The prevalence of non-alcoholic fatty liver disease (NAFLD) is increasing worldwide, with up to 33 % of adults being affected in Western countries( Reference Byrne 1 ). This alarming increase in the number of individuals affected is related to the contemporary pandemic of obesity fostered by an unhealthy dietary pattern and a sedentary lifestyle, consequently leading to an increase in the risk of death( Reference Adams, Schatzkin and Harris 2 ). NAFLD patients exhibit signs of liver inflammation and increased hepatic lipid accumulation( Reference Adams, Lymp and St Sauver 3 ). In addition, the development of NAFLD in obese individuals is closely associated with insulin resistance and other metabolic disorders and thus might be of clinical relevance( Reference Spruss and Bergheim 4 ). We and others demonstrated that not only overfeeding but also particular nutrients such as fructose and SFA promote NAFLD in both animals and humans( Reference Bergheim, Weber and Vos 5 – Reference Thuy, Ladurner and Volynets 10 ). Our findings might explain, at least in part, why many, but by far not all, obese individuals develop the metabolic syndrome and associated diseases. In addition, we provided evidence for the involvement of the intestinal serotonergic system in the development of NAFLD and obesity( Reference Haub, Kanuri and Volynets 7 , Reference Haub, Ritze and Ladel 11 , Reference Weber, Volynets and Kanuri 12 ).
Intestinal serotonin (5-hydroxytryptamine, 5-HT) is released by enterochromaffin cells and neurons and is regulated via the serotonin re-uptake transporter (SERT)( Reference Wade, Chen and Jaffe 13 ). The SERT is located on epithelial cells and neurons in the intestine( Reference Wade, Chen and Jaffe 13 ). Previously, we had tested the hypothesis that the dysregulation of 5-HT due to a diet-induced reduction or a genetically induced loss of the SERT is involved in monosaccharide-induced NAFLD( Reference Haub, Kanuri and Volynets 7 ). We clearly demonstrated that impairment of SERT promotes the fructose- and glucose-induced damage of the intestinal barrier leading to lipopolysaccharide (LPS) translocation and liver inflammation causing NAFLD( Reference Haub, Kanuri and Volynets 7 ). Our findings are consistent with those of studies showing that 5-HT is a key regulator not only of intestinal motility( Reference Gershon 14 ) but also of inflammation( Reference Haub, Ritze and Bergheim 15 , Reference Bischoff, Mailer and Pabst 16 ) and intestinal permeability( Reference Nylander and Pihl 17 – Reference Yamada, Inui and Hayashi 19 ). 5-HT is synthesised from the amino acid tryptophan (TRP). Therefore, TRP supplementation is thought to enhance 5-HT synthesis; however, the substrate seems to be a minor regulator of synthesis compared with the rate-determining enzymes tryptophan hydroxylase (TPH)1 and TPH2( Reference Noguchi, Nishino and Kido 20 ). Nevertheless, in earlier studies, TRP supplementation has been shown to result in elevated small-intestinal 5-HT concentrations as well as in increased duodenal and ileal motility( Reference Ozer, Gonul and Ercan 21 , Reference Ozer, Gonul and Take 22 ). Concerning the development of NAFLD, studies in laying hens have suggested that TRP supplementation reduces hepatic lipid accumulation( Reference Akiba, Takahashi and Horiguchi 23 , Reference Rogers and Pesti 24 ). Interestingly, another study has revealed that, on the one hand, 5-HT supports liver regeneration after partial hepatectomy and, on the other hand, promotes steatohepatitis( Reference Lesurtel, Soll and Graf 25 ). Until today, studies concerning TRP supplementation are patchy and confusing. Thus, in the present study, we aimed to investigate the effect of TRP supplementation on NAFLD. In particular, we were interested in determining whether TRP supplementation affects the translocation of LPS from the intestine to the liver and the subsequent development of NAFLD. The present results indicate TRP supplementation to be a new approach for attenuating diet-induced fatty liver disease.
Materials and methods
Animals and treatments
Mice were housed in a pathogen-free barrier facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All procedures were approved by the local Institutional Animal Care and Use Committee (Regional Council Stuttgart). C57BL/6J mice aged 6–8 weeks were obtained from Janvier (Le-Genes-St-isle, France). The animals were divided into four groups of five to six mice and were fed different diets ad libitum over an 8-week period: (a) control diet (C group) (water and mouse breeding (MZ) diet; sniff®, containing: 6 % fat, 23 % protein, 5·2 % sugars, vitamins and minerals); (b) NAFLD-inducing fructose-rich diet (N group) (30 % fructose solution and enriched MZ diet containing 7·7 % fat, 27·6 % protein, 8·2 % sugars and adjusted vitamins and minerals); (c) control diet supplemented with TRP (C+TRP group) (0·4 g/kg body weight corresponding to 0·24 g/100 g diet); (d) fructose-rich diet supplemented with TRP (N+TRP group). The two animal groups fed the fructose-rich diets consumed about 50 % less food (see Table 1), as has been reported earlier( Reference Bergheim, Weber and Vos 5 ), and were therefore fed a more energy-dense MZ diet to compensate partially for the reduced food intake. The amount of TRP administered was selected according to the method of Rogers & Pesti( Reference Rogers and Pesti 24 ), who demonstrated reduced hepatic lipid accumulation in laying hens after TRP supplementation. Liquid and food intakes, as well as body weight, were recorded weekly. After 8 weeks of feeding, the mice were anaesthetised with 80 mg/kg body weight of ketamine and 6 mg/kg body weight of xylazine, both administered intraperitoneally; blood was collected from the portal vein just after anaesthetisation. After killing the mice, tissue samples of the small intestine and liver were collected and frozen immediately in liquid N2 or fixed in paraformaldehyde and frozen in OCT Tissue-Tek (Sakura Finetek) for further investigation.
Table 1 Effect of tryptophan supplementation on physiological parameters§ (Mean values with their standard errors; n 5–6)
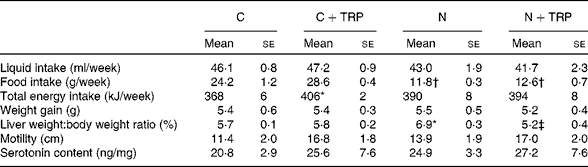
C, control diet group; C+TRP, control diet group with tryptophan supplementation; N, non-alcoholic fatty liver disease mice fed a fructose-rich diet; N+TRP, non-alcoholic fatty liver disease mice fed a fructose-rich diet with tryptophan supplementation.
* Mean values were significantly different from those of the control group (P< 0·05).
† Mean values were significantly different from those of the C+TRP group (P< 0·05).
‡ Mean value was significantly different from that of the N+TRP group (P< 0·05).
§ The detailed feeding protocols of the four animal groups are described in the Materials and methods section.
Determination of intestinal motility
A charcoal solution (0·5 g charcoal, 0·25 g gum Arabic and 5 ml NaCl (0·9 %)) was orally administered to the mice at a concentration of 15 μl/kg body weight 10 min before killing to determine small-intestinal motility. The migration of the charcoal solution from the pylorus to the small intestine was measured once the intestine was removed and gently, without tension, stretched.
Determination of hepatic TAG concentrations
Liver tissue samples weighing 50–100 mg were homogenised in ice-cold double-concentrated PBS. Tissue lipids were extracted with methanol (100 μl)–chloroform (200 ml), dried and resuspended in 5 % fat-free bovine serum albumin. Colorimetric assessment of TAG concentrations was carried out using a commercially available kit (Randox). Values were normalised to protein concentrations determined using the Bradford assay in the liver homogenates (Bio-Rad Laboratories).
Oil Red O and haematoxylin and eosin staining of liver tissue sections
To determine hepatic lipid accumulation, paraformaldehyde-fixed liver tissue sections (10 μm) were stained with Oil Red O (Sigma) for 10 min, washed and counterstained with haematoxylin (Sigma) for 45 s. Liver histology was assessed using haematoxylin and eosin-stained liver tissue sections. Representative photomicrographs were captured at 400 × (Oil Red O) or 200 × (haematoxylin and eosin) magnification using a system incorporated in a microscope (Axiovert 200M; Zeiss). The Kleiner et al. ( Reference Kleiner, Brunt and Van Natta 26 ) scoring system for NAFLD was applied to two selected mice for each group( Reference Kleiner, Brunt and Van Natta 26 ). Selection criteria for the mice were hepatic TAG values that were close to the mean values of each group. The semi-quantitative analysis suggested by Kleiner et al. ( Reference Kleiner, Brunt and Van Natta 26 ) was used for determining the grade of steatosis (0–3), hepatocyte ballooning (0–2), lobular inflammation (0–2) and fibrosis (0–4). For studying the histological features of NAFLD, eight haematoxylin and eosin-stained liver tissue pictures per mouse were screened. The hepatic histological feature scores obtained at the onset of NAFLD are given in Table 2. In addition, the NAFLD activity score proposed by Kleiner et al. ( Reference Kleiner, Brunt and Van Natta 26 ), which is the sum of the scores obtained for steatosis, lobular inflammation and hepatocyte ballooning, was determined (Table 2)( Reference Kleiner, Brunt and Van Natta 26 ).
Table 2 Kleiner scoring system* for non-alcoholic fatty liver disease (NAFLD; n 2)
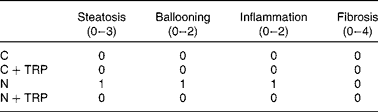
C, control diet group; C+TRP, control diet group with tryptophan supplementation; N, NAFLD-inducing fructose-rich diet group; N+TRP, NAFLD-inducing fructose-rich diet group with tryptophan supplementation.
* The Kleiner et al. ( Reference Kleiner, Brunt and Van Natta 26 ) scoring system was used to analyse the development of NAFLD in haematoxylin and eosin-stained liver tissue sections. The NAFLD activity score for the N group was 3 (sum of the scores of steatosis, ballooning and inflammation), whereas the score was 0 for all other groups, meaning no histological NAFLD features were detected.
Lipopolysaccharide assay
Plasma samples derived from portal vein blood were heated at 73°C for 20 min. The plasma concentrations of LPS were determined using a commercially available limulus amebocyte lysate assay with a concentration range of 0·015–1·2 endotoxin units/ml (Charles River Laboratories International, Inc.).
Determination of duodenal serotonin concentrations
Frozen duodenal tissue samples were homogenised (about 30 s) in ice-cold 0·05 m-HCl containing 0·1 % ascorbic acid and centrifuged at 14 000 rpm for 5 min at 4°C. The supernatant was immediately acylated (37°C for 15 min in a water-bath) and 5-HT concentrations were determined within 1 h to ensure stability. The concentrations of 5-HT (expressed in ng/mg tissue weight) in the supernatant were determined at room temperature (18–25°C) using an enzyme immunoassay kit (sensitivity of 2·68 ng/ml) according to the manufacturer's instructions (IBL).
Immunoblotting
To prepare total tissue protein lysates, snap-frozen small intestine samples were homogenised in a lysis buffer (20 mm-3-morpholin-4-ylpropane-1-sulfonic acid (MOPS), 150 mm-NaCl, 1 mm-EDTA, 1 % Nonidet P-40, 1 % sodium deoxycholate and 0·1 % SDS) containing a protease inhibitor mix (Roche). Protein lysates (30 μg protein/well) were separated on a 10 % SDS–polyacrylamide gel and transferred onto Hybond™-P polyvinylidene difluoride membranes (GE). The resulting blots were then blocked for 45 min at room temperature with 1 % skimmed milk for the detection of SERT, 5 % skimmed milk for the detection of occludin or 2·5 % bovine serum albumin solution for the detection of β-actin. After blocking, the blots were incubated overnight at 4°C with primary antibodies against SERT (1:500; Santa Cruz Biotechnology) or occludin (1:500; Zymed Laboratories) and β-actin (1:750; Cell Signaling, New England Biolabs GmbH) as the reference protein. The blots were washed and incubated for 45 min at room temperature with horseradish peroxidase-conjugated secondary antibodies (all from Cell Signaling). The bands were visualised using the SuperSignal Western Dura Kit (Pierce, Thermo Fischer). To ensure equal loading, all the blots were stained with Ponceau red (Sigma-Aldrich), and the signals were normalised to β-actin signals. Protein bands were densitometrically analysed using the software AlphaEaseFC (FluorChem™) from Alpha Innotech.
RNA isolation and real-time RT-PCR
Total RNA was extracted from duodenal tissue samples using the TriFast™ reagent (PEQLAB). RNA concentrations were determined spectrophotometrically, and 0·25 μg of total RNA was reverse-transcribed using the iScript 8 DNA synthesis Kit (Bio-Rad Laboratories) followed by a DNAse digestion step (Fermentas). PCR primers were designed using the Primer3 software (TPH1: forward CATCAGCCGAGAACAGTTGA; reverse TTCGGATCCATACAACAGCA; 18S: forward ATCAGATACCGTCGTAGTTC; reverse CCAGAGTCTCGTTCGTTAT; Whitehead Institute for Biomedical Research). SsoFast EvaGreen Supermix (Bio-Rad Laboratories) was used to prepare the PCR mix. The amplification reactions were carried out in an iCycler (Bio-Rad Laboratories) with forty cycles of a two-step PCR (denaturation at 95°C for 35 s, denaturation at 95°C for 5 s, and annealing/extension at 62°C for 10 s). The fluorescence intensity of each sample was measured at each temperature change to monitor the amplification of the target gene. The comparative threshold cycle (CT) method was used to determine the amount of target gene, normalised to that of an endogenous reference gene (18S) and relative to a calibrator (2− ΔΔC t ). The purity of PCR products was verified by melting curve analysis and gel electrophoresis.
Statistical analysis
Results are reported as means, with their standard errors. ANOVA or Kruskal–Wallis test was used if variances calculated with Bartlett's test varied significantly. If there were significant differences between the groups, we used as a post hoc test an unpaired, two-tailed t test or t test with Welch's correction (if variances differed significantly), as we were interested in comparing the C and N groups as well as the N and N+TRP groups. A P value < 0·05 was determined as the level of significance before the start of the study. For calculation and graph design, the software GraphPad Prism 5 (GraphPad Software) was used.
Results
Effect of tryptophan supplementation on physiological parameters
Liquid intake and weight gain were similar in the four dietary groups (Table 1). Independent of TRP supplementation, we observed, as has been shown previously( Reference Bergheim, Weber and Vos 5 ), a reduction in solid food intake by 48·5–51·0 % in mice given drinking-water containing fructose (fructose-rich diet) compared with the food intake in mice given plain drinking-water.
Effect of tryptophan supplementation on hepatic lipid accumulation
The liver weight:body weight ratio (P= 0·014) was significantly increased in the N group (Fig. 1(a)). TRP supplementation reduced the liver weight:body weight ratio to normal levels (P= 0·009; Fig. 1(a)). As has been shown previously( Reference Bergheim, Weber and Vos 5 ), here fructose-rich diet-fed mice exhibited a dramatic increase in overall hepatic lipid accumulation compared with the fructose-rich diet-fed mice in which hepatic lipid accumulation was reduced by TRP supplementation (Fig. 1(b) and (d)). Similarly, fructose-rich diet-fed mice exhibited a higher degree of steatosis, cellular ballooning and lobular inflammation compared with mice fed the fructose-rich diet with TRP supplementation (Fig. 1(e); Table 2). The NAFLD activity score was 3 for the N group and 0 for all the other groups (Table 2). Nevertheless, hepatic TAG concentrations were not significantly reduced in the TRP-supplemented N group (Fig. 1(c)).

Fig. 1 Effect of tryptophan (TRP) supplementation on hepatic lipid accumulation. Liver weight:body weight ratio expressed in percentage (a), fat droplet area quantified by Oil Red O staining (b) and TAG concentrations (c). Values are means, with their standard errors represented by vertical bars (n 4–6). C, control diet; C+TRP, control diet with TRP supplementation; N, non-alcoholic fatty liver disease (NAFLD) mice fed a fructose-rich diet; N+TRP, NAFLD mice fed a-fructose-rich diet with TRP supplementation. Mean value was significantly different from that of the C group: * P< 0·05, ** P< 0·01, *** P< 0·001. Mean value was significantly different from that of the N group: † P< 0·05, †† P< 0·01. Liver tissue sections were stained using Oil Red O dye (400 × ) (d) and haematoxylin and eosin (200 × ) (e). Representative photomicrographs of the liver tissue sections are shown. Steatosis (black arrow heads) and cellular ballooning (white arrow heads) are marked for the N group.
Effect of tryptophan supplementation on tight-junction protein expression
We measured the expression of occludin, a tight-junction protein thought to be important for the epithelial barrier function in the small intestine( Reference Brun, Castagliuolo and Di Leo 27 ). Measurements in the duodenum, the first site of sugar absorption, revealed that occludin protein expression in the N group was significantly reduced (P= 0·0007) compared with that in the control group (Fig. 2(a)). Interestingly, TRP supplementation normalised duodenal occludin protein expression in the N group (P= 0·0009; Fig. 2(a)). By contrast, in the ileum, despite a tendency for occludin protein expression to decrease in the N and N+TRP groups, TRP supplementation seemed to have no influence (Fig. 2(b)). The concentrations of portal plasma LPS were almost 7-fold increased in the N group compared with that in the control group (P= 0·005; Fig. 2(c)). However, TRP supplementation led to no significant reduction in LPS concentrations in the N group (Fig. 2(c)).

Fig. 2 Effect of tryptophan (TRP) supplementation on occludin protein expression in the duodenum. Occludin protein expression was quantified by Western blotting in the duodenum (a) and ileum (b). Representative blot images of occludin and β-actin as well as quantitative analysis results of the blots are shown ((a) and (b)). Lipopolysaccharide (LPS; endotoxin units (EU)/ml) concentrations were measured in portal vein plasma (c). Values are means, with their standard errors represented by vertical bars (n 4–6). C, control diet; C+TRP, control diet with TRP supplementation; N, non-alcoholic fatty liver disease (NAFLD) mice fed a fructose-rich diet; N+TRP, NAFLD mice fed a fructose-rich diet with TRP supplementation. Mean value was significantly different from that of the C group: ** P< 0·01, *** P< 0·001. ††† Mean value was significantly different from that of the N group (P< 0·001).
Effect of tryptophan supplementation on the intestinal serotonergic system
To determine whether measurable alterations in the intestinal serotonergic system were associated with the protective effect of TRP, SERT protein concentrations, TPH1 mRNA expression and 5-HT concentrations were quantified in the duodenum. Confirming our own previous data( Reference Haub, Kanuri and Volynets 7 ), we observed a significant reduction in SERT protein concentrations by 56·4 % in the N group compared with that in the control group (P= 0·002; Fig. 3). TRP supplementation increased SERT protein (P= 0·02) expression in mice fed the fructose-rich diet (Fig. 3). As expected, TPH1 mRNA expression was increased (P= 0·01) in the N group, but it was not normalised by TRP supplementation in addition to fructose-rich diet feeding (data not shown). TRP supplementation had an influence neither on motility nor on overall 5-HT concentrations in the duodenum (Table 1).

Fig. 3 Effect of tryptophan (TRP) supplementation on duodenal serotonin re-uptake transporter (SERT) protein expression. SERT protein expression in the duodenum was measured by Western blotting. Representative blot images of SERT and β-actin as well as the quantitative analysis results of the blots are shown. Values are means, with their standard errors represented by vertical bars (n 4–6). C, control diet; C+TRP, control diet with TRP supplementation; N, non-alcoholic fatty liver disease (NAFLD) mice fed a fructose-rich diet; N+TRP, NAFLD mice fed a fructose-rich diet with TRP supplementation. ** Mean value was significantly different from that of the C group (P< 0·01). † Mean value was significantly different from that of the N group (P< 0·05).
Discussion
There is now growing experimental evidence that alterations in the intestinal barrier resulting in an increased translocation of bacterial products contribute to the development of NAFLD( Reference Haub, Kanuri and Volynets 7 , Reference Brun, Castagliuolo and Di Leo 27 , Reference Cani, Bibiloni and Knauf 28 ). However, the triggers causing barrier impairment, LPS influx into the liver and hepatic lipid accumulation are largely unknown. We and others have validated that particular dietetic factors such as sugars, in particular, fructose( Reference Bergheim, Weber and Vos 5 ), and/or SFA( Reference Malhi and Gores 8 , Reference Tetri, Basaranoglu and Brunt 9 ) play a role, but the underlying mechanisms have been described only rudimentarily.
Recent findings reported by our group have suggested that the intestinal serotonergic system, and especially SERT and 5-HT assessed in the present study, might be involved in the regulation of intestinal permeability and thus translocation of gut-derived bacterial LPS( Reference Haub, Kanuri and Volynets 7 ). Such data raised the idea that the intestinal serotonergic system could be an interesting pharmacological target for the prevention of fatty liver disease.
Therefore, we chose the 5-HT precursor TRP as a dietary supplement, which has been investigated in various animal models( Reference Ozer, Gonul and Take 22 , Reference Akiba, Takahashi and Horiguchi 23 ) and has no major side effects on liquid or food intake or on weight gain( Reference Fears and Murrell 29 ). In the present study, we observed an increase in energy intake after TRP supplementation in the control mice but not in the NAFLD mice with TRP supplementation. The increase in energy intake did not have an effect on weight gain, which might be, among others, due to a slight but not significantly increased motility in the TRP-treated control mice.
In the present study, we clearly demonstrated the protective effect of TRP supplementation on the stabilisation of the intestinal barrier through the enhancement of occludin expression and the obvious reduction of fatty acid accumulation in the liver. The protective effect of TRP supplementation in our NAFLD model was not associated with an attenuation of intestinal translocation of bacterial LPS, possibly because of the partial normalisation of the tight junction impairment only in the duodenum but not in the ileum. Interestingly, l-TRP administration in the rat ileum has been found to reduce net Na+ and fluid absorption, supposingly due to morphological changes( Reference Teichberg, Wapnir and Zdanowicz 30 ). Indeed, these ileal effects have been observed only at a concentration higher than 20 mm-l-TRP, whereas lower concentrations have been found to rather stimulate net absorption( Reference Teichberg, Wapnir and Zdanowicz 30 ). As TRP was administered orally and not directly into the ileum in the present study, we observed no influence of TRP supplementation on intestinal barrier functions.
Earlier studies in rats have investigated the effect of TRP supplementation on fatty acid metabolism in the liver, confirming an increase in serum TAG concentrations but not in hepatic TAG concentrations after a single injection (50 mg/kg body weight) or a treatment over 7 d (2·5 g/kg diet)( Reference Fears and Murrell 29 , Reference Sakurai, Miyazawa and Shindo 31 ). In agreement with these findings, we found neither an increase nor a reduction in hepatic TAG concentrations or liver weight in the TRP-treated control mice. On the other hand, TRP supplementation reduced steatosis, cellular ballooning and lobular inflammation in the liver of the NAFLD mice. In close relation to our findings, a recent study has indicated positive effects of indoleamine 2,3-dioxygenase, an enzyme that mediates the catabolism of l-TRP to l-kynurenine, on liver inflammation and fibrosis( Reference Nagano, Shimizu and Hara 32 ). In earlier studies, TRP supplementation has been found to attenuate the development of NAFLD in laying hens( Reference Akiba, Takahashi and Horiguchi 23 , Reference Rogers and Pesti 24 ).
We not only found that TRP supplementation attenuated NAFLD but also found that it increased the lower intestinal expression of SERT in the N group. Lower intestinal expression of SERT in our NAFLD model was accompanied by liver steatosis, increased portal plasma LPS concentrations and tight-junction protein loss in the small intestine, as has been postulated here and previously( Reference Haub, Kanuri and Volynets 7 ). Similarly, SERT knockout mice have been found to develop liver damage on being fed a glucose-rich diet, which induced no or minor damages in C57BL/6J wild-type mice( Reference Haub, Kanuri and Volynets 7 ). Our data indicated that fructose is more harmful than glucose in this respect, but in animals lacking SERT, glucose becomes equally harmful with regard to fatty liver disease as fructose in wild-type mice. Therefore, we suggest that SERT and thus the serotonergic system play a role in sugar-induced liver damage. Possibly, loss of SERT in the intestine leads to a dysregulation of 5-HT signalling that may evoke an enhanced inflammatory response as determined in IL-10 knockout and SERT/IL-10 double-knockout mice( Reference Haub, Ritze and Bergheim 15 ). The latter were characterised by high concentrations of TNF-α, which modulates tight-junction proteins via myosin light-chain kinase activation( Reference Turner 33 ). Therefore, we assume that the induction of SERT might be an important mechanism, besides occludin enhancement, with regard to how TRP might attenuate NAFLD at the intestinal level (Fig. 4).

Fig. 4 Hypothesis on the possible influence of tryptophan (TRP) on non-alcoholic fatty liver disease (NAFLD). In our NAFLD model, we observed a decreased expression of serotonin (5-HT) re-uptake transporter (SERT) in the small intestine that might result in fortified signalling of bioavailable 5-HT in small intestinal tissue compared with that in the control group. Either via 5-HT or by other yet unknown mechanisms, the NAFLD-inducing fructose-rich diet (N group) used in the present study supposingly caused a decrease in occludin expression in the upper small intestine resulting in an impaired epithelial barrier and a consecutive increased translocation of bacteria ![]() or bacterial products such as lipopolysaccharides (LPS, □). In the N group, TRP supplementation normalised reduced SERT expression and assumingly improved barrier function in the upper intestine by enhancing occludin expression. Changes within the serotonergic system or the intestinal barrier might influence the reduction of hepatic lipid accumulation in our NAFLD model (effect of TRP supplementation (+Tryptophan) is shown with arrowheads; EC, enterochromaffin cells; TPH1, TRP hydroxylase 1.
or bacterial products such as lipopolysaccharides (LPS, □). In the N group, TRP supplementation normalised reduced SERT expression and assumingly improved barrier function in the upper intestine by enhancing occludin expression. Changes within the serotonergic system or the intestinal barrier might influence the reduction of hepatic lipid accumulation in our NAFLD model (effect of TRP supplementation (+Tryptophan) is shown with arrowheads; EC, enterochromaffin cells; TPH1, TRP hydroxylase 1.
In contrast to previous findings reported by Ozer et al. ( Reference Ozer, Gonul and Ercan 21 , Reference Ozer, Gonul and Take 22 ) no significant differences in small-intestinal 5-HT concentrations or motility were detected among the dietary groups. Ozer et al. ( Reference Ozer, Gonul and Ercan 21 , Reference Ozer, Gonul and Take 22 ) reported 5-HT concentrations and duodenal and ileal motility to be significantly increased in mice treated with TRP (0·1 g/kg body weight per 24 h, intraperitoneally, 7 d), which might have resulted from the different doses and routes of administration (oral v. intraperitoneal). However, it is unclear whether overall concentrations of 5-HT in tissues are a reliable marker for 5-HT signalling. Therefore, we determined the mRNA concentration of TPH1, the rate-limiting enzyme of 5-HT synthesis, which was significantly increased on feeding the fructose-rich diet but not normalised by TRP supplementation. Nevertheless, we assume that TPH1 might be a more relevant marker for bioavailable 5-HT at the intercellular level than the overall 5-HT tissue concentration. Of course, other mechanisms such as central effects of TRP cannot be excluded and could contribute to the beneficial effects of TRP in the experimental NAFLD model used in the present study.
Our data provide the first evidence that oral supplementation of the amino acid TRP attenuates NAFLD in mice. In conclusion, we report that modulation of the upper intestinal barrier and the intestinal serotonergic system via TRP supplementation might be an important mechanism of protection from the development of NAFLD in mice. Adverse effects of TRP supplementation were not observed in our mouse model; however, these have to be considered when testing this approach in human subjects.
Acknowledgements
The present study was supported by grants from the ‘Center for Nutritional Medicine’ Tübingen/Hohenheim, Germany (TP7.AI to SCB), and the Competence Network of Obesity, group ‘Obesity and GI tract’, funded by the Federal Ministry of Education and Research (grant number 01GI0843 to SCB). The ‘Center for Nutritional Medicine’ Tübingen/Hohenheim and the Competence Network of Obesity had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: Y. R. and S. C. B. conceived and designed the experiments; Y. R., G. B., M. B. and A. H. carried out the experiments; Y. R., G. B., M. B. and A. H. analysed the data; Y. R. and S. C. B. interpreted the results of the experiments; Y. R. prepared the figures; Y. R. and A. H. drafted the manuscript; Y. R. and S. C. B. edited and revised the manuscript; Y. R., G. B., M. B., A. H. and S. C. B. approved the final version of the manuscript.
None of the authors has any conflicts of interest to declare.








