A major production expense for beef cow/calf enterprises is associated with the development of replacement heifers and their associated feed inputs(Reference Freetly, Ferrell and Jenkins1, Reference Clark, Creighton and Patterson2). The present paradigm for the development of replacement heifers implies that heifers need to obtain a certain body weight (BW, 60–65 % of mature weight) and be pubertal by 14 months of age in order to give birth to their first calf by 24 months of age in most North American production systems, while globally other production systems may target a more liberal time frame beyond 24 months of age for heifers to have their first offspring. It is believed by cow/calf producers in the Northern Great Plains, USA, that achievement of this goal requires providing additional feed resources above those provided by native rangelands from weaning to breeding. Appropriate development of replacement heifers is crucial in order to obtain puberty(Reference Ferrell3–Reference Short and Bellows6), promote lifetime productivity and optimise milking ability(Reference Pinney, Stephens and Pope7, Reference Swanson8). Low nutrient intake following weaning can delay the onset of puberty(Reference Arije and Wiltbank4–Reference Short and Bellows6, Reference Clanton and Zimmerman9), while very high levels of nutrition following weaning may reduce lifetime productivity, longevity in a cow herd and limit the milking ability of heifers(Reference Pinney, Stephens and Pope7, Reference Swanson8). Researchers(Reference Clanton, Jones and England10) concluded that producers have many options of developing heifers as long as the necessary weight is achieved by breeding (14 months of age). Therefore, producers encounter the challenge of obtaining reproductive competency (i.e. puberty) in heifers while minimising input (i.e. harvested feedstuffs) costs. However, over the last few decades, it has become evident that the size of mature cow has increased(Reference Arango, Cundiff and Van Vleck11, Reference Dib, Van Vleck and Spangler12), which increases nutrient requirements, making it even more difficult to economically feed heifers to achieve the standard BW by breeding. Reports provide evidence that harvested feed inputs can be reduced without sacrificing reproductive performance(Reference Freetly, Ferrell and Jenkins1) by targeting a lesser BW (% of mature BW) for replacement females(Reference Funston and Deutscher13).
To evaluate the metabolic influence of reduced feeding of harvested feedstuffs during the heifer development period, an evaluation of the efficiency of tissue nutrient uptake of specific energetic metabolites can be evaluated (i.e. glucose and acetate). Glucose tolerance tests (GTT) are frequently used to evaluate the efficiency of tissues to incorporate glucose. Hepatic, pancreatic and small intestinal tissues express GLUT2, which is primarily responsible for maintaining homeostatic status of glucose in the normal physiological state. The GLUT2 transporter is insulin independent and in the hepatic tissue regulates excess peripheral glucose by removing it from circulation, while pancreatic β-cells of the islets of Langerhans along with glucokinase monitor glucose concentrations and may help regulate insulin secretion(Reference Hughes, Quaade and Johnson14–Reference Antoine, Lefrancois-Martinez and Le Guillou17). The insulin-dependent GLUT4 found in muscle and adipose tissue(Reference Hocquette, Castiglia-Delavaud and Graulet18) is present when higher physiological concentrations of blood glucose are detected(Reference Roche, Sheahan and Chagas19). Therefore, a GTT that delivers a high physiological dose of glucose can indirectly measure the efficiency of both the GLUT2 (initial response of glucose uptake by hepatic and pancreatic tissues) and more importantly GLUT4 (extended response directed to muscle and adipose via pancreatic insulin release and subsequent tissue responsiveness to insulin), and in the case of the present study evaluate glucose incorporation with regard to heifers fed at two different planes of nutrition and at two different physiological production ages. The question arises is whether animals that are produced from and developed on different planes of dietary intake may become conditioned to have tissues that respond differently to metabolic signals (i.e. insulin). Tissues that are insulin insensitive lack the ability to signal GLUT and have them translocate to tissue cell membranes. In grazing livestock, this can occur when forages senesce and ruminal acetate concentration becomes much greater than propionate(Reference Cronje, Nolan and Leng20). Conversely, in ruminants if acetate accumulates from an inadequate supply of glucogenic precursors for hepatic gluconeogenesis, this may exacerbate tissue insulin insensitivity through the production of ketones and NEFA(Reference Dresner, Laurent and Marcucci21–Reference Waterman, Butler, Hess, DelCurto and Bowman25).
Hepatic ketogenesis occurs at similar rates with no discrimination in fed, non-pregnant, non-lactating goats, sheep and dairy cows, and hepatic tissue release of β-hydroxybutyrate increases in late gestation and early lactation(Reference Heitmann, Dawes and Sensenig26). Acetate irreversible loss tests (AILT) indirectly evaluate the efficiency of energy metabolism in ruminants. Acetate, an endproduct of ruminal fermentation, is a precursor for lipogenesis in adipose tissue, where acetyl-CoA is used to synthesise fat. Acetate is also an intermediate in the tricarboxylic acid cycle, where acetyl-CoA is utilised to regenerate citrate and eventually ATP. Acetate utilisation is dependent upon the supply of intracellular glucose, which is the sum of glucose supply and insulin sensitivity. When the lipogenic pathway (incorporation of acetate into long-chain fatty acids in adipose tissue) and tricarboxylic acid cycle (incorporation of acetate to convert oxaloacetate to citrate) are operating less efficiently, excess acetate is either oxidised as a substrate in futile cycles or directed towards the synthesis of ketones(Reference Armentano27, Reference MacRae and Lobley28). Therefore, the rate of irreversible loss or half-life of acetate into peripheral tissues will identify the glucogenic potential of the diet(Reference Cronje, Nolan and Leng20) and identify how efficient acetate is being incorporated into lipids and utilised in the tricarboxylic acid pathway since acetate moves into cells passively via the extracellular–intracellular concentration gradient. AILT allows for an indirect measure of the rate of acetate utilisation.
The objective of the present study was to evaluate energy status by comparing glucose and acetate kinetics in maternal tissues by conducting GTT and AILT on heifers born from dams that received two different winter supplementation treatments in utero and then were reared on two post-weaning nutritional development programmes.
Materials and methods
Study area
The present study was conducted at the Fort Keogh Livestock and Range Research Laboratory (LARRL) located approximately 1·6 km west of Miles City, MT, USA (46°22′N 105°5′W). The LARRL encompasses 22 500 ha and has an average elevation of 730 m, which includes rolling hills and barren land with small intersecting streams that drain into large permanent rivers. Precipitation and temperature patterns varied during 2003 through 2006 when the study took place (Fig. 1). Predominant grass genera at study sites include grama (Bouteloua), needlegrass (Hesperostipa) and wheatgrass (Pascopyron) within a mixed grass-dominated rangeland(Reference Küchler29).

Fig. 1 Monthly precipitation (![]() ) and temperature (■) from January 2003 to December 2006 and their corresponding 69 years average precipitation and temperature (
) and temperature (■) from January 2003 to December 2006 and their corresponding 69 years average precipitation and temperature (![]() precipitation,
precipitation, ![]() temperature) in Miles City, MT, USA. Annual precipitation was 280, 240, 380 and 270 mm, respectively, for 2003, 2004, 2005 and 2006 with a 69-year average annual precipitation of 340 mm. Initiation of metabolic challenges began in May of each year followed by a second challenge in October of the subsequent year as indicated by dashed lines. Information obtained from Western Regional Climate Center(44) for monthly and historical average annual precipitation and temperature.
temperature) in Miles City, MT, USA. Annual precipitation was 280, 240, 380 and 270 mm, respectively, for 2003, 2004, 2005 and 2006 with a 69-year average annual precipitation of 340 mm. Initiation of metabolic challenges began in May of each year followed by a second challenge in October of the subsequent year as indicated by dashed lines. Information obtained from Western Regional Climate Center(44) for monthly and historical average annual precipitation and temperature.
Herd management
The LARRL Institutional Animal Care and Use Committee approved all animal handling and experimental procedures used in the present study.
The heifers were a stable composite gene combination population composed of 1/2 Red Angus, 1/4 Charolais and 1/4 Tarentaise. Heifers represent a randomly selected population produced by mating composite dams and sires with consideration given to minimising inbreeding but without emphasis on production traits or dam nutritional treatment. The heifers were born over a 2-year period from dams that were fed harvested feed from mid-to-late gestation (approximately 80 d before parturition) that provided an adequate dam winter supplementation (ADEQ) or marginal dam winter supplementation (MARG) level of supplemental nutrition. The ADEQ and MARG treatments were designed to complement dormant protein-deficient forage from nutrient analysis collected on rumen and oesophageal diet extrusa samples (file data at LARRL). In brief, supplemental feed was supplied either daily or every other day to deliver on average 1·8 kg/d for each ADEQ cow and 1·2 kg/d for each MARG cow. The only exception was during days when access to pasture forage was limited due to snow cover when cows were fed at a rate equivalent to 10·9 or 9·1 kg alfalfa hay/d for each cow in the ADEQ or MARG treatments, respectively(Reference Roberts, Waterman and Geary30). This composite gene combination herd is subjected to a minimum of five cattle handling days per year (pre-calving in early March, pre-breeding in early June, autumn pregnancy check in mid September, weaning in mid-October and allocation to winter treatments in early December), at which time BW and body condition scores are assessed. Body condition scores (1, emaciated and 9, extremely obese) were assigned by two experienced technicians(Reference Herd and Sprott31, Reference Wagner, Lusby and Oltjen32).
A complete description of the experimental design and protocol has been reported(Reference Roberts, Waterman and Geary30, Reference Roberts, Paisley and Geary33). In brief, at weaning, the heifers were stratified into groups of six based on weaning weight. Each group was randomly assigned to one of the twenty-two to twenty-four pens. Each pen measures approximately 5·8 × 11 m and contains six individual feed stanchions equipped with electronic Calan gates (American Calan, Inc., Northwood, NH, USA) to accommodate individual feed delivery and consumption. The heifers were adapted to the pen environment and mechanical operation of Calan gates for approximately 30 d. During adaptation, the heifers were provided daily ad libitum access to the experimental diet (approximately 10·8 kg/d; Table 1). The heifers were randomly assigned to either a control (ad libitum; 100 %) or restricted (80 %) level of feeding within the pens. Control heifers were fed to appetite and 80 % heifers were fed at 80 % of that consumed by 100 % heifers that were adjusted to a common BW basis using the following formula: (0.80 × (mean BW of restricted/mean BW of control) × mean daily feed intake (as-fed basis) of controls over the preceding 28 d period). Heifer BW was measured every 28 d throughout the 140 d development period and feed intake was adjusted using the aforementioned formula.
Table 1 Feedstuffs and chemical composition (DM basis) of diets fed during a 140 d heifer development period in 2004 and 2005
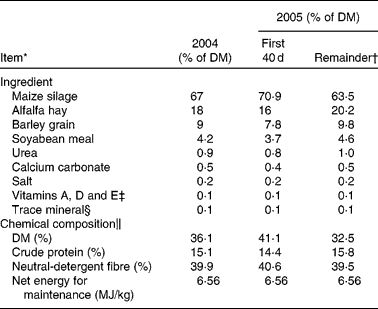
* Adapted from Roberts et al. (Reference Roberts, Paisley and Geary33).
† A change in source of silage resulted in slightly different dietary formulation.
‡ Contained 13 200 000 μg/kg of vitamin A, 22 000 μg/kg of vitamin D and 396 μg/kg of vitamin E.
§ Contained 20 % Mg, 0·2 % K, 2·6 % S, 18 000 parts per million (ppm) of Cu, 60 000 ppm of Zn, 40 000 ppm of Fe, 300 ppm of Se, 60 000 ppm of Mn, 180 ppm of Co and 1140 ppm of I.
∥ Based on analysed chemical composition of individual ingredients.
Orts were removed from the feed bunk and weight was recorded as necessary to ensure that fresh feed was available for each heifer on a daily basis. At the end of the 140 d development period, the heifers were placed into common pens and received ad libitum feed for an additional 50 d to allow for oestrous synchronisation and artificial insemination. Synchronisation and artificial insemination protocols have been described(Reference Roberts, Paisley and Geary33). Following artificial insemination, the heifers were managed as one herd until gestation/winter nutritional treatments were applied. Winter nutritional treatments consisted of separating heifers into two herds – ADEQ or MARG – from November through February. At the beginning of March, just before parturition, all first calf heifers were combined and managed as a single herd receiving the same nutritional management regimen until the subsequent winter feeding regimen when they were separated to receive their gestation/winter nutritional treatments.
Experimental animals
A study initiated in 2001 was designed to investigate the immediate and long-term responses from the offspring of cows permanently assigned to ADEQ or MARG winter nutritional treatments. Each year at weaning, the heifers from the ADEQ and MARG cows are randomly assigned to one of two levels of development (100 or 80 %) for 140 d with no discrimination to dam treatment.
A total of thirty-two (16/year; eight heifers assigned from each nutritional programme (100 and 80 %)) heifers were randomly assigned to receive administration of a GTT and AILT. The heifers used in the present study were born from dams receiving the ADEQ (n 12) or MARG (n 20) winter nutritional treatment. The heifers used in year 1 (2003) were born between days 87 and 93 (average day of 91 (sem 0·4) d; BW 35 (sem 0·88) kg) and weaned on day 281 (190 (sem 0·04) d of age; BW 219 (sem 4·7) kg). The heifers used in year 2 (2004) were born between days 73 and 92 (average day of 83 (sem 1·5) d; BW 34 (sem 1·29) kg) and were weaned on day 297 (sem 214 (sem 1·5) d of age; 203 (sem 6·3) kg).
Metabolic measures of energetic efficiency
Heifer BW and body condition score were recorded on the morning of each metabolic test. The heifers were subjected to the first metabolic test in May (before oestrous synchronisation), a GTT (403 (sem 1·13) d of age) and an AILT (403 (sem 1·13) d of age) at the cessation of the 140 d development period in both years. In consecutive weeks, one half of 100 and 80 % heifers received a GTT, whereas the remaining heifers received AILT. The following week, the heifers were given the opposite metabolic challenge (GTT or AILT) such that all heifers received both the GTT and the AILT within a 10 d period. Subsequently, the second metabolic challenge in November (beginning of last trimester of pregnancy), a GTT (935 (sem 1·50) d of age) and an AILT (945 (sem 1·71) d of age) were conducted on the same animals the following year during their second pregnancy. For this subsequent evaluation, all heifers received a GTT followed by an AILT 7 d later.
On the day of the metabolic tests at 06.00 hours (before offering of feed for the May challenge and before morning grazing bout for the November challenge), the heifers were gathered and transported to a cattle-working facility, and fitted with an in-dwelling jugular catheter. Immediately following catheterisation, the heifers were walked, in a low stress manner, to an individual stall located approximately 45 m adjacent to the chute where in-dwelling jugular catheterisation occurred. The technicians began the metabolic challenges once the heifers were stalled. There was no access to feed or water during the metabolic challenges. For the GTT, a 50 % (w/v) dextrose solution was infused through the catheter at 0·50 ml/kg BW (250 mg glucose/kg BW) using 60 ml syringes. Blood samples were collected into syringes via a jugular in-dwelling catheter at − 1, 0, 3, 6, 9, 12, 15, 20, 40, 60, 80, 100, 120, 140, 160 and 180 min relative to glucose infusion (infusion was immediate after obtaining the 0 min blood sample). During each collection time, 2 ml of blood was initially drawn and discarded to remove saline (0·9 % NaCl) from the catheter. The blood was then subsequently drawn and transferred from syringes into serum separator tubes (9 ml draw serum separator tubes, Corvac™; Tyco Healthcare Group LP, Mansfield, MA, USA). A 5 ml saline flush was then pushed through the catheter and the saline syringe remained attached to the catheter until subsequent collection time. The samples were allowed to coagulate and the serum was harvested after centrifugation at 1500 g for 30 min and stored at − 20°C until analysis.
For AILT, a 20 % (w/v) acetate solution was infused through the catheter at 1·25 ml/kg BW (4·16 mm acetate/kg BW) using 60 ml syringes. Blood samples were collected into syringes via the jugular in-dwelling catheter at − 1, 0, 1, 3, 5, 7, 10, 15, 30, 60 and 90 min relative to acetate infusion (infusion was immediately after obtaining the 0 min blood sample). During each collection time, 2 ml blood was initially drawn and discarded to remove saline (0·9 % NaCl) from the catheter. The blood was then subsequently drawn and transferred from syringes into tubes containing lithium heparin as an additive (7 ml draw, Vacutainer™; Becton Dickson, Franklin Lakes, NJ, USA). Plasma was collected after being centrifuged at 1500 g for 30 min and stored at − 20°C until analysis.
To evaluate whether serum metabolite concentrations were influenced by the nutritional regimen, baseline concentrations were evaluated for glucose, insulin, urea-N and NEFA using − 1 and 0 pre-infusion samples before the GTT. Additionally, baseline plasma acetate concentrations were measured using − 1 and 0 pre-infusion samples before the AILT.
All serum metabolite concentrations were analysed in duplicate aliquots using commercially available kits to measure glucose by the glucose oxidase method (Kit TR15321; Thermo Electron Corporation, Waltham, MA, USA; endpoint with an intra-assay CV of 3·0 % and an inter-assay CV of 5·8 %), urea N by the urease method (Kit TR12321; Thermo Electron Corporation; endpoint with an intra-assay CV of 3·6 % and an inter-assay CV of 7·7 %) and NEFA by the acyl-CoA synthetase-acyl-CoA oxidase (ACS-ACOD) method (Wako Chemicals USA, Inc., Richmond, VA, USA; endpoint with an intra-assay CV of 3·2 % and an inter-assay CV of 1·9 %). A handheld ketone sensor (MediSence®, Precision Xtra™; Abbott Laboratories, Abbott Park, IL, USA) was used to measure serum β-hydroxybutyrate(Reference Byrne, Tieszen and Hollis34). Serum insulin concentrations were measured in duplicate by solid-phase 125I-Insulin RIA (Coat-a count kit; Diagnostic Products, Inc., Los Angeles, CA, USA). The insulin assay had an intra-assay CV of 8·8 % and an inter-assay CV of 14·3 % with 99 % recovery. Acetate was filtered by centrifugation with a centrifugal filter device for 2 h at 5000 g for deproteinisation (Millipore Centricon® YM-10 centrifugal device; Millipore Corporation, Burlington, MA, USA). The filtered supernatant was mixed in a 5:1 ratio with 25 % meta-phosphoric acid containing 2 g/l of 2-ethyl butyric acid as an internal standard. Concentrations of acetate were measured by GC (Thermo Trace GC; Thermo Fisher Scientific, West Palm Beach, FL, USA with a capillary column (15 m × 0·53 mm; RESTEK Stalbilwax®-DA; Bellefonte, PA, USA); temperature ramp 20°C/min from 90°C to 220°C and maintained for 4 min).
Acetate and glucose disappearance, and half-life were estimated for each animal by regression of the logarithmic-transformed metabolite concentrations over time(Reference Kaneko35, Reference Regnault, Oddy and Nancarrow36). Total and incremental (i.e. ignores area beneath baseline values) area under the curve (AUC and IAUC, respectively) was determined for acetate, glucose and insulin concentrations using trapezoidal summation.
Statistical analysis
Data were analysed using the MIXED procedure of the Statistical Analysis Systems statistical software package version 9.1 (SAS Institute, Inc., Cary, NC, USA). A completely randomised block design was used, where a block represented calves born in 2003 and 2004 as follows: calves born in 2003 received metabolic challenges in 2004 and 2005; calves born in 2004 received metabolic challenges in 2005 and 2006. The statistical model included fixed effects of dam winter nutritional treatment (the in utero winter nutritional treatment; ADEQ and MARG), heifer development treatment (100 and 80 %), metabolic challenge (immediately after the heifer development period and when gestating their second calf) and their interactions (i.e. dam winter nutritional treatment × heifer development treatment; dam winter nutritional treatment × metabolic challenge; heifer development treatment × metabolic challenge; dam winter nutritional treatment × heifer development treatment × metabolic challenge). Only significant P ≤ 0·05 interactions are reported. The RANDOM statement included heifer within block × heifer treatment × dam treatment. Average daily gain from birth to weaning was calculated and used as a covariate in the model. A total of four heifers each year failed to conceive or calve and were eliminated from the analysis for the second metabolic challenge. Values are expressed as means with standard errors and a P ≤ 0·05 separating means was considered significantly different.
Results
Body weight and condition
BW was similar for dam winter nutritional treatment (P = 0·53) and tended to be greater (P = 0·076) throughout the study for 100 v. 80 % treated heifers; however, as the heifers aged, BW increased from the first to the second metabolic challenge (P < 0·001; Table 2). The body condition score was similar for the dam winter nutritional treatment (P = 0·86). A heifer treatment × age at metabolic challenge interaction was observed for the body condition score (P = 0·053), which indicated that 100 % treated heifers lost body condition as age increased between the metabolic challenges, whereas 80 % treated heifers maintained the same condition (4·5–4·0 (sem 0·17) v. 4·1–4·2 (sem 0·19), respectively, for 100 and 80 % heifer development treatments for age at the first and second metabolic challenges; Table 2).
Table 2 Body weight (BW), body condition and baseline serum metabolites from glucose tolerance tests and acetate irreversible loss test conducted on heifers immediately after the 140 d development period and again approximately 17 months later in the autumn when heifers were pregnant with their second calf

ADEQ, adequate dam winter supplementation; MARG, marginal dam winter supplementation, BCS, body condition score.
* An evaluation of heifers born from dams receiving ADEQ or MARG winter nutritional treatments.
† A comparison of heifers developed on an ad libitum (100 %) or reduced (80 %; fed at 80 % of that consumed by controls adjusted to a common BW) 140 d heifer development diet.
‡ Age at first metabolic challenge, 403 (sem 1·13) d; age at second metabolic challenge, 935 (sem 1·50) d for the glucose tolerance test and 945 (sem 1·71) d for the acetate irreversible loss test.
§ Values represent number of heifers at the time of first and second (in parentheses) metabolic challenges.
‖ Heifer development treatment × metabolic challenge interaction (P = 0·05).
Baseline metabolites
Baseline serum glucose concentrations were similar for dam winter nutritional treatment (P = 0·62) and heifer development treatment (P = 0·46; Table 2). There was a 22·6 % decrease in baseline glucose concentrations as age between the metabolic challenges increased (P < 0·001). Baseline serum insulin concentrations tended to be greater (P = 0·08) for dam winter nutritional treatment when the heifers were born from MARG dams compared with the heifers born from ADEQ dams. However, baseline insulin concentrations did not differ due to heifer development treatment (P = 0·30). Similar to baseline glucose concentrations, there was a 73·9 % decrease (P < 0·001) in baseline insulin concentrations as age at metabolic challenge increased (Table 2).
Baseline serum NEFA concentrations were similar for dam winter nutritional treatment (P = 0·21) and heifer development treatment (P = 0·57). Serum NEFA concentrations increased (P < 0·001) by 15·5 % as age increased from the first to the second metabolic challenge. Baseline serum β-hydroxybutyrate concentrations were similar for dam winter nutritional treatment (P = 0·32), heifer development treatment (P = 0·99) and age at metabolic challenge (P = 0·65). Baseline serum urea N concentrations were similar for dam winter nutritional treatment (P = 0·18), heifer development treatment (P = 0·52) and age at metabolic challenge (P = 0·83). Baseline serum acetate concentrations were also similar for dam winter nutritional treatment (P = 0·21), heifer development treatment (P = 0·48) and age at metabolic challenge (P = 0·29; Table 2).
Response to glucose tolerance test
The mean profiles of glucose and insulin relative to the infusion of glucose or acetate at the first metabolic challenge when the heifers averaged 403 d of age and again at the second metabolic challenge when the heifers averaged 935 d of age are presented in Fig. 2. Peak glucose concentrations, following a bolus dose of glucose, were similar for dam winter nutritional treatments (P = 0·99), heifer treatments (P = 0·88) and age at metabolic challenges (P = 0·83). A dam winter nutritional treatment × age at metabolic challenge interaction was observed for time to peak glucose concentration (P = 0·008). Heifers from dams receiving the ADEQ winter treatment had peak glucose concentration that did not differ for age at the first and second metabolic challenges (3·1–3·6 (sem 0·68) min), whereas time to peak glucose concentration for heifers from MARG winter-treated dams was shortened as heifer age increased from the first to second metabolic challenge (5·6–3·1 (sem 0·51) min). Time to peak glucose concentration after infusion was similar for heifer development treatments (P = 0·99; Table 3). Peak insulin concentrations were similar for dam winter nutritional treatments (P = 0·45) and heifer development treatment (P = 0·63). However, peak insulin concentrations decreased (P < 0·001) by 67 % between the metabolic challenges as the age of the heifers increased from the first to second metabolic challenge. Time to peak insulin concentration after glucose infusion was similar for dam winter nutritional treatment (P = 0·96), heifer development treatment (P = 0·16) and metabolic challenge (P = 0·18; Table 3).

Fig. 2 Mean response profile of (a and b) glucose, (c and d) insulin and (e and f) acetate of heifers whose dams received either 1·8 or 1·2 kg/d of winter nutritional supplementation, and then were developed post-weaning at either ad libitum (100 %, ![]() ) or 80 % (
) or 80 % (![]() ) of the ad libitum feed (on a common body weight bases), receiving a glucose (250 mg d-glucose/kg body weight (BW)) and an acetate (4·16 mm acetate/kg BW) tolerance test at 403 d of age (a, c and e) and again at 935 (sem 1·50) d of age for the glucose tolerance test and 945 (sem 1·71) d of age for the acetate irreversible loss test (b, d and f).
) of the ad libitum feed (on a common body weight bases), receiving a glucose (250 mg d-glucose/kg body weight (BW)) and an acetate (4·16 mm acetate/kg BW) tolerance test at 403 d of age (a, c and e) and again at 935 (sem 1·50) d of age for the glucose tolerance test and 945 (sem 1·71) d of age for the acetate irreversible loss test (b, d and f).
Table 3 Peak concentrations, disappearance, half-life and total and incremental area under the curve (AUC and IAUC, respectively) for a glucose tolerance tests and acetate irreversible loss test conducted on heifers immediately after the 140 d development period and again approximately 17 months later in the autumn when heifers were pregnant with their second calf

ADEQ, adequate dam winter supplementation; MARG, marginal dam winter supplementation.
* An evaluation of heifers born from dams receiving ADEQ or MARG winter nutritional treatments.
† A comparison of heifers developed on an ad libitum (100 %) or reduced (80 %; fed at 80 % of that consumed by controls adjusted to a common body weight) 140 d heifer development diet.
‡ Age at the first metabolic challenge, 403 (sem 1·13) d; age at the second metabolic challenge, 935 (sem 1·50) d for the glucose tolerance test and 945 (sem 1·71) d for the acetate irreversible loss test.
§ Values represent number of heifers at time of the first and second (in parentheses) metabolic challenges.
‖ Dam winter nutritional treatment × age at metabolic challenge (P ≤ 0·008).
The disappearance of glucose from peripheral circulation was similar between dam winter nutritional treatments (P = 0·39), heifer development treatments (P = 0·86) and age at metabolic challenges (P = 0·13). Heifers born from MARG dams tended to have a 24·8 % shorter (P = 0·083) glucose half-life than heifers born from ADEQ dams. Glucose half-life did not differ for heifer development treatments (P = 0·34) or age at metabolic challenges (P = 0·65). Glucose AUC (total and incremental) for glucose following the GTT was similar for dam winter nutritional treatments (P = 0·40) and heifer treatments (P = 0·53). Additionally, total glucose AUC tended (P = 0·066) to be lesser during the second metabolic challenge when the heifers were pregnant with their second calf compared with immediately after the heifer development period. Total and incremental insulin AUC did not differ for dam winter nutritional treatments (P = 0·49) or heifer treatments (P = 0·32; Table 3). Both total and incremental insulin AUC were lesser (P < 0·001) during the second metabolic challenge when the heifers were pregnant with their second calf compared with immediately after the heifer development period (Table 3).
Responses from the acetate irreversible loss test
The mean profiles of plasma acetate relative to the infusion of acetate during the AILT at the first metabolic challenge when the heifers averaged 403 d of age and again at the second metabolic challenge when the heifers averaged 945 d of age are presented in Fig. 2. A dam winter nutritional treatment × age at metabolic challenge interaction was measured (P = 0·004) for peak acetate concentration and indicated that heifers born from ADEQ dams had decreased acetate concentrations (12·7–6·8 (sem 1·75) mm) as age increased between the first and second metabolic challenges, whereas heifers born from MARG dams had increased acetate concentrations as age increased from the first to second metabolic challenge (6·6–10·5 (sem 1·30) mm). Peak acetate concentration following acetate infusion was not different for heifer development treatments (P = 0·84). Time to peak acetate concentration after infusion was similar for dam winter nutritional treatments (P = 0·80) and heifer development treatments (P = 0·59). However, as age increased from the first to second metabolic challenge, time to peak acetate concentration was 2·7 min earlier (P = 0·003; Table 3).
Acetate disappearance, half-life and total AUC were similar for dam winter nutritional treatments (P ≥ 0·22), heifer development treatments (P ≥ 0·62) and age at metabolic challenges (P ≥ 0·28; Table 3). A dam winter nutritional treatment × age at metabolic challenge interaction (P = 0·02) for incremental acetate AUC was observed. Heifers from dams receiving the ADEQ winter treatment had incremental acetate AUC that declined as age increased from the first to second metabolic challenge (220–131 (sem 51) mm × 90 min, respectively), whereas incremental acetate AUC for heifers from MARG winter-treated dams increased as heifer age increased from the first to second metabolic challenge (94–220 (sem 38) mm × 90 min, respectively). Additionally, a heifer development treatment × age at metabolic challenge interaction (P = 0·04) for incremental acetate AUC was observed. Heifers receiving the 100 % development treatment had incremental acetate AUC that increased as age increased from the first to second metabolic challenge (108–218 (sem 42) mm × 90 min, respectively), whereas incremental acetate AUC for heifers that received the 80 % development treatment declined as heifer age increased from the first to second metabolic challenge (205–133 (sem 47) mm × 90 min, respectively).
Discussion
Post-weaning development of heifers on the restricted protocol results in a 27 % reduction in the use of harvested feed throughout the 140 d development period(Reference Roberts, Paisley and Geary33, Reference Roberts, Geary and Grings37). This resulted in an approximate savings of US$21 per pregnant heifer(Reference Roberts, Geary and Grings37). Additionally, previous results of heifers in this long-term experiment have consistently shown lesser average daily gain (ADG) and BW gain for 80 % treated heifers compared with 100 % treated heifers beginning approximately 4 weeks after the initiation of the 140 d heifer development period(Reference Roberts, Paisley and Geary33, Reference Roberts, Geary and Grings37). However, ADG from the conclusion of the 140 d development period through 19·5 months of age was greater for 80 % treated heifers, which indicates a period of compensatory gain and potential metabolic efficiency following reduction in feed(Reference Roberts, Paisley and Geary33). The present study represents a small subset of heifers described in previous reports. In agreement with previous reports, heifers that received 100 % treatment weighed more than heifers receiving 80 % treatment at both 403 and 940 d of age when the metabolic challenges were administered. When management purposely reduced nutrient intake by 20 % of what ad libitum (100 %) treated heifers received (on a common BW basis) during the 140 d development period, baseline serum metabolite concentrations were unchanged (P>0·10; Table 2). Serum glucose, insulin, NEFA, urea N or acetate concentrations remained consistent, even though a lower DM intake was imposed (by experimental design) for the 80 % group(Reference Roberts, Paisley and Geary33). Furthermore, 100 and 80 % treated heifers were similar in all measures for GTT and AILT, which suggests that both 100 and 80 % treated heifers have similar rates of gluconeogenesis, insulin sensitivity and release and utilisation of acetate for oxidative metabolism and lipogenesis(Reference Preston and Leng38–Reference Egan40). Our data do not reveal any potential mechanism for compensatory gain observed in 80 % treated heifers following the 140 d heifer development period as reported(Reference Roberts, Paisley and Geary33).
Researchers(Reference Cronje, Nolan and Leng20) have demonstrated that when the glucogenic potential of a diet is low and glucose precursors are added to the diet, acetate irreversible loss is faster and glucose half-life is shorter(Reference Waterman, Sawyer and Mathis24). The present study documents that a reduction in DM intake during a 140 d development period does not have any detrimental impact on glucose supply, insulin release or tissue sensitivity of acetate utilisation in 80 % treated heifers compared with 100 % treated heifers.
However, differences were measured due to changes in age and physiological state between the two metabolic challenges that were separated by 17 months. Lower pre-infusion (i.e. baseline) concentrations of glucose and insulin from the first and second metabolic challenges partially reflect the diet quality changes that occurred. At the time of the first metabolic challenge, the heifers had completed a 140 d development trial while consuming a high-quality maize silage-based diet in confinement, whereas the second metabolic challenge that occurred approximately 17 months later when the heifers were consuming mostly dormant rangeland native forages. In ewes, the peripheral concentration of insulin and glucose-stimulated insulin release decreased as gestation advanced(Reference Regnault, Oddy and Nancarrow36), and in cattle, both season or quality of forage and physiological state (gestation) have shown decreased responses in glucose uptake and insulin responsiveness(Reference Waterman, Grings and Geary41). Differences in diet quality between the metabolic challenges also explain the greater NEFA concentrations observed during the second metabolic challenges due to likely mobilisation of adipose storage. The serum metabolite concentrations observed at the second metabolic challenge are comparable with concentrations previously reported for cattle grazing dormant rangelands(Reference Waterman, Grings and Geary41). Interestingly, the peak insulin concentration and insulin AUC in the second GTT (in November when the heifers are grazing dormant forages) were substantially lower than those measured in the first GTT (Fig. 2). This may indicate that the pancreatic release of insulin was greater in heifers at the termination of the 140 d heifer development period and the infusion of glucose was accompanied by a longer sustained pancreatic release of insulin at the first metabolic challenge.
The glucogenic potential of the diet during the second metabolic challenge should have been lower when the heifers were grazing dormant rangelands. Therefore, it would be expected that a higher ruminal acetate:propionate ratio would be observed when the ruminants graze rangelands(Reference Adams, Cochran and Currie42) compared with the maize silage-based diets consumed during the 140 d heifer development period. From this scenario, a slower acetate irreversible loss could be expected if precursors for gluconeogenesis were limiting(Reference Cronje, Nolan and Leng20, Reference Preston and Leng38, Reference Jarrett and Filsell43). However, differences in acetate irreversible loss from approximately 403 d of age to 940 d age were not realised. Previous research(Reference Waterman, Grings and Geary41) has demonstrated that energetic efficiency can be altered due to season and quality of forage being consumed. If any changes in acetate irreversible loss were occurring, it did not alter due to dam winter nutrition or heifer development treatment in the present study.
Potential existed for heifers to differ in glucose and acetate irreversible loss due to carry-over effects from fetal development due to nutritional management treatments imposed on their dams. Since no differences were detected, this would indicate that potential in utero effects did not carry over later in life. A trend (P = 0·083) for the glucose half-life to be shorter for heifers calved from MARG dams needs to be further investigated because it could potentially lead to a limited explanation for improved energy efficiency (Table 3).
In conclusion, heifers receiving a 20 % reduction in winter feed provision have comparable indicators of energetic efficiency as heifers that were fed to appetite. This outcome was consistent at both the termination of a 140 d development period and again approximately 17 months later when the heifers were pregnant with their second calf. These results support other production findings(Reference Roberts, Paisley and Geary33, Reference Roberts, Geary and Grings37) that there are opportunities to reduce the amount of harvested feed fed and associated input costs while maintaining sustainable production. Future research may conclude that further reduction in harvested feed inputs may be attainable, which will not only lower overall production costs but also optimise economic feasibility.
Acknowledgements
The present study was funded by the United States Department of Agriculture, Agricultural Research Service (Project no. 5434-31000-014-00D). The United States Department of Agriculture, Agricultural Research Service, Northern Plains Area, is an equal opportunity/affirmative action employer. All agency services are available without discrimination. The present study was conducted under a cooperative agreement between the United States Department of Agriculture, Agricultural Research Service and the Montana Agricultural Experiment Station. Mention of a proprietary product does not constitute a guarantee or warranty of the product by the United States Department of Agriculture, Montana Agricultural Experiment Station or the authors, and does not imply its approval to the exclusion of other products that also may be suitable. R. C. W., A. J. R., T. W. G., E. E. G., L. J. A. and M. D. M. designed the study; R. C. W., A. J. R., T. W. G., E. E. G. and L. J. A. conducted the study; R. C. W. wrote the manuscript; R. C. W. and A. J. R. had primary responsibility for the final content. All authors read and approved the final manuscript. R. C. W. gratefully acknowledges W. Kelly, S. Reil, S. Bellows, B. Shipp, K. Neary, M. Woods, D. Armstrong, C. R. Harris, D. Phelps, T. Mott, R. J. Hubbard, M. Landers and H. Stroh for their technical assistance. The authors declare that there are no conflicts of interest.







