standard errors represented by vertical bars. ** Mean value was significantly different from that of the PDX diet (P< 0·01). ‡ Mean value was significantly different from that of the SCF diet (P< 0·05). ∥∥ Mean value was significantly different from that of the ISO control diet (P< 0·01). ISO, isoenergetic (![]() ); FULL, full energetic (
); FULL, full energetic (![]() ); PDX, polydextrose (
); PDX, polydextrose (![]() ); SCF, soluble maize fibre (
); SCF, soluble maize fibre (![]() ).
).
Energy expenditure
EE over 24 h was not significantly different between the four diets (data not shown). There were also no differences in SMR during the second night, DIT and activity-induced EE (data not shown). On average, the subjects were in energy balance on consuming the FULL diet (+0·19 (sem 0·17) MJ/d, P= 0·3) and were in negative energy balance on consuming the PDX ( − 0·83 (sem 0·17) MJ/d, P< 0·001), isoenergetic ( − 0·78 (sem 0·17) MJ/d, P< 0·001) and SCF ( − 0·60 (sem 0·18) MJ/d, P= 0·003) diets. There was a significant difference in energy balance between the FULL diet and the other three diets (isoenergetic, PDX and SCF diets) (P< 0·001, post hoc FULL v. PDX: P< 0·001, FULL v. ISO: P< 0·001, FULL v. SCF: P< 0·001). This may be explained by the lower amount of energy in both fibre diets and the isoenergetic diet.
Substrate oxidation
Over 24 h, carbohydrate oxidation was reduced after consumption of the PDX and SCF diets than after consumption of the FULL diet (Fig. 4(a)). Fat oxidation was increased after consumption of both fibre diets and the isoenergetic diet than after consumption of the FULL diet over 24 h (Fig. 4(d)). The effect on substrate oxidation was mainly observed during the day (Fig. 4(b) and (e)). During the night, consumption of the isoenergetic diet resulted in a lower carbohydrate oxidation rate and a higher fat oxidation rate when compared with that of the FULL and PDX diets (Fig. 4(c)–(f)).
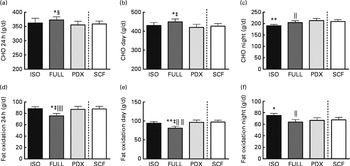
Fig. 4 Carbohydrate oxidation (CHO) and fat oxidation measured over 24 h in a respiration chamber. CHO oxidation measured during (a) 24 h, (b) daytime and (c) night-time. Fat oxidation measured during (d) 24 h, (e) daytime and (f) night-time. Repeated-measures ANOVA was carried out with least square difference post hoc testing. Values are means (n 16), with their standard errors represented by vertical bars. Mean value was significantly different from that of the PDX diet: * P< 0·05, ** P< 0·01. ‡ Mean value was significantly different from that of the SCF diet (P< 0·05). § Mean value was marginally significantly different from that of the SCF diet (P< 0·1). Mean value was significantly different from that of the ISO control diet: ∥ P< 0·05, ∥∥ P< 0·01. ISO, isoenergetic; FULL, full energetic; PDX, polydextrose; SCF, soluble maize fibre.
Hydrogen excretion
Hydrogen concentration was significantly higher after consumption of the PDX diet over 24 h than after consumption of both control diets (Fig. 5). Consumption of the SCF diet also resulted in a higher breath hydrogen concentration compared with that of the FULL diet. This indicates a higher colonic fermentation rate after consumption of both fibre diets. There was no difference between the two fibre diets. The higher breath hydrogen response after consumption of the PDX and SCF diets decreased after dinner and was similar to that observed after consumption of the control diets.
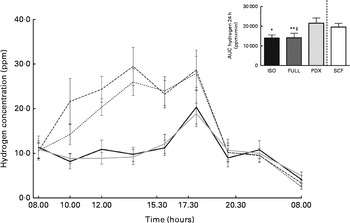
Fig. 5 Breath hydrogen measurement over 24 h. Breath hydrogen concentration response and AUC over 24 h. Repeated-measures ANOVA was carried out with least square difference post hoc testing using the integrated responses (AUC). Values are means (n 18), with their standard errors represented by vertical bars. Mean value was significantly different from that of the PDX diet: * P< 0·05, ** P< 0·01. ‡ Mean value was significantly different from that of the SCF diet (P< 0·05). ISO, isoenergetic (![]() ); FULL, full energetic (
); FULL, full energetic (![]() ); PDX, polydextrose (
); PDX, polydextrose (![]() ); SCF, soluble maize fibre (
); SCF, soluble maize fibre (![]() ); ppm, parts per million.
); ppm, parts per million.
Visual analogue scale and well-being questionnaires
The desire to eat and feeling of hunger were significantly lower after consumption of the PDX diet than after consumption of both control diets over 24 h (Fig. 6(a) and (b)). There were no significant differences observed between men and women. The feelings of fullness and satiety were higher after consumption of the PDX diet than after consumption of both control diets over 24 h, but this effect was only observed in women (Fig. 6(c)–(e)). The effect on appetite was only observed for the PDX diet since subjects who consumed the SCF diet exhibited a tendency towards a greater desire to eat (P= 0·09) and less feelings of fullness (P= 0·001) and satiety (P= 0·002) compared with those who consumed the PDX diet.
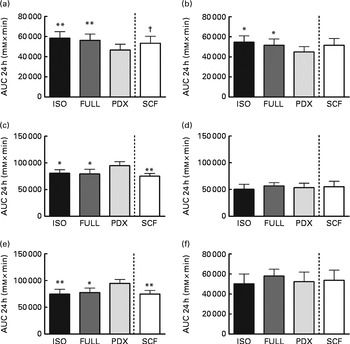
Fig. 6 Appetite scores measured over 24 h. (a) Desire to eat. (b) Hunger. (c, d) Fullness in women and men. (e, f) Satiety in women and men. Repeated-measures ANOVA was carried out with least square difference post hoc testing using the integrated responses (AUC). Values are means (n 16), with their standard errors represented by vertical bars. Mean value was significantly different from that of the PDX diet: * P< 0·05, ** P< 0·01. † Mean value was marginally significantly different from that of the PDX diet (P< 0·1). ISO, isoenergetic; FULL, full energetic; PDX, polydextrose; SCF, soluble maize fibre.
The subjects scored no feelings of nausea, burping, headache, vomiting and dizziness in all the conditions. Only one male subject reported abdominal bloating in all the conditions. The same subject reported stomach–intestinal cramps in all the conditions except in the PDX condition. Diarrhoea was scored present by one female in the PDX condition, one female in the SCF condition and one male in both the PDX and SCF conditions (data not shown). The urge to defecate was the highest in the SCF condition with an average score of 1·9 (sem 0·3) on a total score of 10 measured over 24 h compared with 1·5 (sem 0·2) in both the PDX and full energetic conditions (diet P= 0·02, post hoc FULL v. SCF P= 0·05, PDX v. SCF P= 0·01).
Discussion
Food components with health-promoting properties are gaining widespread interest. The present study shows that replacing available carbohydrates with the soluble fibres PDX and SCF reduces the peak glucose response, which is accompanied by a reduction in postprandial insulin response compared with the FULL diet. Furthermore, increased circulating NEFA concentrations concomitant with a higher fat oxidation rate were observed. This was mainly attributed to the lower energetic value and the more negative energy balance of the high-fibre diets and not to the fibres per se. Nevertheless, the PDX diet exhibited favourable effects on appetite, which appeared to be a specific characteristic of PDX independent of energy balance. The mechanisms responsible for the effect of PDX on appetite sensations as well as the differential effect exerted by SCF remain to be elucidated. The effects on appetite and fat oxidation can be regarded as a beneficial change in metabolic profile in relation to body weight control and insulin sensitivity if these effects persist over longer periods of time under ad libitum feeding conditions.
The replacement of available carbohydrates with PDX or SCF reduced insulin responses in the postprandial state, translating into a reduced insulin response over the whole day for the SCF diet compared with the FULL diet. However, no major effects on interstitial and plasma glucose responses were observed during the day after consumption of both fibre diets; only the peak glucose responses after breakfast and lunch were lower compared with those after consumption of the FULL diet. During the night, consumption of the PDX diet resulted in the highest glucose response. Previously, two studies have reported no effects of PDX on plasma glucose or insulin kinetics when PDX was given in a pure form or as a drink with a mixed meal( Reference Craig, Holden and Troup 33 , Reference Schwab, Louheranta and Torronen 34 ). However, other studies have reported a lower glucose response when PDX was incorporated into chocolates, sweetened dried cranberries and strawberry jam( Reference Shimomura, Maeda and Nagasaki 35 – Reference Wilson, Luebke and Morcomb 37 ). Kendall et al. ( Reference Kendall, Esfahani and Hoffman 11 ) reported lower postprandial glucose and insulin responses for SCF when given as a lemonade drink. These studies investigated postprandial glucose and insulin concentrations after consumption of a single meal over a limited period of time rather than those after consumption of two meals (breakfast and lunch containing PDX or SCF) during daytime as has been done in the present study.
Furthermore, we investigated the effect of both fibres on substrate oxidation and EE. For the PDX and SCF diets, a higher fat oxidation rate and a lower carbohydrate oxidation rate were observed compared with those for the FULL diet. As indicated above, this increased fat oxidation was accompanied by reduced insulin responses (only significant for the SCF diet over the whole day) and consequently higher NEFA concentrations, confirming our initial hypothesis that a fibre-induced reduction in insulin concentrations may result in increased circulating NEFA concentrations from an increased fat oxidation. Nevertheless, the increased fat oxidation was mainly attributed to the lower energetic value and not to the fibres per se, since consumption of the isoenergetic control diet also resulted in a higher fat oxidation rate compared with that of the FULL diet. Sparti et al. ( Reference Sparti, Milon and Di Vetta 38 ) studied the effect of two different isoenergetic diets containing high- or low-unavailable carbohydrates on 24 h substrate oxidation in lean subjects and reported no differences in total substrate oxidation between the two diets. In another study, substrate oxidation was measured for 5 h after ingestion of a meal containing resistant starch or rapidly digestible starch, with a difference of 103 kJ in energy content between the former and the latter meal. Resistant starch intake resulted in a lower glucose oxidation rate and a higher fat oxidation rate( Reference Tagliabue, Raben and Heijnen 39 ). In line with our results, the difference in energy content between the resistant starch and rapidly digestible starch meals may have contributed to the increase in fat oxidation.
There were no differences in 24 h EE between the diets as in previous studies, in which the meal or diet was supplemented with dietary fibres and also no differences in 24 h EE were reported( Reference Sparti, Milon and Di Vetta 38 , Reference Poppitt, Livesey and Elia 40 – Reference Ryttig, Lammert and Nielsen 42 ). SMR, DIT and activity-induced EE were not different between the fibre and control diets. There is still a lot of controversy about the effect of dietary fibres on DIT. Some studies have reported no effect of dietary fibres on DIT( Reference Tagliabue, Raben and Heijnen 39 , Reference Ritz, Krempf and Cloarec 43 ), while others have reported reduced DIT( Reference Scalfi, Coltorti and D'Arrigo 44 , Reference Raben, Christensen and Madsen 45 ). The explanation for the discrepant results is not entirely clear, but they may be related to differences in the methodology used for determining DIT, subject characteristics and different fibre characteristics.
Commensal bacteria ferment dietary fibres. The gases produced by fermentation are absorbed into the circulation and diffuse into the pulmonary alveolus. Therefore, measurement of hydrogen concentration in the breath reflects the anaerobic bacterial fermentation of dietary fibres in the colon( Reference Chinda, Nakaji and Fukuda 14 , Reference Le Marchand, Wilkens and Harwood 46 ). In the present study, we indeed showed that hydrogen concentration in the breath was significantly higher after consumption of the PDX and SCF diets than after consumption of both control diets, indicating a higher colonic fermentation rate after consumption of both fibre diets. There were no differences in hydrogen concentration between both fibre diets. Because colonic fermentation is an incomplete combustion, disregarding the fermentation process could lead to errors in the estimation of substrate oxidation from indirect calorimetry data( Reference Heresbach, Flourie and Briet 47 , Reference Ritz, Cloarec and Beylot 48 ). However, in an experiment designed to quantify the measurement error in substrate oxidation due to the occurrence of fermentation, Poppitt et al. ( Reference Poppitt, Livesey and Faulks 49 ) estimated the maximal error in carbohydrate oxidation after ingestion of 58 g unavailable carbohydrates to be 2 %. Also, Heresbach et al. ( Reference Heresbach, Flourie and Briet 47 ) reported that when the additional excretion of breath hydrogen is < 20 parts per million, the magnitude of error in the measurement of VCO2 is small, on average. Although it is important to measure hydrogen excretion in whole-body calorimetric studies in which fermentable carbohydrates are part of the diet, the effects on the calculation of EE and fuel oxidation in humans are small( Reference Poppitt, Livesey and Elia 40 ), and they had no impact on the conclusions of the present study.
Furthermore, the effect of both fibres on several measures of appetite sensation was investigated. In the present study, the desire to eat and feeling of hunger were lowest after consumption of the PDX diet than after consumption of all the other diets. The feelings of fullness and satiety were higher after consumption of the PDX diet than after consumption of both control diets, but this was observed only in women. Sex differences in satiety responses have been noted in previous studies, in which women have reported higher post-meal satiety ratings and exhibited a reduced food intake( Reference Cornier, Salzberg and Endly 50 , Reference Hess, Birkett and Thomas 51 ). The effect on appetite has not been observed for SCF. The importance of energy density in satiation and satiety has been described by Drewnowski( Reference Drewnowski 52 ). In general, fibre-rich diets have a reduced energy density, which is related to the ability of the fibre to add bulk and weight to the diet. Therefore, incorporation of fibres into the diet may be one strategy for inducing fullness or satiety while consuming less energy. A lower energy density was observed for both fibre diets (PDX and SCF), but the satiety-inducing effect was observed only for the PDX diet, indicating that this effect was not due to energy density per se but due to a PDX-specific effect. Peripheral fatty acid oxidation has long been implicated in the control of eating( Reference Scharrer and Langhans 53 ). Nevertheless, since the favourable effects on appetite were a specific characteristic of PDX, while the increased fat oxidation was mainly determined by a negative energy balance, other mechanisms may be responsible for the PDX-induced reduction in appetite. Several factors may influence appetite such as the intrinsic physical properties of dietary fibres (bulking, gel formation and viscosity), modulation of gastric motor function, increased mastication, changes in the levels of gut hormones (ghrelin or glucagon-like peptide-1 (GLP-1)) and blunting of postprandial glucose and insulin responses( Reference Papathanasopoulos and Camilleri 54 – Reference Kristensen and Jensen 56 ). Additionally, it has been hypothesised that colonic fermentation resulting in increased production of SCFA could stimulate colonic L-cells to produce several appetite-regulating hormones such as peptide YY and GLP-1( Reference Kristensen and Jensen 56 ). The mechanisms responsible for this remain to be elucidated.
In conclusion, the replacement of available carbohydrates with PDX or SCF reduced the peak glucose response and postprandial insulin responses and increased circulating NEFA concentrations, with an increased fat oxidation after consumption of both fibre diets. The last effect was mainly attributed to the lower energetic value of the diets and not to the fibres per se. Interestingly, PDX incorporated into food products may be of special interest since it has a beneficial effect on appetite control. However, the present study only reports the acute effects, and further long-term studies are warranted to evaluate whether replacing available carbohydrates with PDX in ad libitum feeding conditions may have a significant effect on body weight control and improve metabolic profile in the long term.
Acknowledgements
The authors thank all the subjects for participating in the present study. They are grateful to Wendy Sluijsmans, Gabby Hul and Hasibe Aydeniz for their excellent analytical support. They thank Tate and Lyle, Kaiseraugst, Villeneuve d'Ascq, France, for providing the PDX and SCF test products. The present study was supported by Tate and Lyle, Kaiseraugst, Villeneuve d'Ascq, France. The authors' responsibilities were as follows: E. E. B. and E. K. designed the research; E. K. conducted the research; E. K., J. S. and P. F. S. analysed the data; E. K. performed the statistical analyses; E. K. wrote the paper; E. E. B. had primary responsibility for the final content; P. F. S. and E. E. B. reviewed/edited the manuscript. There are no conflicts of interest.










