Thyroid cancer is the most common tumour type of the endocrine system(Reference DeLellis1). Since the 1970s, thyroid cancer incidence rates have increased worldwide(Reference DeLellis1). In contrast to Western countries, where breast cancer is the most common cancer type in women, thyroid cancer is the most common cancer type in adult Korean women(Reference Ferlay, Shin and Bray2, 3). The age-standardised incidence rate of thyroid cancer in Korean women (86·1/100 000) is higher than that of other countries and worldwide (22·3/100 000 for the USA, 11·5/100 000 for Europe, 8·3/100 000 for Japan, 3·0/100 000 for China and 6·6/100 000 worldwide)(Reference Ferlay, Shin and Bray2). Furthermore, the incidence of this cancer type in Korean women has increased remarkably over time from 11·9/100 000 in 1999 to 80·2/100 000 in 2008(3).
The best-established risk factors for thyroid cancer are exposure to radiation(Reference Dal Maso, Bosetti and La Vecchia4) and a history of benign thyroid nodules or adenomas(Reference Franceschi, Preston-Martin and Maso5). Exposure to radiation and benign thyroid nodules or adenomas may induce thyroid cancer through gene rearrangements(Reference Elisei, Romei and Vorontsova6) or mutations(Reference Kondo, Ezzat and Asa7). However, to our knowledge, only one cohort study reported some possible risk factors such as family history of cancer, BMI and hormonal factors for benign thyroid nodules(Reference Wong, Ron and Gieriowski8), while little is known about lifestyle factors that may increase or decrease the risk of benign thyroid nodules or adenomas. Some dietary factors have also been reported to be related to thyroid cancer risk including fruit intake(Reference Markaki, Linos and Linos9), vegetable intake(Reference Markaki, Linos and Linos9–Reference Memon, Varghese and Suresh12), cruciferous vegetable intake(Reference Dal Maso, Bosetti and La Vecchia4, Reference Galanti, Hansson and Bergstrom10–Reference Truong, Baron-Dubourdieu and Rougier13), fish intake(Reference Memon, Varghese and Suresh12–Reference Mack, Preston-Martin and Bernstein16), glycaemic index, glycaemic load(Reference Randi, Ferraroni and Talamini17), micronutrients(Reference D'Avanzo, Ron and Vecchia18), iodine(Reference Truong, Baron-Dubourdieu and Rougier13, Reference Horn-Ross, Morris and Lee15) and nitrate(Reference Kilfoy, Zhang and Park19, Reference Ward, Kilfoy and Weyer20). Among dietary factors, fruits and vegetables have been reported to have protective effects against many different cancers(Reference Vainio and Bianchini21, Reference Marmot, Atinmo and Byers22). Some epidemiological studies have investigated the effect of fruit and vegetable intake on thyroid cancer risk. However, those studies were conducted primarily in Western countries(Reference Dal Maso, Bosetti and La Vecchia4, Reference Markaki, Linos and Linos9–Reference Bosetti, Negri and Kolonel11) and their findings were inconsistent(Reference Dal Maso, Bosetti and La Vecchia4).
Vegetables can be consumed raw, cooked or pickled; these latter two procedures may destroy the antioxidants present in raw vegetables(Reference Kim, Lim and Lee23–Reference Kaur and Kapoor26). Thus, the effect of raw vegetables on cancer risk might be masked if total vegetable consumption, including all raw, cooked and pickled vegetables, is examined. Nevertheless, previous studies have not determined the effect of raw vegetable consumption on cancer risk separately, except for a Greek study that reported an inverse association between raw vegetable dietary pattern and thyroid cancer(Reference Markaki, Linos and Linos9).
Although the effects of fruit intake on thyroid cancer risk have been investigated in a few previous studies, inconsistent results were reported. This could be because different fruits have different effects on different cancer types. A Norwegian study reported an increased risk of thyroid cancer with citrus fruit consumption, but no association with apple intake(Reference Galanti, Hansson and Bergstrom10). A Greek study reported that fresh tomatoes and lemons may reduce thyroid cancer risk (P for trend = 0·002 for tomatoes; P for trend = 0·001 for lemons). Our objective in the present study was to investigate the association between total and raw vegetable intake and fruit intake on benign and malignant thyroid cancer risk in a Korean population.
Materials and methods
Cases and controls
From June 2008 to June 2010, cases and controls were recruited at Hanyang University Medical Center in Seoul, South Korea. All participants were women between the ages of 20 and 70 years. All potential subjects were examined by ultrasonography to confirm the presence of thyroid cancer.
Malignant cases were diagnosed by fine needle aspiration biopsy and ultrasonography. About 90 % of malignant cases were papillary carcinoma. Benign cases were subjects who had benign nodules or adenomas in ultrasonography and/or fine needle aspiration biopsy. We included 111 histologically confirmed malignant cases and 115 benign cases in this study.
Subjects were excluded from the study if they had any history of cancer (ten cases), or an estimated total energy intake < 2093 kJ/d (500 kcal/d) or >16 744 kJ/d (4000 kcal/d) (eighteen cases and one control). Controls were patients who visited the otolaryngology or orthopaedic surgery departments of the same hospital. Patients with hormone-related diseases, abnormal thyroid hormone levels (thyroxine, thyroid-stimulating hormone) or thyroid nodules found by ultrasonography were excluded. Cases and controls were matched by their age (within 2 years) (111 pairs of malignant cases and controls and 115 pairs of benign cases and controls). All interviews were conducted in the hospital and the response rates for malignant and benign cases combined and controls were 70·4 and 41·0 %, respectively. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board of Hanyang University Medical Center. Written informed consent was obtained from all participants.
Data collection
Cases and controls were interviewed using questionnaires to determine patients' general characteristics, menstrual and reproductive history, family history of thyroid cancer, smoking and drinking habits, intake of multivitamins and average time spent exercising.
Dietary data were collected using a quantitative FFQ, modified from the validated FFQ(Reference Kim, Lee and Ahn27), with visual aids such as food photographs and models to demonstrate item-specific units. Kim et al. (Reference Kim, Lee and Ahn27) evaluated the validity and reproducibility of semiquantitative FFQ by administering it twice at an interval of 2 years and comparing it with four or five 24 h recalls collected at intervals of 3 months. The Pearson's correlation coefficients between energy-adjusted nutrient intakes averaged 0·44 on the first questionnaire v. 24 h recalls and 0·37 on the second questionnaire v. 24 h recalls for fourteen nutrients (protein, fat, carbohydrate, fibre, Ca, P, Fe, Na, K, vitamin A, thiamin, riboflavin, niacin and ascorbic acid). The reproducibility of this FFQ determined by the correlation of the nutrient intakes was greater than 0·5. Regarding reliability of the modified FFQ, using our data for controls, the Cronbach's α was 0·78 for food frequency and 0·87 for its portion size.
Subjects were asked by trained interviewers to recall their usual intake of 121 food items over a period of 12 months beginning from 3 years before the time of the interview(Reference Willett28). Portion sizes were open-ended questions and standard units were used for all food items. All frequencies were standardised into ‘times per d’ by using the conversion factors 30·4 d/month and 4·3 d/week. Daily food intake was calculated using the standardised frequency per d and the amount of food per standard unit. Daily nutrient and energy intake were estimated using the daily food intake and recipe and nutrient database(29). Nutrient intakes were adjusted for total energy intake by the residual method to avoid bias due to the simple relationship between nutrient intake and total energy intake(Reference Willett28). Total vegetable intake was estimated by summing the daily consumption of pickled vegetables (cabbage kimchi, radish kimchi, watery radish kimchi, cucumber kimchi, sesame leaf kimchi and jangajji), raw vegetables (lettuce, Korean cabbage, cabbage, cucumber, pepper, carrot, sesame leaf, onion, radish, crown daisy, water dropwort, chicory, broccoli, celery and garlic), cooked vegetables (soyabean sprouts, mungbean sprouts, spinach, eggplant and bracken), mushrooms and seaweeds. Total fruit intake was estimated by totalling the daily intake of fourteen fruits (persimmon, tangerine, orange, kiwi, melon, grape, strawberry, watermelon, pear, apple, banana, peach, tomato and fruit juice).
Statistical analyses
Cases and controls were compared using the Wilcoxon signed rank test for continuous variables and the McNemar test for categorical variables. Data were analysed according to quartiles of daily intake (g/d) and average consumption frequency (times/week) of total vegetables, raw vegetables, total fruits and all single fruits. Among single fruits, only fruits significantly associated with malignant cancer or benign thyroid disease are presented in the tables. The OR and corresponding 95 % CI were obtained using conditional logistic regression analysis. The general linear model and Cochran–Mantel–Haenszel analysis were used to assess potential confounders among controls. Trend tests were conducted by treating the median values of each quartile of daily fruit and vegetable intake or the average consumption frequency of the specific fruit or vegetable as continuous values in each of the models. Variables that were significantly different between cases and controls and which showed significant linear trends across quartiles of daily intake of total vegetables, raw vegetables, total fruits, persimmons and tangerines were considered as potential confounders and adjusted in the analyses. Antioxidants such as vitamin C, β-carotene, and folate, which were highly correlated with fruit and vegetable intake (r p>0·40), were not included in multiple logistic regression models as potential confounders. All multiple logistic regression models included parity (yes/no), energy (quartile) for malignant pair analyses and energy (quartile) for benign pair analyses. Additionally, Na (quartile) was added in the total vegetable model, whereas Na (quartile) and vitamin E (quartile) were added in the raw vegetable model, and age at menarche (years) was added in the persimmon model. Parity (yes/no) was added in the tangerine model. Statistical analyses were conducted using SAS (version 9.1) software.
Results
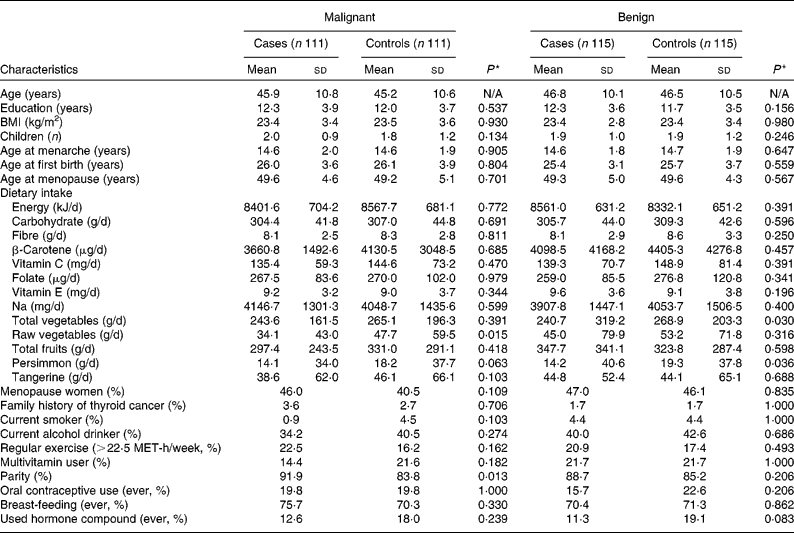
Table 1 shows the general characteristics of the cases and controls. The average ages of malignant cases and controls were 45·9 and 45·2 years, respectively, and those of benign cases and controls were 46·8 and 46·5 years, respectively. Parity was significantly higher in malignant cases than controls. Daily intake of raw vegetables was higher in controls than in malignant cases (34·1 g/d for malignant cases v. 47·7 g/d for controls). Daily intake of total vegetables and persimmons were higher in controls than benign cases (240·7 g/d for benign cases v. 268·9 g/d for controls in total vegetables intake; 14·2 g/d for benign cases v. 19·3 g/d for controls in persimmon intake). Daily intake of energy, carbohydrates, fibre, β-carotene, vitamin C, folate, vitamin E, Na, total fruits and tangerines did not differ between cases and controls for either malignant pairs or benign pairs.
Table 1 General characteristics of the study subjects with and without thyroid cancer† (Mean values and standard deviations or proportions)

N/A, not applicable; MET, metabolic equivalent.
* Wilcoxon signed rank test for continuous variables and McNemar test for categorical variables.
† All nutrient intakes are total energy-adjusted values.
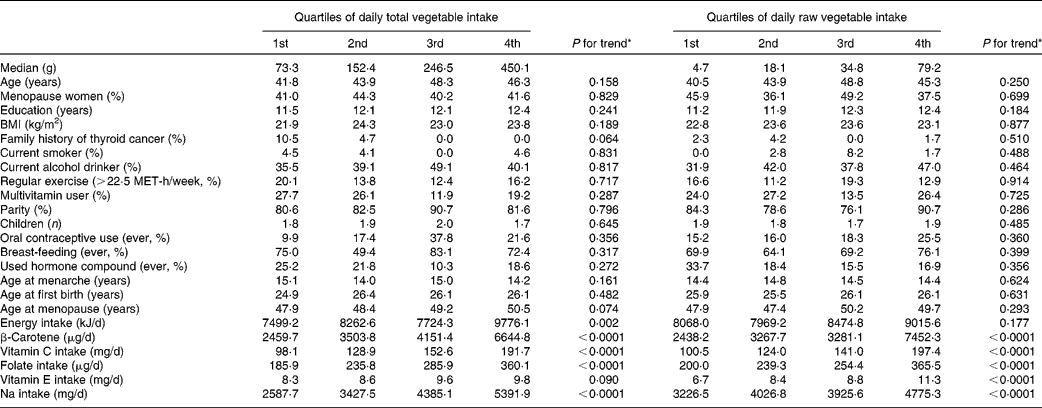
Potential confounding factors determined by using the quartiles of daily total vegetable and raw vegetable intake are shown in Table 2. Dietary intake of β-carotene, vitamin C, folate and Na increased across quartiles of total vegetable intake and quartiles of raw vegetable intake.
Table 2 Potential confounders according to quartile of intake of total vegetables and raw vegetables among controls†‡

MET, metabolic equivalent.
* P -values for trends were determined by the general linear model for continuous variables and by the Cochran–Mantel–Haenszel test for categorical variables.
† All results except median and age were adjusted for age, and all nutrient intakes are total energy-adjusted values.
‡ Values are expressed as mean or percentage according to quartile of fruit and vegetable intake.
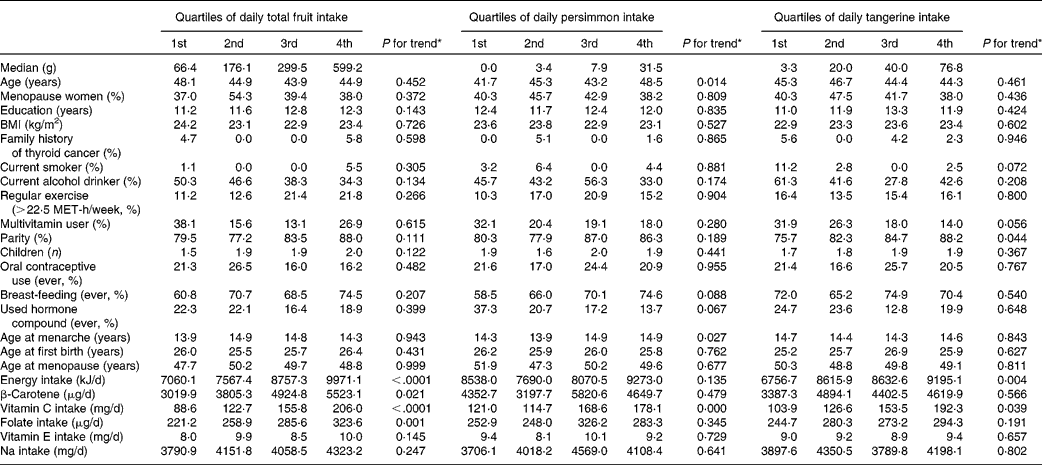
Potential confounding factors determined by using the quartiles of daily total fruit, persimmon and tangerine intake are shown in Table 3. Average age and age at menarche increased across quartiles of daily persimmon intake. Vitamin C increased across quartiles of total fruit intake, persimmon intake and tangerine intake. Daily intake of β-carotene and folate increased across quartiles of total fruit intake.
Table 3 Potential confounders according to quartile of intake of fruit, persimmons and tangerines among controls†‡

MET, metabolic equivalent.
* P -values for trends were determined by the general linear model for continuous variables and by the Cochran–Mantel–Haenszel test for categorical variables.
† All results except median and age were adjusted for age, and all nutrient intakes are total energy-adjusted values.
‡ Values are expressed as mean or percentage according to quartile of fruit and vegetable intake.
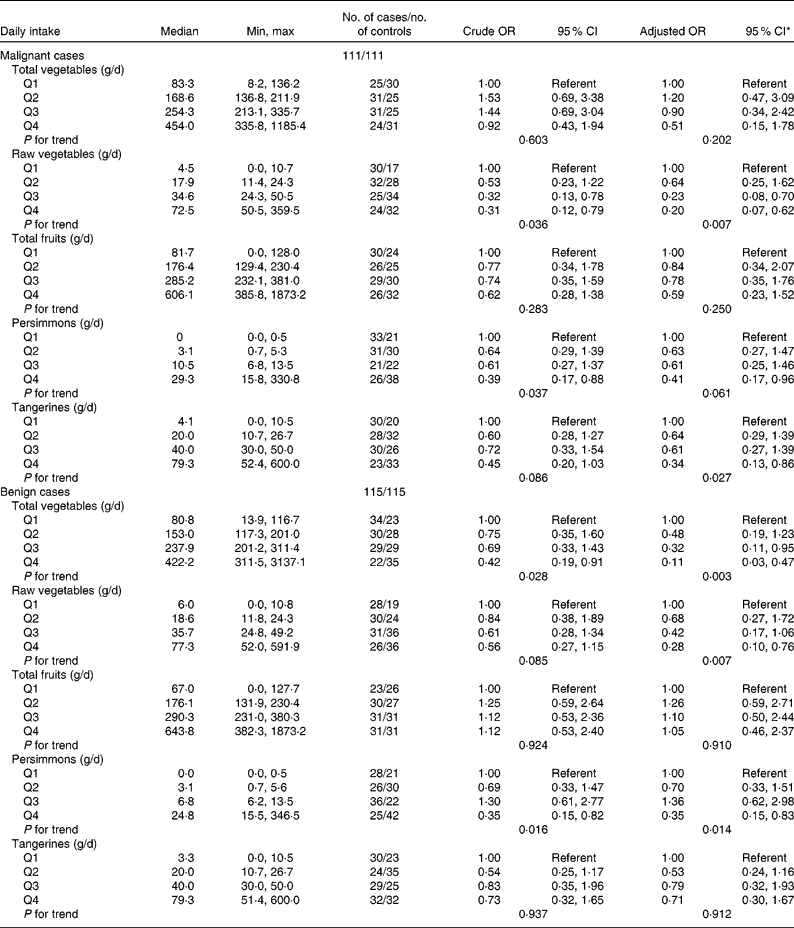
The association between vegetable and fruit intake and thyroid cancer risk is shown in Table 4. Total vegetable intake was inversely associated with benign thyroid cancer risk (OR 0·42; 95 % CI 0·19, 0·91; P for trend = 0·028 in benign cases). After adjusting for potential confounders (energy intake and Na intake), the significant inverse association remained (OR 0·11; 95 % CI 0·03, 0·47; P for trend = 0·003 in benign cases). A significant inverse association between raw vegetable intake and thyroid cancer risk was found (OR 0·31; 95 % CI 0·12, 0·79; P for trend = 0·036 in malignant cases and OR 0·56; 95 % CI 0·27, 1·15; P for trend = 0·085 in benign cases). The inverse association between raw vegetable intake and thyroid cancer risk remained (OR 0·20; 95 % CI 0·07, 0·62; P for trend = 0·007 in malignant cases and OR 0·28; 95 % CI 0·10, 0·76; P for trend = 0·007 in benign cases) after adjusting for potential confounders (parity, energy intake, vitamin E intake and Na intake). An inverse association between thyroid cancer risk and persimmon intake was found in both malignant and benign cases (OR 0·39; 95 % CI 0·17, 0·88 for the highest quartile; P for trend = 0·037 in malignant cases and OR 0·35; 95 % CI 0·15, 0·82 for the highest quartile; P for trend = 0·016 in benign cases). After adjusting for potential confounders, the significant inverse associations remained (OR 0·41; 95 % CI 0·17, 0·96 for the highest quartile; P for trend = 0·061 in malignant cases and OR 0·35; 95 % CI 0·15, 0·83 for the highest quartile; P for trend = 0·014 in benign cases). An inverse association between tangerine intake and malignant thyroid cancer risk was found after adjusting for potential confounders, but there was no significant association between daily tangerine intake and benign thyroid cancer risk (OR 0·34; 95 % CI 0·13, 0·86 for the highest quartile; P for trend = 0·027 in malignant cases). There were no associations between thyroid cancer risk and daily intake of total vegetables or total fruits in the present study.
Table 4 Thyroid cancer according to quartiles of vegetable and fruit intake in multiple logistic regression models (Odds ratios and 95 % confidence intervals)

Min, minimum; max, maximum.
* All adjusted OR models included parity (yes/no), energy (quartile) in the malignant cancer model. Energy (quartile) was adjusted for in the benign cancer model. The model for total vegetables was additionally adjusted by Na intake (quartile). The model for raw vegetables was additionally adjusted for Na intake (quartile) and vitamin E intake (quartile). The model for persimmons was additionally adjusted for age at menarche (years). The model for tangerines was additionally adjusted for parity (yes/no).
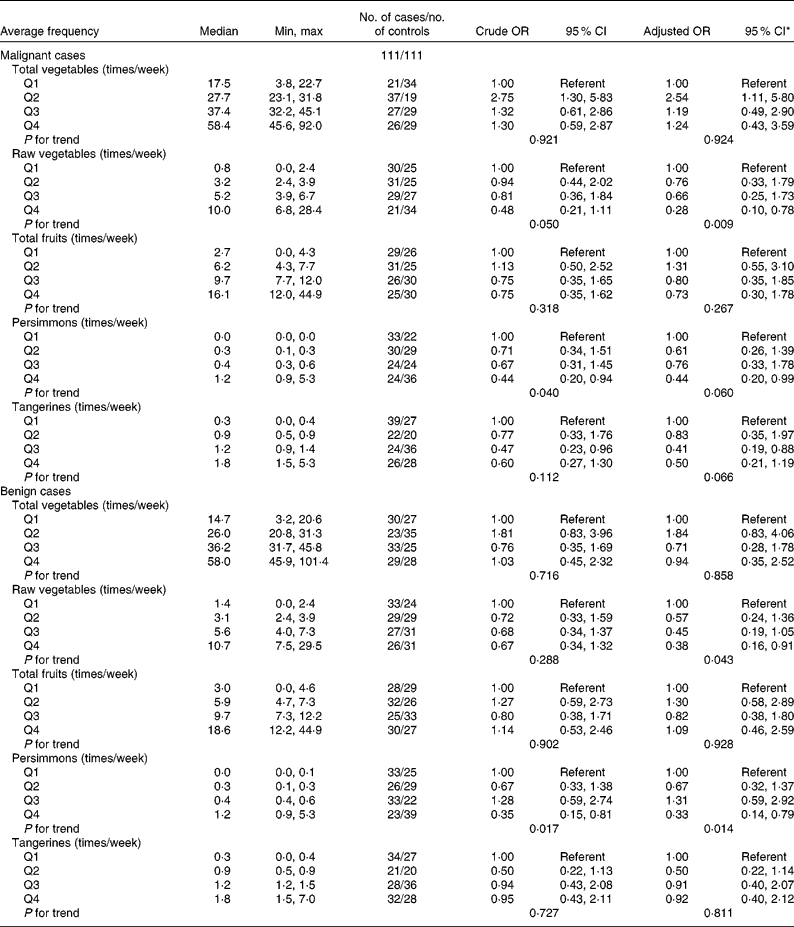
The association between thyroid cancer risk and the average frequency of vegetable and fruit intake is shown in Table 5. There was a trend for an inverse association between thyroid cancer risk and the average frequency of raw vegetable intake in malignant and benign cases after adjusting for potential confounders. The frequency of raw vegetable intake was inversely associated with thyroid cancer risk (OR 0·28; 95 % CI 0·10, 0·78 for the highest quartile; P for trend = 0·009 in malignant cases and OR 0·38; 95 % CI 0·16, 0·91 for the highest quartile; P for trend = 0·043 in benign cases). A high frequency of persimmon intake was inversely associated with thyroid cancer risk (OR 0·44; 95 % CI 0·20, 0·94 for the highest quartile; P for trend = 0·040 in malignant cases and OR 0·35; 95 % CI 0·15, 0·81 for the highest quartile; P for trend = 0·017 in benign cases). The inverse association between the average frequency of persimmon intake and thyroid cancer risk remained after adjusting for potential confounders (OR 0·44; 95 % CI 0·20, 0·99 for the highest quartile; P for trend = 0·060 in malignant cases and OR 0·33; 95 % CI 0·14, 0·79 for the highest quartile; P for trend = 0·014 in benign cases). We did not find a significant association between thyroid cancer risk and the frequency of total vegetable intake, total fruit intake or tangerine intake. The additional adjustments for the risk factors reported in previous studies (education, family history of thyroid cancer, BMI, current smoker and current alcohol drinker) did not substantially change the results (data not shown).
Table 5 Thyroid cancer according to quartiles of the average frequency of vegetable and fruit consumption in multiple logistic regression models (Odds ratios and 95 % confidence intervals)

Min, minimum; max, maximum.
* All adjusted OR models included parity (yes/no), energy (quartile) for the malignant cancer model. Energy (quartile) was adjusted for in the benign cancer model. The model for frequency of consumption of total vegetables was additionally adjusted for Na intake (quartile). The model for frequency of consumption of raw vegetables was additionally adjusted for Na intake (quartile) and vitamin E intake (quartile). The model for frequency of consumption of persimmons was additionally adjusted for age at menarche (years). The model for tangerines was additionally adjusted for parity (yes/no).
Discussion
Given that the effects of vegetable and fruit intake on thyroid cancer risk have not been well characterised, we aimed to examine the association between vegetable and fruit intake and the risk of thyroid cancer. We found that consumption of raw vegetables, persimmons and tangerines had a significant protective effect against thyroid cancer. Raw vegetable and persimmon intake had a dose–response effect on both malignant cases and benign cases in terms of both frequency of consumption as well as amount consumed. The amount of tangerines consumed showed a dose–response association with malignant cases.
According to the fourth Korean National Health and Nutrition Survey (KNHANES 2007–9), the average intake of total vegetables and fruits by Korean women is 293·8 g/d and 209·6 g/d, respectively. In the present study, the consumption of total vegetables by malignant cases and benign cases (243·6 g/d for malignant cases, 240·7 g/d for benign cases and 270·5 g/d for controls) was lower than that reported by KNHANES (293·8 g/d). The total fruit intake of cases and controls (297·4 g/d for malignant cases, 347·7 g/d for benign cases and 325·4 g/d for controls) was higher than that reported by KNHANES (209·6 g/d). With regard to persimmon intake, cases and controls had a lower intake than that reported by KNHANES (14·1 g/d for malignant cases, 14·2 g/d for benign cases, 17·8 g/d for controls, v. 27·7 g/d for KNHANES). However, cases and controls had a higher tangerine intake than that reported by KNHANES (38·6 g/d for malignant cases, 44·8 g/d for benign cases, 45·1 g/d for controls, v. 28·2 g/d for KNHANES). The level of vegetable consumption by Korean adults has been reported to be relatively high(Reference Popkin30, Reference Lee, Popkin and Kim31). The amount of vegetables consumed per person per year in Korea in 2005 was relatively high at 695 g for Korea, almost twice that of the USA (344 g) but lower than that of Greece (807 g)(32). When the intake of fruits and vegetables was combined (503·4 g/d in the fourth KNHANES), the intake by Koreans based on national surveys was higher than that of Europeans(33, Reference Joffe and Robertson34).
Several meta-analyses of the association between fruit and vegetable intake and the risk of cancers including breast cancer(Reference Gandini, Merzenich and Robertson35), colon cancer(Reference Koushik, Hunter and Spiegelman36), oral cancer(Reference Pavia, Pileggi and Nobile37) and gastric cancer(Reference Lunet, Valbuena and Vieira38) have found that vegetable intakes were inversely associated with the risk of breast cancer(Reference Gandini, Merzenich and Robertson35), oral cancer(Reference Pavia, Pileggi and Nobile37) and gastric cancer(Reference Lunet, Valbuena and Vieira38), but the site-specific associations of colon cancer were not consistent(Reference Koushik, Hunter and Spiegelman36) and that the fruit intakes were significantly related to oral cancer(Reference Pavia, Pileggi and Nobile37) and gastric cancer risk(Reference Lunet, Valbuena and Vieira38). Fruits and vegetables may exert their protective effects via antioxidant mechanisms, by serving as substrates for the formation of anti-neoplastic agents, by inhibiting nitrosamine formation, and/or by altering hormone metabolism, among other possible mechanisms(Reference Vainio and Bianchini21, Reference Steinmetz and Potter39). Furthermore, multiple substances present in fruits and vegetables such as antioxidant vitamins (β-carotene, vitamin A, vitamin E, vitamin C), dietary fibres, micronutrients and other phytochemicals may protect against cancer(Reference La Vecchia, Altieri and Tavani40). Several studies have also investigated the association between thyroid cancer risk and consumption of vegetables, especially in western countries(Reference Dal Maso, Bosetti and La Vecchia4, Reference Markaki, Linos and Linos9–Reference Memon, Varghese and Suresh12, Reference Mack, Preston-Martin and Bernstein16). These studies reported an inverse association between thyroid cancer risk and raw vegetable consumption(Reference Dal Maso, Bosetti and La Vecchia4, Reference Galanti, Hansson and Bergstrom10–Reference Memon, Varghese and Suresh12). In the present study, we focused on the effect of raw vegetables to obtain additional information about the inverse, dose–response associations between the amount and frequency of raw vegetable intake and thyroid cancer risk (more than 24·3 g/d) in malignant and benign cases. However, unlike the intake of raw vegetables, the intake of total vegetables and the frequency of vegetable consumption were not associated with malignant thyroid cancer. More than 50 % of the total vegetables consumed in Korea are pickled or cooked based on the results of the third KNHANES in 2005; the consumption of pickled and cooked vegetables may attenuate the effects of raw vegetables, possibly due to the carcinogenic effect of salt in pickled vegetables(Reference Ying and Sanders25) and/or the destruction of the anti-carcinogenic activity of raw vegetables by processing and storage under acidic and oxygen-rich conditions(Reference Kaur and Kapoor41).
No consistent association between fruit intake and thyroid cancer risk has been found in previous studies. Markaki et al. (Reference Markaki, Linos and Linos9) reported that high fruit consumption had a protective effect on thyroid cancer risk (OR 0·68, P = 0·01). In contrast, studies in Kuwait(Reference Memon, Varghese and Suresh12) and Northern Europe(Reference Galanti, Hansson and Bergstrom10) showed no association between the amount of fruit consumed and thyroid cancer risk. We found no significant association between total fruit intake and thyroid cancer risk but among fruits, persimmon and tangerine intakes were inversely associated with thyroid cancer risk. Diospyros kaki Thunb. (Ebenaceae), more commonly known as persimmon, is widely distributed in East Asian countries such as Korea, Japan and China(Reference Sakanaka, Tachibana and Okada42). Persimmon contains many bioactive agents such as flavonoids, tannins, carotenoids, antioxidant vitamins and minerals(Reference Mallavadhani, Panda and Rao43, Reference Kawase, Motohashi and Satoh44) that may mediate anti-carcinogenic processes. Furthermore, the anti-oxidative activity of persimmon has been examined both in vitro (Reference Kawase, Motohashi and Satoh44–Reference Jang, Jo and Bae47) and in vivo (Reference Ahn, Jeon and Lee45). Among the extracts of persimmon, tannins in particular may increase catalase and superoxide dismutase activity, thereby reducing oxidative damage to cells(Reference Ahn, Jeon and Lee45). As another explanation for the anti-carcinogenic effects of persimmon, 24-hydroxyursolic acid from persimmon may inhibit cell proliferation by activating AMP-activated protein kinase signalling, resulting in growth inhibition and cell apoptosis(Reference Khanal, Oh and Thuong46). However, there is very limited evidence available regarding the association between persimmon intake and cancer risk in epidemiological studies(Reference Shannon, Ray and Wu48). In the present study, both the amount of persimmon consumed (more than 15·8 g/d) and the frequency of consumption (approximately once per d) had a significant inverse relationship with thyroid cancer risk. These results are consistent with those reported by a case–control study in China; the authors of that study reported that persimmon intake significantly reduced breast cancer risk (OR 0·60; 95 % CI 0·42, 0·86; P for trend = 0·006)(Reference Shannon, Ray and Wu48). Tangerine (Citrus reticulate Blanco) is a citrus fruit that is a good source of bioactive compounds such as flavonoids, vitamin C, carotenoids, limonene and citric acid(Reference Vainio and Bianchini21). Citrus flavonoids are known to have strong anti-proliferative activities in human cancer cells(Reference Manthey and Guthrie49). Citrus flavonoids may function as anticancer agents by inhibiting enzymes such as protein kinases involved in mitogenic signal transduction(Reference Manthey, Guthrie and Grohmann50). Epidemiological studies have been conducted in the past to determine if there is a relationship between citrus fruit intake, including tangerine intake, and thyroid cancer risk(Reference Galanti, Hansson and Bergstrom10), colon, rectum cancer risk(Reference Franceschi, Parpinel and La Vecchia51) and breast cancer risk(Reference Shannon, Ray and Wu48, Reference Franceschi, Parpinel and La Vecchia51). However, no significant negative association was observed in any of these previous studies(Reference Shannon, Ray and Wu48, Reference Franceschi, Parpinel and La Vecchia51).
The present study had several limitations. First, it was a hospital-based case–control study, and thus, the results may not be generalisable to the general population(Reference Breslow and Day52). Second, recall bias is one of the important potential biases in a case–control study. The differential recall between cases and controls may induce biased results(Reference Gordis53). However, in the present study, almost all cases were interviewed before diagnosis so that differential recall biases may not be substantial. Third, the sample size was not sufficient to draw definitive conclusions and we were not able to analyse the differences in risk factors according to the subtype of thyroid cancer (papillary, follicular, medullary, anaplastic). The number of pairs assuming α = 0·05, β = 0·10 and an OR of 2·0 (or 0·5) was 281 pairs(Reference Schlesselman and Stolley54). However, the final sample size was smaller than the calculated number. Therefore, a weak relationship (OR < 2·0 or 0·5 < OR < 1·0) may not have been detected in the present study. Fourth, although the semiquantitative FFQ has been validated in previous studies, we did not perform the additional validity–reliability study for the modified version and for food items. Fifth, among the non-dietary risk factors for thyroid cancer, the best-established cause of thyroid cancer is exposure to ionising radiation, particularly during early childhood(Reference DeLellis1, Reference Dal Maso, Bosetti and La Vecchia4). We could not measure the extent of radiation exposure. In future studies, radiation in everyday life including medical radiation needs to be assessed and to be analysed for the association with thyroid cancer. Sixth, we could not examine the association between benign thyroid nodules or adenomas and malignant thyroid cancer risk in the present study, because we recruited controls who did not have thyroid nodules or thyroid hormone diseases. In the analyses, after excluding pairs with history of any thyroid disease in cases (twenty-nine pairs in malignant cancer and forty-three pairs in benign thyroid nodules or adenomas), the association between fruit and vegetable intake and thyroid cancer risk did not substantially change. To the best of our knowledge, ours is the first study to focus on benign thyroid cases and to use controls without any nodules in the thyroid gland.
The findings in the present study suggest that high consumption of raw vegetables, persimmons and tangerines may decrease thyroid cancer risk. In addition, consumption of raw vegetables and these fruits may help prevent thyroid cancer. However, our findings need to be confirmed in a large-scale cohort study.
Acknowledgements
The present study was supported by the Korean Ministry of Science and Technology (grant nos M10418020002-08N1802-00210 and M10418020002-09N1802-00210). The authors wish to thank all subjects for their dedication and commitment. M. K. K. designed and supervised the execution of the study. S. K. J. performed the data analyses and wrote the manuscript with K. K. In addition, K. T. and G. K. contributed to the data preparation. All authors participated in the interpretation of the results and the editing of the manuscript. None of the authors had a personal or financial conflict of interest.