Intake of added sweeteners, essentially of table sugar and high-fructose corn syrup, is markedly increased today compared with the 1960s(Reference Tappy and Lê1, Reference Malik, Schulze and Hu2). Table sugar consists of sucrose which is a disaccharide composed of fructose and glucose. Similarly, high-fructose corn syrup contains about 42–55 % of fructose and about 45–58 % of glucose in monosaccharide form. Several epidemiological studies have related high dietary fructose intake to prevalent obesity and overweight-associated diseases such as the metabolic syndrome, type 2 diabetes and fatty liver. In addition, overconsumption of fructose has been shown to cause obesity in rats(Reference Tappy and Lê1–Reference de Moura, Ribeiro and de Oliveira9).
There are important differences between fructose and glucose metabolism. Fructose, after intestinal uptake, is mainly removed from the blood stream by the liver in an insulin-independent manner. It is used for intrahepatic production of glucose, fatty acids or lactate. Newly synthesised NEFA are exported from the liver in the form of VLDL-TAG(Reference Tappy and Lê1, Reference Bray, Nielsen and Popkin3, Reference Abdel-Sayed, Binnert and Lê10). In contrast, glucose, once absorbed, is predominantly transported to peripheral tissues, where it is taken up into cells mediated via insulin.
The aim of the present exploratory study was to investigate and compare the effects of very high fructose and very high glucose in hyperenergetic diets on (1) intrahepatic- and intramyocellular lipids (IMCL), (2) visceral fat and (3) insulin resistance and plasma lipids. We studied young to middle-aged, normal- and overweight individuals and used a randomised design.
Experimental methods
Study design and diet
The TUebingen Fructose Or Glucose (TUFROG) study is an exploratory, prospective, randomised, single-blinded, outpatient, intervention study. Inclusion criteria were age 20–50 years, BMI 20–35 kg/m2, physical health and not more than 1 h sports/week. Exclusion criteria were pregnancy, any relevant illness (i.e. diabetes, dyslipidaemia, endocrine disease, coronary artery disease, malignancy, gastrointestinal disease and psychological disease), fructose intolerance, medication, metal implants (e.g. pacemaker, metal heart valve), regular alcohol consumption ≥ 10 g/d and claustrophobia. The participants received 150 g (2512 kJ (600 kcal)/d) of fructose or 150 g of glucose (2512 kJ (600 kcal)/d) for 4 weeks. They were not told whether they received fructose or glucose. The sugar was provided in identical plastic packs of 50 g and had to be dissolved in water (50 g sugar in 250 ml water). The participants were instructed to consume the sugar in addition to a balanced weight-maintaining diet (50 % carbohydrates, 35 % fat and 15 % protein). Fructose or glucose was ingested three times a day (morning, midday and evening) with the main meals. Dietary counselling was provided by an experienced dietitian according to the guidelines of the German Society of Nutrition. There were three visits at the study laboratory: a screening examination at the beginning of the study and visits 1 and 2 (clinical examination, blood withdrawal, oral glucose tolerance test (OGTT), MRI and magnetic resonance spectroscopy) at 2 and 6 weeks after the screening examination, respectively. Moreover, study participants were contacted via telephone at day 14 of the intervention to determine whether the fructose or glucose was well tolerated and regularly consumed. At the screening examination, the decision about inclusion of a participant was made by a physician based on the inclusion–exclusion criteria mentioned above. Furthermore, participants had a fructose test drink and received dietary counselling for 1 h. After a run-in phase of 2 weeks between screening examination and visit 1 in which the subjects were instructed to keep an isoenergetic diet (50 % carbohydrates, 35 % fat and 15 % protein), the participants were randomised to the fructose or glucose intervention group, and dietary counselling for 1 h was repeated. Restricted randomisation (blocking) was performed with a computerised random number generator. We did not use stratification. Random number generation was performed by an information technology manager who was not clinically involved in the study. The random allocation sequence was concealed from the physicians enrolling the study participants. The information whether a participant had to be allocated to the glucose or fructose intervention was provided by a coordinator according to the randomisation sequence at visit 1. The laboratory personnel and the radiologists (J. M. and F. S., quantification of the body fat compartments) were blinded to the type of intervention. The other health care providers/data collectors/outcome adjuncators/data analysts knew about the type of intervention after randomisation had been performed(Reference Schulz, Altman and Moher11). We aimed to assess compliance with the dietary prescription by close telephone contact. The participants were instructed to immediately inform the investigators in case of problems with the intake of fructose or glucose. For this purpose, they were provided a calling card. Furthermore, compliance was evaluated by interview at visits 1 and 2. In addition, the subjects were asked to fill out food intake records on 3 d in each week of the study. Food intake records were incomplete in four participants. The data on the remaining sixteen subjects were analysed for energy intake and composition of the diet (percentage of carbohydrate, fat and protein) by a trained dietitian using DGE PC® software (GOE mbH, Linden, Germany; www.goe-software.de). The study was approved by the local ethics committee and was conducted in accordance with the Declaration of Helsinki. Informed written consent was obtained from all participants. Recruitment began in April 2008.
Body composition and body fat distribution
Total body fat was measured by the bioelectrical impedance method (RJL, Detroit, MI, USA)(Reference Lukaski, Johnson and Bolonchuk12). Subcutaneous abdominal fat and visceral adipose tissue were measured by MRI applying an axial T1-weighed fast spin echo technique with a 1·5 T whole-body MR imager (Magnetom Sonata; Siemens Healthcare, Erlangen, Germany) in the complete abdominal region, ranging from the head of the femur to the head of the humerus. Slice thickness was 10 mm, with a gap of 10 mm, between subsequent slices. Approximately thirty-five slices were recorded for each volunteer, depending on size(Reference Machann, Thamer and Schnoedt13).
Quantitative analysis of liver fat and intramyocellular lipids
Liver fat as well as IMCL of the tibialis anterior muscle was determined by localised proton magnetic resonance spectroscopy, applying a single-voxel stimulated echo acquisition mode (STEAM) technique with short echo time as described previously(Reference Machann, Thamer and Schnoedt14, Reference Thamer, Machann and Bachmann15).
Oral glucose tolerance test
We performed standard 75 g OGTT after a 10 h overnight fast. Venous plasma samples were obtained at 0, 30, 60, 90 and 120 min for the determination of plasma glucose and insulin.
Analytical procedures
Blood glucose was determined using a bedside glucose analyser based on a glucose-oxidase method (Yellow Springs Instruments, Yellow Springs, CO, USA). Insulin was analysed by microparticle enzyme immunoassay (Abbott Laboratories, Tokyo, Japan). Total, HDL- and LDL-cholesterol concentrations were measured with a standard colorimetric method on a Bayer analyser (Bayer Health Care, Leverkusen, Germany), and TAG and NEFA were quantified with an enzymatic method (Wako Chemicals, Neuss, Germany). Uric acid was measured with an enzymatic method on the automatic ADVIA 1650 analyser (Siemens Medical Solutions Diagnostics). All analytical procedures were performed about 10 min after blood withdrawal at the central laboratory facility of the University Hospital Tübingen, Germany.
Calculations
Energetic requirements were calculated using sex-specific equations presented by Mifflin et al. (Reference Mifflin, St Jeor and Hill16): males – resting energy expenditure = 9·99 × weight − 4·92 × age+6·25 × height+5; females – resting energy expenditure = 9·99 × weight − 4·92 × age+6·25 × height − 161. Total energy expenditure was computed by multiplication of the resting energy expenditure with a physical activity level of 1·6 ( ≤ 1 h of sports/week)(Reference Brooks, Butte and Rand17). Insulin sensitivity was estimated from the OGTT as proposed by Matsuda & DeFronzo(Reference Matsuda and DeFronzo18):
Furthermore, we used the homeostasis model assessment of insulin resistance = Ins0 × Gluc0/22·5(Reference Matthews, Hosker and Rudenski19).
Statistical analysis
The clinical and biochemical characteristics and estimated energy requirements of the study participants are presented as means with their standard errors separately for men and women. Comparisons of the baseline characteristics, estimated energy requirements, and dietary intake between the fructose and glucose groups were performed with the t test or the χ2 test for continuous and categorical variables, respectively. Changes in metabolic parameters in response to the 4-week high-hexose diets were studied with the two-sided paired samples t test. For body-weight gain and body-fat gain, we used the one-sided paired samples t test because both were expected to rise with extra energy. Data that were not normally distributed (Shapiro–Wilk W test) were transformed logarithmically (base e). ANCOVA was used to compare the changes in metabolic parameters (e.g. change in liver fat between visits 1 and 2) between the fructose and glucose intervention groups, with study group as the main factor and the metabolic parameter of interest at baseline (e.g. liver fat at visit 1) as covariate (two-sided tests). To estimate the treatment effect, differences in least-squares means and the corresponding 95 % CI were calculated based on the ANCOVA models(Reference Schulz, Altman and Moher11). Due to the exploratory nature of the present study, the P values were not corrected for multiple testing. The JMP statistical software package 4.0 (SAS Institute, Cary, NC, USA) was used.
Results
Subjects
A total of thirty-four individuals were screened (Fig. 1). Of this cohort, five subjects did not meet the inclusion criteria and four subjects declined participation. The remaining twenty-five subjects were randomised to either the fructose (n 12) or the glucose (n 13) intervention. There were two and three dropouts in the fructose and glucose groups, respectively (Fig. 1).

Fig. 1 Enrolment of the participants and completion of the study.
Baseline characteristics, energy requirements and diet
A total of twelve males and eight females completed the study. They had a mean age of 30·5 (sem 2·0) years and a mean BMI of 25·9 (sem 0·5) kg/m2. The estimated mean resting and daily energy expenditures and the portions of the hexose supplementation compared with the participants' daily energy requirements were not different in the fructose and glucose intervention groups (Tables 1 and 2). Energy intake and the composition of the diet (percentage of carbohydrate, fat and protein) during the run-in phase and during the intervention period were not different in the fructose and glucose intervention groups either (all P>0·2).
Table 1 Baseline characteristics of the study participants
(Mean values with their standard errors)

IMCL, intramyocellular lipids; HOMA, homeostasis model assessment.
* P values were significantly different between the fructose and glucose groups (two-sided t test or the χ2 test for continuous and categorical variables, respectively).
† Fructose group: men n 6 and women n 2; glucose group: men n 5 and women n 5.
‡ Fructose group: men n 5 and women n 3; glucose group: men n 4 and women n 5.
§ Estimated according to Matsuda.
Table 2 Energy requirements of the study participants
(Mean values with their standard errors)
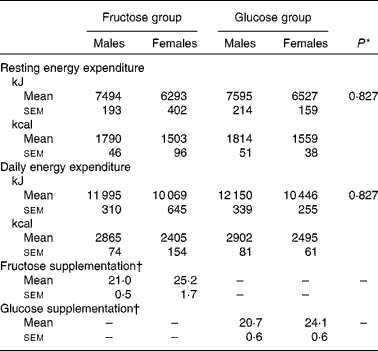
* P value for difference between the fructose and glucose groups (two-sided t test).
† Expressed as a percentage of daily energy expenditure (calculated from individual patient data).
Changes in body weight, fat depots and blood pressure in response to very-high-fructose or very-high-glucose diet
Body weight appeared to increase in the glucose but not in the fructose intervention group. The treatment effect of glucose and fructose on weight change was not different (Table 3). We did not observe strong alterations in total fat mass, visceral and subcutaneous abdominal fat, liver fat, IMCL of the tibialis anterior muscle and blood pressure in either group. Treatment effects of fructose and glucose on the changes in total fat mass, visceral and subcutaneous abdominal fat, liver fat, IMCL of the tibialis anterior muscle and blood pressure were not different (Table 3; see the supplementary figure available online at http://www.journals.cambridge.org/bjn).
Table 3 Changes in weight, fat depots and blood pressure in response to the very-high-fructose or very-high-glucose diet
(Mean values with their standard errors)

LSM, least-squares mean; IMCL, intramyocellular lipids.
* Absolute difference between visits 1 and 2.
† Treatment effect of fructose intervention compared with glucose intervention.
‡ P value (not corrected for multiple testing) for the change between visits 1 and 2 calculated with paired samples t test (one-sided in the case of body-weight gain and body-fat gain, two-sided in the case of systolic and diastolic blood pressure).
§ Difference in LSM between fructose and glucose intervention (calculated with ANCOVA with correction for baseline values).
∥ P value (not corrected for multiple testing) for the difference in change between fructose and glucose intervention (calculated with ANCOVA with correction for baseline values, two-sided).
¶ Fructose group n 8; glucose group n 10.
** Fructose group n 8; glucose group n 9.
Changes in glucose and lipid metabolism and in plasma uric acid in response to very-high-fructose or very-high-glucose diet
Insulin sensitivity estimated according to Matsuda seemed to decrease both in the fructose and glucose intervention groups. These results were supported by the changes in the homeostasis model assessment index (Table 4; see the supplementary figure, available online at http://www.journals.cambridge.org/bjn). We did not observe pronounced changes in plasma NEFA, total cholesterol, LDL-cholesterol, HDL-cholesterol and uric acid in either group (Table 3; see the supplementary figure, available online at http://www.journals.cambridge.org/bjn). Plasma TAG markedly increased in the fructose group with a trend towards a difference between interventions (Table 4; see the supplementary figure, available online at http://www.journals.cambridge.org/bjn).
Table 4 Changes in glucose and lipid metabolism and uric acid in response to the very-high-fructose or very-high-glucose diet
(Mean values with their standard errors)

LSM, least-squares mean; HOMA, homeostasis model assessment.
* Absolute difference between visits 1 and 2.
† Treatment effect of fructose intervention compared with glucose intervention.
‡ P value (not corrected for multiple testing) for the change between visits 1 and 2 calculated with paired samples t test (two-sided).
§ Difference in LSM between fructose and glucose intervention (calculated with ANCOVA with correction for baseline values).
∥ P value (not corrected for multiple testing) for the difference in change between fructose and glucose intervention (calculated with ANCOVA with correction for baseline values, two-sided).
¶ Estimated according to Matsuda.
Discussion
The main findings of the present study in young to middle-aged healthy subjects were (1) 4 weeks of very high fructose and very high glucose in hyperenergetic diets did not markedly increase liver fat, visceral fat and IMCL of the tibialis anterior muscle. (2) Treatment effects of glucose and fructose on the changes in liver fat, visceral fat and IMCL of the tibialis anterior muscle were not different. (3) Both very high fructose and very high glucose in hyperenergetic diets appeared to induce a decrease in insulin sensitivity with no difference between interventions. (4) Very high fructose intake but not very high glucose intake caused an elevation of plasma TAG. Of relevance, the alterations in metabolic parameters induced by both very high fructose and very high glucose in hyperenergetic diets were within the physiological range.
A meta-analysis has demonstrated that the effects of fructose intake on glucose and lipid metabolism are dose-dependent(Reference Livesey and Taylor20). Daily consumption of more than 50 g of fructose is suggested to be relevant in the pathogenesis of the metabolic syndrome(Reference Johnson, Segal and Sautin21). Fructose intake of more than 100 g/d is regarded as very high. Participants of the TUFROG study in addition to a balanced diet were administered 150 g of fructose or glucose/d for 4 weeks. This large amount was chosen to metabolically challenge the organism. Consequently, the present results do not reflect the metabolic effects of moderate monosaccharide intake in ‘real life’.
In agreement with previous studies, very high fructose intake was associated with a marked increase in plasma TAG, most probably caused by an up-regulation of hepatic de novo lipogenesis and TAG secretion and a decreased clearance of VLDL-TAG(Reference Livesey and Taylor20–Reference Ngo Sock, Lê and Ith26). On the contrary, NEFA were not strongly altered during the 4 weeks of the very-high-fructose or -glucose diet.
Our main objective was to investigate and compare the effects of very-high-fructose and very-high-glucose diets on hepatic lipid content and insulin resistance. An earlier study did not report an increase in ectopic lipid deposition in seven young healthy males receiving a hyperenergetic fructose diet (1·5 g/kg body weight per d) for 4 weeks(Reference Lê, Faeh and Stettler23). However, in three very recent studies, 7 d hyperenergetic fructose diets (3·5 g/kg fat-free mass per d) were associated with an increase in intrahepatic lipids again in healthy males (partly offspring of patients with type 2 diabetes) and particularly when combined with saturated fat(Reference Lê, Ith and Kreis25–Reference Sobrecases, Lê and Bortolotti27). Due to the availability of a control group receiving a very-high-glucose diet, it was possible to specifically address the difference between the effects of glucose and fructose on liver fat. Interestingly, the treatment effect of very high fructose and very high glucose in hyperenergetic diets on the relatively modest changes in liver fat was not different in participants of the TUFROG study. This finding in a cohort of males and females extends the results of a recently published study, which has been performed in males only(Reference Ngo Sock, Lê and Ith26). The data suggest that at least in healthy subjects, fructose and glucose have no majorly different impact on hepatic lipid content.
Both very high fructose and very high glucose in hyperenergetic diets appeared to induce a decrease in insulin sensitivity estimated from the OGTT in participants of the TUFROG study. Several previous studies have addressed the effect of fructose intake on insulin sensitivity(Reference Faeh, Minehira and Schwarz22–Reference Ngo Sock, Lê and Ith26, Reference Beck-Nielsen, Pedersen and Lindskov28). In eight young healthy subjects, addition of 250 g of fructose per day to the usual diet continued for 1 week was accompanied by a significant reduction of insulin sensitivity(Reference Beck-Nielsen, Pedersen and Lindskov28). A very-high-glucose diet (250 g/d) did not cause significant changes in insulin sensitivity in the present study(Reference Beck-Nielsen, Pedersen and Lindskov28). Another study in seven healthy males with a cross-over design reported that a 6 d hyperenergetic fructose diet (3 g/kg body weight per d) was associated with a decrease in hepatic and adipose tissue insulin sensitivity(Reference Faeh, Minehira and Schwarz22). Furthermore, a 7 d hyperenergetic fructose regimen (3·5 g fructose/kg fat-free mass per d) induced hepatic insulin resistance in a cohort of twenty-four healthy young males (partly offspring of subjects with type 2 diabetes) compared with an isoenergetic diet in a cross-over design(Reference Lê, Ith and Kreis25). Likewise, 7 d hyperenergetic fructose or glucose interventions (3·5 g/kg fat-free mass per d) induced an increase in hepatic glucose output v. an isoenergetic control diet in a cross-over study in eleven healthy males(Reference Ngo Sock, Lê and Ith26). In contrast, insulin sensitivity remained unchanged in a study in seven young healthy males after a 4-week hyperenergetic fructose diet (1·5 g/kg body weight per d) compared with an isoenergetic balanced diet using a longitudinal design(Reference Lê, Faeh and Stettler23).
Unlike a recent trial by Stanhope et al. (Reference Stanhope, Schwarz and Keim24), a very-high-fructose diet did not induce visceral obesity in the TUFROG collective. This may be due to the fact that participants of the TUFROG study were younger and had a lower BMI and therefore less metabolic risk. In addition, the study by Stanhope et al. had a longer duration of intervention (10 v. 4 weeks). Furthermore, the subjects consuming fructose in the study by Stanhope et al. (Reference Stanhope, Schwarz and Keim24) gained a significant amount of body weight and body fat.
Body weight did not increase during fructose intervention in our cohort. This observation has also been made by Lê et al. (Reference Lê, Faeh and Stettler23). Bray et al. (Reference Bray, Nielsen and Popkin3) initially raised the hypothesis that fructose may be related to obesity. This possible relationship has been suggested to be accounted for by the fact that fructose consumption does not provoke endogenous secretion of leptin(Reference Stanhope, Schwarz and Keim24). Clinical studies investigating the implications of fructose on food intake are controversial(Reference Rodin, Reed and Jamner29, Reference Akhavan and Anderson30).
Fructose-induced hyperuricaemia has been hypothesised to be a causal factor in the pathogenesis of the metabolic syndrome(Reference Johnson, Perez-Pozo and Sautin31). However, we did not observe an increase in the plasma uric acid concentration in the fructose group.
It has been reported that the increase in plasma TAG and the decrease in insulin sensitivity in response to high dietary fructose intake were only apparent in males(Reference Couchepin, Lê and Bortolotti32, Reference Tran, Jacot-Descombes and Lecoultre33). The sample size of the present study was too small to investigate possible differences in the metabolic effects of fructose between males and females.
The main limitation of the present study is that we performed multiple tests, which would require a strict correction, e.g. Bonferroni with a conservative significance level of P ≤ 0·001. Our approach was to clearly declare the study as exploratory only. Therefore, the findings have observational character and will definitely need to be reproduced and confirmed or challenged in larger cohorts in order to achieve a higher level of evidence. Furthermore, the TUFROG study was performed in an outpatient setting, which does not allow precise assessment of the compliance with the dietary instructions and with the intake of fructose or glucose. Nevertheless, the marked increase in plasma TAG in response to a very-high-fructose diet suggests that the bulk of carbohydrate supplementation was also ingested in the fructose intervention group. We also used a randomised single-blinded design to reduce confounding due to malcompliance. It represents another limitation of the TUFROG study that the number of participants was relatively small. However, the sample size was similar or even larger compared with previous highly recognised studies in the field(Reference Lê, Faeh and Stettler23). Of note, one would have to include 500 subjects (PS power and sample size calculations; biostat.mc.vanderbilt.edu) to detect a significant difference in the change of liver fat content of 0·07 % signal between the glucose and fructose groups. It also has to be mentioned that MRI data were available for the trunk only (visceral fat and subcutaneous abdominal fat). The bioelectric impedance analysis was used for the quantification of the whole body fat, which may cause imprecision. Finally, our findings are restricted to young to middle-aged healthy individuals at low metabolic risk.
In summary, our data suggest that very high fructose and very high glucose in hyperenergetic diets do not have different effects on insulin resistance and hepatic lipid content. However, the conclusions drawn from the present small exploratory study need to be validated in larger cohorts.
Acknowledgements
All authors participated in the design of the study. S. U. and G. S. were responsible for the recruitment of the participants and performed the OGTT. The fat depots were quantified by J. M. and F. S. Data analysis was performed by A. F., S. U. and G. S. The manuscript was written by G. S. and A. F., and was critically revised by J. M., F. S., N. S. and H. U. H. All authors read and approved the manuscript. We thank all study participants for their cooperation. Furthermore, we gratefully acknowledge the help and excellent technical assistance of A. Bury, M. Graf, B. Horrer, E. Kollmar, S. Kümmerle, H. Luz and A. Vosseler. The study was supported by a grant from the German Research Foundation (KFO 114/2) and a grant (grant no. 4 AI) from the Zentrum Ernährungsmedizin Tübingen-Hohenheim. Funding did not include industrial sponsorship. The authors have no conflict of interest to declare.







