It has been shown that infection with Helicobacter pylori leads to both acute and chronic inflammation in the gastric mucosa and produces reactive oxygen species in areas that it colonises. Reactive oxygen species can release harmful products accumulating in the gastric mucosa, and further cause gastric epithelial dysplasia and carcinogenesis(Reference Sasazuki, Hayashi and Nakachi1, Reference Calvino-Fernández, Benito-Martínez and Parra-Cid2). Therefore, it is necessary to continuously regenerate antioxidant capacity and provide homoeostasis in gastric mucosa.
Vitamin C (ascorbic acid) and vitamin E (tocopherol) can act as potent antioxidants to reduce reactive oxygen species in the gastric mucosa, as well as to inhibit nitrosoamine formation. Moreover, vitamin E has an antioxidant effect via impairing the lipid peroxidation pathway(Reference Sugimoto, Yoshida and Nakamura3). A possible relationship between H. pylori infection and antioxidant vitamins is under investigation. In in vitro assays, high concentration of vitamin C could inhibit the growth of H. pylori (Reference Zhang, Wakisaka and Maeda4). In in vivo assays, the feeding of vitamins C and E could reduce the gastric H. pylori loads in animal models(Reference Zhang, Wakisaka and Maeda4–Reference Sun, Girgensone and Leanderson7). These observations make antioxidant vitamins an interesting objective in H. pylori eradication.
It is still unknown whether triple therapy (proton pump inhibitors (PPI) or bismuth subcitrate along with two antibiotics selected from clarithromycin, metronidazole, amoxicillin and tetracycline) supplemented with vitamins C and E could improve the rate of H. pylori eradication. Several experimental and clinical studies(Reference Kaboli, Zojaji and Mirsattari8–Reference Koçkar, Oztürk and Bavbek15) have shown different results. We therefore performed a meta-analysis of randomised controlled trials (RCT) to evaluate the effect of the addition of vitamins C and E to standard therapy on the eradication rate of H. pylori infection.
Methods
Study design
Studies were accepted based on the following criteria: study design – RCT; study population – infection with H. pylori identified by rapid urease test or histological examination; intervention – drug regimen of a PPI and/or bismuth in combination with two antibiotics; comparison intervention – the same regimen plus vitamin C and/or vitamin E; and outcome – eradication of H. pylori. We excluded review articles, retrospective analyses, case reports as well as studies that were only reported as abstracts. If a study that met the selection criteria had missing data, we contacted the authors in an attempt to obtain these data. Approval for all study procedures was obtained from the ethics committee of the host institution.
Literature search
There were two investigators who independently searched the published RCT in the PubMed (US National Library of Medicine, Bethesda, MD, USA) (1980 to present), EMBASE (Reed Elsevier PLC, Amsterdam, The Netherlands) (1980 to present) and Cochrane Library (2011, Issue 1) databases. Bibliographies of all relevant studies and recent review articles were scanned to identify further citations. We also searched for unpublished and ongoing trials in clinicaltrials.gov and controlled-trials.com. The search terms were ‘Helicobacter pylori’ and ‘vitamin’. There were no language restrictions.
Data extraction
There were two independent observers who used standardised forms to independently extract data. Recorded data included the demographic characteristics of the patients, tests for confirming infection of H. pylori, protocol for pharmacological therapy, eradication rate of H. pylori and any reported side effects of the therapy. The quality of all selected articles was ranked in accordance with the Jadad composite scale(Reference Kjaergard, Villumsen and Gluud16). According to this scale, low-quality studies had a score of ≤ 2 and high-quality studies had a score of ≥ 3(Reference Kjaergard, Villumsen and Gluud16). Allocation concealment was assessed with the classification of the Cochrane Collaboration. Disagreements remaining after contact with authors were resolved by consensus.
Statistical analysis
If several trials were available for a specific topic, we performed meta-analysis with the software RevMan 4.2.10 (provided by the Cochrane Collaboration, Oxford, UK). The risk ratio was presented with a 95 % CI. We used χ2 tests to assess statistical heterogeneity and the Higgins I 2 statistic to determine the percentage of total variation across studies due to heterogeneity. If the I 2 statistic was ≤ 50 %, the fixed effect model was used to pool studies; otherwise, the random effects model was used.
Results
Literature search
The search strategy generated fifty-two studies. From these, we retrieved a preliminary short list of eleven papers for detailed evaluation, of which six papers were eligible for this meta-analysis (Fig. 1)(Reference Sezikli, Cetinkaya and Sezikli10–Reference Koçkar, Oztürk and Bavbek15).

Fig. 1 Identification of trials for inclusion.
Study characteristics
A total of 811 participants were enrolled in the six studies (Table 1). Of the six trials, three including 323 patients were randomised to eradication therapy v. eradication therapy plus vitamins C and E (159 in the eradication therapy and 164 in the eradication therapy plus vitamins C and E group)(Reference Sezikli, Cetinkaya and Sezikli10–Reference Everett, Drake and White12). There were no significant differences between the two groups with regard to age, sex and endoscopic or pathological diagnosis. Another three trials were included for the analysis of eradication therapy v. eradication therapy plus only vitamin C (247 patients in the eradication therapy and 241 patients in the eradication therapy plus vitamin C group)(Reference Zojaji, Talaie and Mirsattari13–Reference Koçkar, Oztürk and Bavbek15). The demographic characteristics of the two groups also had no differences.
Table 1 Characteristics of studies included in meta-analysis

RUT, rapid urease test; UBT, urea breath test.
Quality of methods
Table 2 showed the quality of the included studies as assessed by the Jadad score, and allocation concealment was assessed with the classification of the Cochrane collaboration. Of the six included RCT, five(Reference Sezikli, Cetinkaya and Sezikli10, Reference Chuang, Sheu and Huang11, Reference Zojaji, Talaie and Mirsattari13–Reference Koçkar, Oztürk and Bavbek15) had a high risk of bias. None of the trials reported on the generation of a randomisation list. Only one trial performed double-blind outcome assessment and adequate allocation concealment(Reference Everett, Drake and White12). All trials had a clear explanation for withdrawals and dropouts in each group.
Table 2 Methodological quality of trials included in meta-analysis

ITT, intention-to-treat.
Eradication regimen v. eradication regimen plus vitamins C and E
A total of three RCT compared the efficacy of the eradication regimen v. eradication regimen plus vitamins C and E in patients with H. pylori infection(Reference Sezikli, Cetinkaya and Sezikli10–Reference Everett, Drake and White12). Of the three trials, one(Reference Sezikli, Cetinkaya and Sezikli10) used PPI, bismuth subcitrate plus two antibiotics as eradication therapy; the other two(Reference Chuang, Sheu and Huang11, Reference Everett, Drake and White12) used PPI or bismuth subcitrate plus two antibiotics. The daily dosage of vitamin C in different trials varied from 400 to 1000 mg. Vitamin E was used at a dose of 100–400 mg/d. In the different trials, eradication therapy was continued for 1–2 weeks followed by vitamin supplementation for 2–6 weeks (Table 3).
Table 3 Intervention features and results of studies included in meta-analysis

ITT, intention-to-treat.
In the eradication therapy group, H. pylori eradication was achieved in 56·67–60 % of patients. In the eradication therapy plus vitamins C and E group, the eradication rate varied between 40 and 91·25 % during the study period. Available information on the eradication rate for three studies was included in the meta-analysis. Our meta-analysis detected a non-significant difference in the eradication rate of H. pylori (eradication regimen 59·12 % v. eradication regimen plus vitamins C and E 69·51 %, risk ratio 0·93 (95 % CI 0·56, 1·53), P = 0·76, Fig. 2), showing that vitamins C and E supplements to the H. pylori eradication regimen might have no effect on the treatment of H. pylori infection. The test for heterogeneity indicated that the studies were significantly heterogeneous, so the random effects model was used.
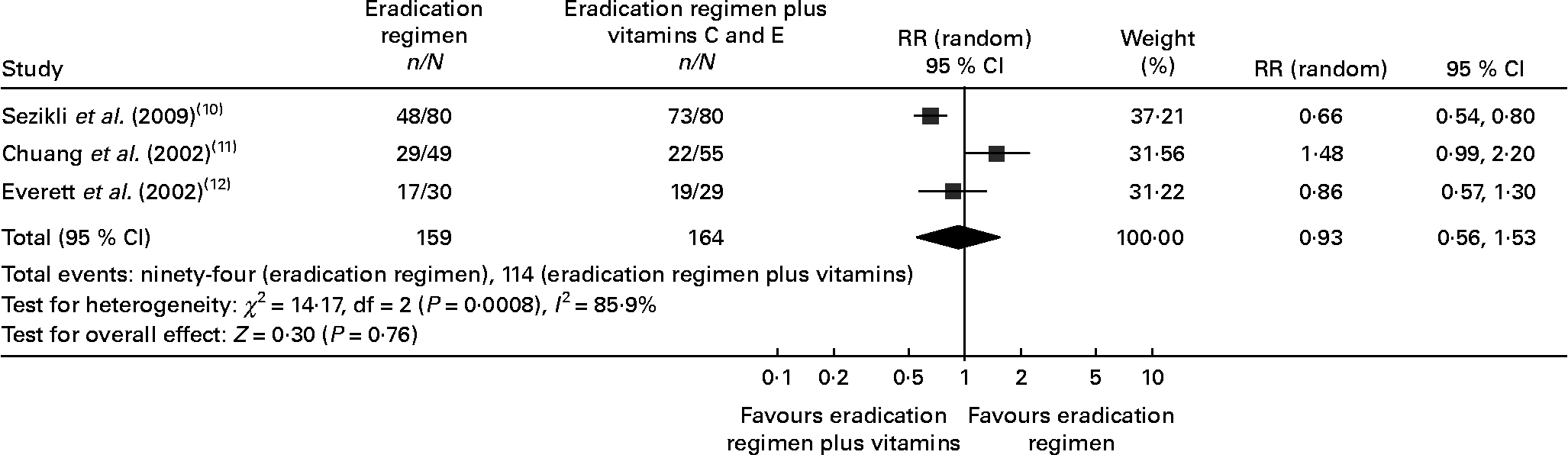
Fig. 2 Forest plots for eradication rate comparison: eradication regimen v. eradication regimen plus vitamins C and E. RR, risk ratio.
Eradication regimen v. eradication regimen plus vitamin C
A total of three RCT compared the eradication regimen v. eradication regimen plus vitamin C only in patients with H. pylori infection(Reference Zojaji, Talaie and Mirsattari13–Reference Koçkar, Oztürk and Bavbek15). Of the three trials, one(Reference Zojaji, Talaie and Mirsattari13) used PPI, bismuth subcitrate plus two antibiotics as eradication therapy; the other two(Reference Chuang, Sheu and Kao14, Reference Koçkar, Oztürk and Bavbek15) used PPI plus two antibiotics. The mean dose of vitamin C varied from 500 to 1000 mg/d. In different trials, the patients received the eradication therapy or eradication therapy plus vitamin C for 1 or 2 weeks (Table 3).
In the eradication therapy group, the eradication rate of H. pylori varied from 48·77 to 66·67 %. In the eradication therapy plus vitamin C group, the eradication rate in individual trials varied from 50 to 78·69 %. We performed a meta-analysis to evaluate the eradication rate as the outcome measure for all three trials. No difference was observed between the two groups (eradication regimen 54·25 % v. eradication regimen plus vitamin C 74·69 %, risk ratio 0·83 (95 % CI 0·58, 1·19), P = 0·32, Fig. 3). The test for heterogeneity indicated that the studies were statistically heterogeneous, so the random effects model was used to pool studies.
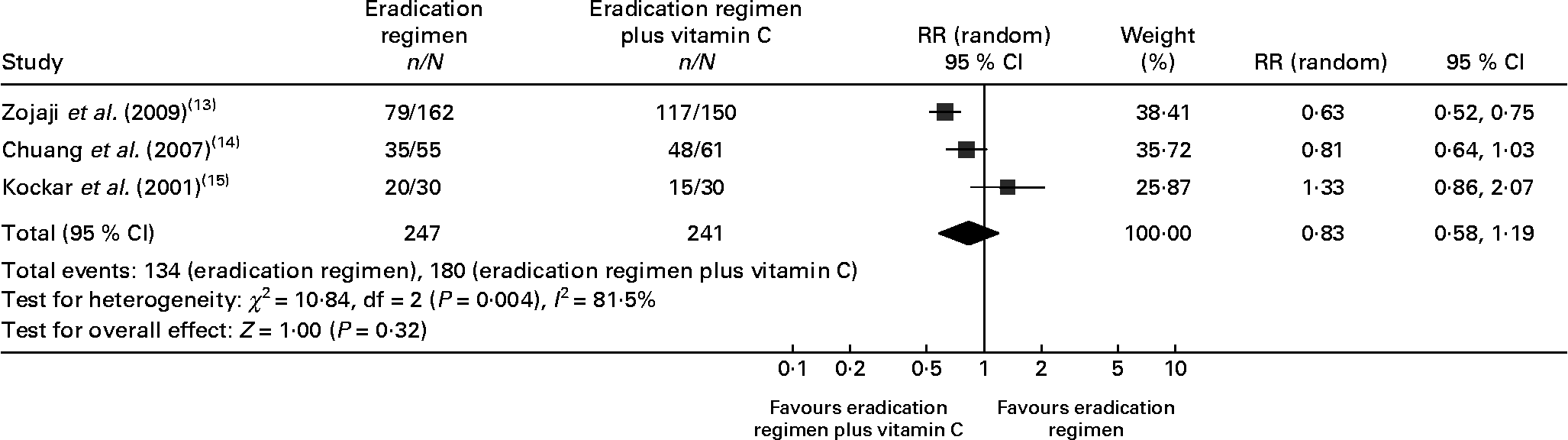
Fig. 3 Forest plots for eradication rate comparison: eradication regimen v. eradication regimen plus vitamin C. RR, risk ratio.
Adverse events
The most frequent adverse effects were nausea, diarrhoea, headache and skin rash. Because of a skin rash, one patient in the eradication therapy plus vitamins C and E group had to discontinue the therapy(Reference Chuang, Sheu and Huang11). There were no serious adverse events. Most of the patients tolerated these drugs well.
Discussion
Helicobacter pylori infections play a major role in the development of peptic ulcers, chronic gastritis, gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma(Reference Kandulski, Selgrad and Malfertheiner17). Approximately 50 % of the world's population is infected with H. pylori (Reference Go18). Eradication criteria defined in the Maastricht III Report is now commonly used for H. pylori eradication(Reference Suzuki, Nishizawa and Hibi19). However, increasing antimicrobial resistance, falling eradication rates, high treatment costs and drugs side effects have led to the necessity of the development of new treatment modalities.
The microenvironment created by H. pylori in the gastric mucosa protected these bacteria from gastric acid and a host of defensive mechanisms. Moreover, H. pylori infection under this microenvironment promotes oxidative DNA damage to gastric mucosa by reactive oxygen species(Reference Naito and Yoshikawa20). Therefore, it is necessary to develop remedies combining with antibiotics for impairing this microenvironment. Some studies and clinical experiments suggest that vitamins C and E could inhibit the growth of H. pylori and increase the effects of the antibiotics(Reference Zhang, Wakisaka and Maeda4–Reference Sun, Girgensone and Leanderson7, Reference Sasazuki, Sasaki and Tsubono21, Reference Jarosz, Dzieniszewski and Dabrowska-Ufniarz22). There are several suggestions for the mechanism of vitamins C and E. First, vitamins C and E impair the microenvironment of H. pylori by attenuating oxidative stress and oxidative DNA damage in the gastric mucosa, thus inhibiting the growth of H. pylori. Second, vitamins C and E may strengthen the immune system and provide an appropriate environment for antibiotics to affect the bacteria. However, the opposite has also been observed by several investigators. Kamiji & Oliveira(Reference Kamiji and Oliveira9) found that the administration of vitamin C did not alter the bacterial load in the H. pylori-infected patients. Similarly, a high dose of vitamin C had not been shown to protect vitamin C-deficient gulo( − / − ) mice against H. pylori-induced gastritis and gastric premalignancy(Reference Lee, Wang and Chien23). Therefore, whether the eradication regimen supplemented with vitamins C and E could improve the H. pylori eradication rate remains uncertain.
The purpose of the present review was to provide additional insight into the options for treating the infection of H. pylori, focusing on the effect of the addition of vitamins C and E to the eradication regimen in the H. pylori-infected patients. Few RCT have tested the effects of antioxidant vitamins for H. pylori, and none of the existing trials was methodologically rigorous. This is the first meta-analysis to compile all available RCT comparing the eradication regimen with the eradication regimen plus antioxidant vitamins for H. pylori infection. The present results showed that vitamins C and/or E supplements to the H. pylori eradication regimen had no effect on improving the H. pylori eradication rate. Of the six RCT included in our meta-analysis, two trials demonstrated that the treatment plus vitamins was superior to the treatment alone. The reasons for this discrepancy were not clear, other than the possibility of the addition of bismuth subcitrate to triple therapy in these two trials. High heterogeneity was found among the trials, limiting the quality of meta-analysis; hence, we provided a more conservative estimate of a treatment effect by using a random effect model. The present results should be interpreted with some caution while considering the relatively few trials and significant heterogeneity.
The reasons that the addition of vitamins C and E to the eradication regimen did not increase H. pylori eradication rate might be as follows: first, antioxidant vitamin supplements might have either a beneficial or a harmful effect for H. pylori eradication. It was reported that vitamin C could mediate the formation of genotoxins from lipid hydroperoxides in the absence of transition metal ions(Reference Lee, Oe and Blair24). Second, the observation that vitamin C inhibits the in vitro growth of H. pylori at concentrations of 2048, 512 and 128 μg/ml at pH values of 7·4, 6·0 and 5·5 is of particular interest, and the inhibitory activity of vitamin C might be pH dependent(Reference Zhang, Wakisaka and Maeda4). Therefore, PPI might have a negative effect on the H. pylori inhibitory activity of vitamin C through elevating intra-gastric pH.
The eradication rate of H. pylori was poor in some studies included in our meta-analysis(Reference Sezikli, Cetinkaya and Sezikli10–Reference Koçkar, Oztürk and Bavbek15). Several factors, including patient compliance, bacterial density and antimicrobial susceptibility of H. pylori, contributed to the unsatisfying eradication rate of the standard treatment. A triple regimen without the use of PPI in the trial by Everett et al. (Reference Everett, Drake and White12) could be another reason for explaining the low eradication rate.
This meta-analysis has several limitations. The studies included are of relatively poor quality, with five of the six studies having a Jadad score of < 3. None of the RCT described the generation of a random sequence. Only one study included in this meta-analysis performed double blinding and allocation concealment. Thus, the reliability of the evidence presented in the present study is clearly low. An additional limitation is the small number of available studies and an overall sample size of fewer than 1000 patients included in the meta-analysis, which does not allow for conclusive statements on the effectiveness of vitamin supplements to H. pylori eradication regimen for treating H. pylori infection. Considering the low and moderate quality of evidence and limited data, ongoing and future trials for the effect of vitamins on H. pylori eradication might affect the present results.
In summary, based on the available data, vitamins C and/or E supplements to H. pylori eradication regimens could not improve the eradication rate of H. pylori. However, these data do not allow for definitive conclusions about the effectiveness of antioxidant vitamins on H. pylori eradication, owing to the small sample size and low-to-moderate methodological quality. In future studies, more rigorous double-blind and larger RCT to study the effects of vitamin supplements to H. pylori eradication regimen are required. The findings from our meta-analysis may allow for a better planning of those trials, sample size estimation, definition of treatment protocol and selection of trial end points.
Acknowledgements
G. L., L. L. and L. C. conceived the study design; L. L. and C. Y. searched and selected the trials, extracted, analysed and interpreted the data; G. L., L. L. and L. C. drafted the manuscript. All authors read and approved the final version of the manuscript. The authors have no conflicts of interest. The study was supported by the science and technology plan projects of Zhejiang Province (grant nos 2008C33053 and 2009C33010).








